Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.


206
6
C
H
A
P
T
E
R
ENGINEERING CONTEXT Up to this point, our study of the sec-
ond law has been concerned primarily with what it says about systems undergoing
thermodynamic cycles. In this chapter means are introduced for analyzing systems from
the second law perspective as they undergo processes that are not necessarily cycles.
The property entropy plays a prominent part in these considerations. The objective of the
present chapter is to introduce entropy and show its use for thermodynamic analysis.
The word energy is so much a part of the language that you were undoubtedly familiar
with the term before encountering it in early science courses. This familiarity probably
facilitated the study of energy in these courses and in the current course in engineering
thermodynamics. In the present chapter you will see that the analysis of systems from a
second law perspective is conveniently accomplished in terms of the property entropy.
Energy and entropy are both abstract concepts. However, unlike energy, the word entropy is
seldom heard in everyday conversation, and you may never have dealt with it quantitatively
before. Energy and entropy play important roles in the remaining chapters of this book.
Using Entropy
chapter objective
6.1 Introducing Entropy
Corollaries of the second law are developed in Chap. 5 for systems undergoing cycles while
communicating thermally with two reservoirs, a hot reservoir and a cold reservoir. In the
present section a corollary of the second law known as the Clausius inequality is introduced
that is applicable to any cycle without regard for the body, or bodies, from which the cycle
receives energy by heat transfer or to which the cycle rejects energy by heat transfer. The
Clausius inequality provides the basis for introducing two ideas instrumental for analyses of
both closed systems and control volumes from a second law perspective: the property entropy
(Sec. 6.2) and the entropy balance (Secs. 6.5 and 6.6).
The Clausius inequality states that for any thermodynamic cycle
(6.1)
where Q represents the heat transfer at a part of the system boundary during a portion of the
cycle, and T is the absolute temperature at that part of the boundary. The subscript “b” serves
as a reminder that the integrand is evaluated at the boundary of the system executing the cycle.
The symbol indicates that the integral is to be performed over all parts of the boundary and
~
a
dQ
T
b
b
0
Clausius inequality
6.1 Introducing Entropy 207
over the entire cycle. The equality and inequality have the same interpretation as in the
Kelvin–Planck statement: the equality applies when there are no internal irreversibilities as
the system executes the cycle, and the inequality applies when internal irreversibilities are
present. The Clausius inequality can be demonstrated using the Kelvin–Planck statement of
the second law (see box).
DEVELOPING THE CLAUSIUS INEQUALITY
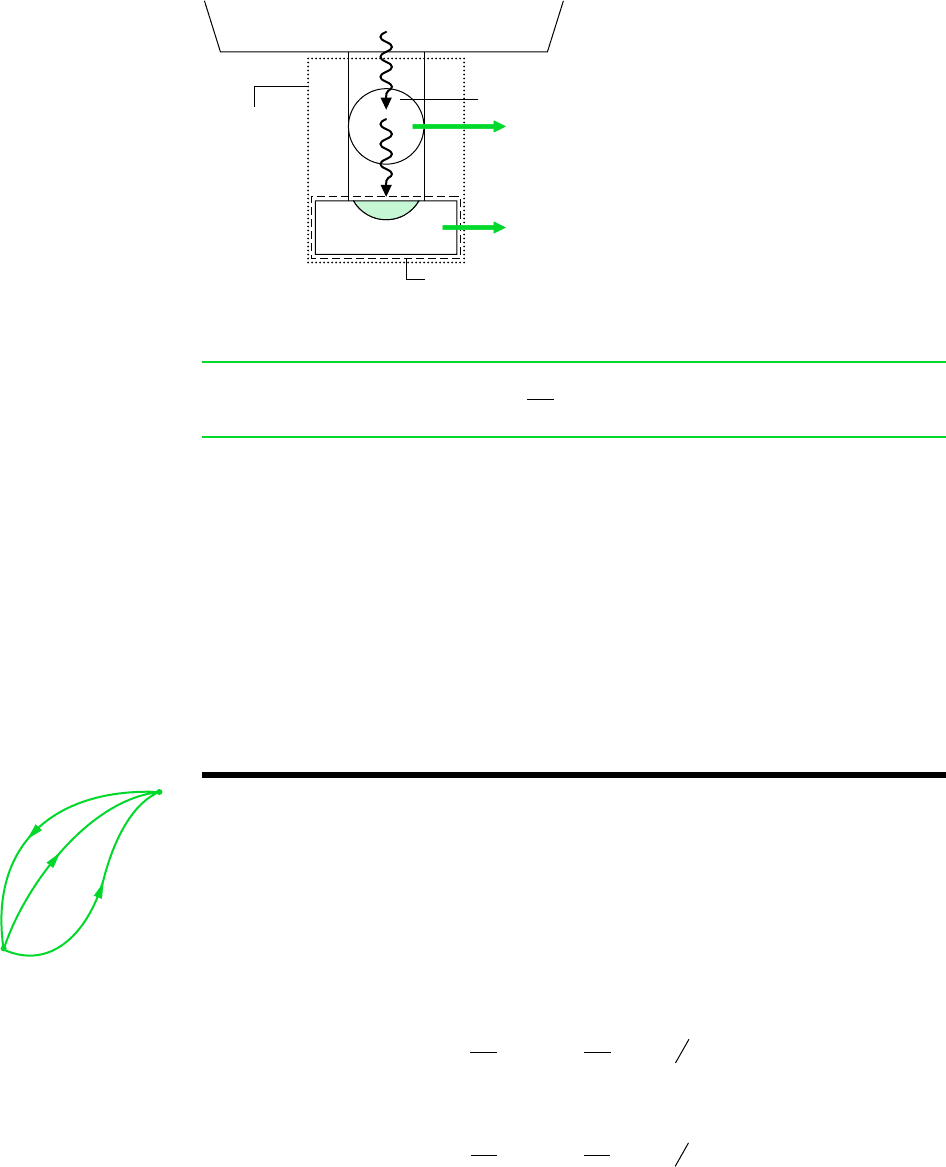
The Clausius inequality can be demonstrated using the arrangement of Fig. 6.1. A sys-
tem receives energy Q at a location on its boundary where the absolute temperature
is T while the system develops work W. In keeping with our sign convention for heat
transfer, the phrase receives energy Q includes the possibility of heat transfer from
the system. The energy Q is received from (or absorbed by) a thermal reservoir at
T
res
. To ensure that no irreversibility is introduced as a result of heat transfer between
the reservoir and the system, let it be accomplished through an intermediary system
that undergoes a cycle without irreversibilities of any kind. The cycle receives energy
Q from the reservoir and supplies Q to the system while producing work W. From
the definition of the Kelvin scale (Eq. 5.6), we have the following relationship between
the heat transfers and temperatures:
(a)
As temperature may vary, a multiplicity of such reversible cycles may be required.
Consider next the combined system shown by the dotted line on Fig. 6.1. An en-
ergy balance for the combined system is
where W
C
is the total work of the combined system, the sum of W and W, and dE
C
denotes the change in energy of the combined system. Solving the energy balance for
W
C
and using Eq. (a) to eliminate Q from the resulting expression yields
Now, let the system undergo a single cycle while the intermediary system undergoes
one or more cycles. The total work of the combined system is
(b)
Since the reservoir temperature is constant, T
res
can be brought outside the integral. The
term involving the energy of the combined system vanishes because the energy change
for any cycle is zero. The combined system operates in a cycle because its parts exe-
cute cycles. Since the combined system undergoes a cycle and exchanges energy by
heat transfer with a single reservoir, Eq. 5.1 expressing the Kelvin–Planck statement
of the second law must be satisfied. Using this, Eq. (b) reduces to give Eq. 6.1, where
the equality applies when there are no irreversibilities within the system as it executes
the cycle and the inequality applies when internal irreversibilities are present. This in-
terpretation actually refers to the combination of system plus intermediary cycle. How-
ever, the intermediary cycle is regarded as free of irreversibilities, so the only possible
site of irreversibilities is the system alone.
W
C
~
T
res
a
dQ
T
b
b
~
dE
0
C
T
res
~
a
dQ
T
b
b
dW
C
T
res
a
dQ
T
b
b
dE
C
dE
C
dQ¿ dW
C
dQ¿
T
res
a
dQ
T
b
b
Equation 6.1 can be expressed equivalently as
(6.2)
where
cycle
can be viewed as representing the “strength” of the inequality. The value of
cycle
is positive when internal irreversibilities are present, zero when no internal irreversibilities
are present, and can never be negative. In summary, the nature of a cycle executed by a sys-
tem is indicated by the value for
cycle
as follows:
Accordingly,
cycle
is a measure of the effect of the irreversibilities present within the system
executing the cycle. This point is developed further in Sec. 6.5, where
cycle
is identified as
the entropy produced (or generated) by internal irreversibilities during the cycle.
s
cycle
6 0
impossible
s
cycle
7 0
irreversibilities present within the system
s
cycle
0
no irreversibilities present within the system
~
a
dQ
T
b
b
s
cycle
208 Chapter 6 Using Entropy
6.2 Defining Entropy Change
A quantity is a property if, and only if, its change in value between two states is independ-
ent of the process (Sec. 1.3). This aspect of the property concept is used in the present sec-
tion together with Eq. 6.2 to introduce entropy.
Two cycles executed by a closed system are represented in Fig. 6.2. One cycle consists
of an internally reversible process A from state 1 to state 2, followed by internally reversible
process C from state 2 to state 1. The other cycle consists of an internally reversible process
B from state 1 to state 2, followed by the same process C from state 2 to state 1 as in the
first cycle. For the first cycle, Eq. 6.2 takes the form
(6.3a)
and for the second cycle
(6.3b)
In writing Eqs. 6.3, the term
cycle
has been set to zero since the cycles are composed of
internally reversible processes.
a
2
1
dQ
T
b
B
a
1
2
dQ
T
b
C
s
0
cycle
a
2
1
dQ
T
b
A
a
1
2
dQ
T
b
C
s
0
cycle
Reservoir at T
res
Intermediary
cycle
System boundary
δW´
δQ´
δQ
δW
Combined
system
boundary
System
T
Figure 6.1 Illustration used to develop the
Clausius inequality.
C
B
A
2
1
Figure 6.2 Two in-
ternally reversible cycles.

6.3 Retrieving Entropy Data 209
When Eq. 6.3b is subtracted from Eq. 6.3a
This shows that the integral of QT is the same for both processes. Since A and B are ar-
bitrary, it follows that the integral of QT has the same value for any internally reversible
process between the two states. In other words, the value of the integral depends on the end
states only. It can be concluded, therefore, that the integral represents the change in some
property of the system.
Selecting the symbol S to denote this property, which is called entropy, its change is
given by
(6.4a)
where the subscript “int rev” is added as a reminder that the integration is carried out for
any internally reversible process linking the two states. Equation 6.4a is the definition of
entropy change. On a differential basis, the defining equation for entropy change takes
the form
(6.4b)
Entropy is an extensive property.
The SI unit for entropy is J/K. However, in this book it is convenient to work in terms
of kJ/K. Units in SI for specific entropy are kJ/kg K for s and kJ/kmol K for
Since entropy is a property, the change in entropy of a system in going from one state to
another is the same for all processes, both internally reversible and irreversible, between these
two states. Thus, Eq. 6.4a allows the determination of the change in entropy, and once it has
been evaluated, this is the magnitude of the entropy change for all processes of the system
between the two states. The evaluation of entropy change is discussed further in the next
section.
It should be clear that entropy is defined and evaluated in terms of a particular integral
for which no accompanying physical picture is given. We encountered this previously with
the property enthalpy. Enthalpy is introduced without physical motivation in Sec. 3.3.2. Then,
in Chap. 4, enthalpy is shown to be useful for thermodynamic analysis. As for the case of
enthalpy, to gain an appreciation for entropy you need to understand how it is used and what
it is used for.
s
.
##
dS a
dQ
T
b
rev
int
S
2
S
1
a
2
1
dQ
T
b
rev
int
a
2
1
dQ
T
b
A
a
2
1
dQ
T
b
B
6.3 Retrieving Entropy Data
In Chap. 3, we introduced means for retrieving property data, including tables, graphs, equa-
tions, and the software available with this text. The emphasis there is on evaluating the prop-
erties p, v, T, u, and h required for application of the conservation of mass and energy
principles. For application of the second law, entropy values are usually required. In this
section, means for retrieving entropy data are considered.
definition of entropy
change
units for entropy

6.3.1 General Considerations
The defining equation for entropy change, Eq. 6.4a, serves as the basis for evaluating en-
tropy relative to a reference value at a reference state. Both the reference value and the ref-
erence state can be selected arbitrarily. The value of the entropy at any state y relative to the
value at the reference state x is obtained in principle from
(6.5)
where S
x
is the reference value for entropy at the specified reference state.
The use of entropy values determined relative to an arbitrary reference state is satisfac-
tory as long as they are used in calculations involving entropy differences, for then the ref-
erence value cancels. This approach suffices for applications where composition remains con-
stant. When chemical reactions occur, it is necessary to work in terms of absolute values of
entropy determined using the third law of thermodynamics (Chap. 13).
ENTROPY DATA FOR WATER AND REFRIGERANTS
Tables of thermodynamic data are introduced in Sec. 3.3 for water and several refrigerants
(Tables A-2 through A-18). Specific entropy is tabulated in the same way as considered there
for the properties v, u, and h, and entropy values are retrieved similarly.
VAPOR DATA. In the superheat regions of the tables for water and the refrigerants, specific
entropy is tabulated along with v, u, and h versus temperature and pressure.
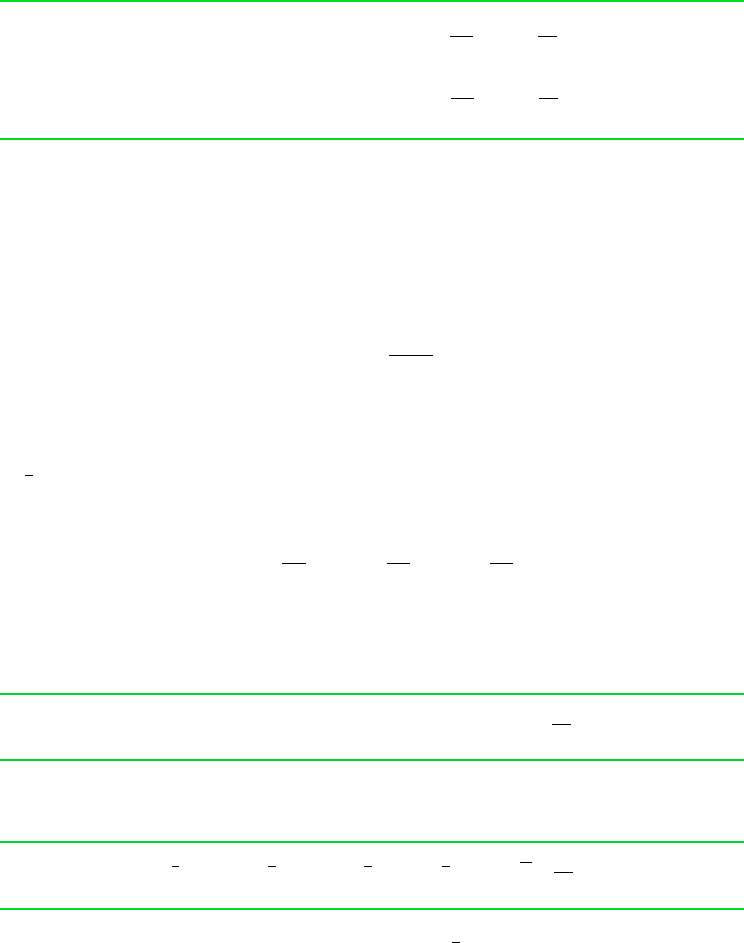
for example. . . consider two states of water. At state 1 the pressure is 3 MPa and the
temperature is 500C. At state 2, the pressure is 0.3 MPa and the specific entropy is the same
as at state 1, s
2
s
1
. The object is to determine the temperature at state 2. Using T
1
and p
1
,
we find the specific entropy at state 1 from Table A-4 as s
1
7.2338 kJkg K. State 2 is
fixed by the pressure, p
2
0.3 MPa, and the specific entropy, s
2
7.2338 kJkg K. Returing
to Table A-4 at 0.3 MPa and interpolating with s
2
between 160 and 200C results in
T
2
183C.
SATURATION DATA. For saturation states, the values of s
f
and s
g
are tabulated as a func-
tion of either saturation pressure or saturation temperature. The specific entropy of a two-
phase liquid–vapor mixture is calculated using the quality
(6.6)
These relations are identical in form to those for v, u, and h (Sec. 3.3). for example. . .
let us determine the specific entropy of Refrigerant 134a at a state where the temperature
is 0C and the specific internal energy is 138.43 kJ/kg. Referring to Table A-10, we see that
the given value for u falls between u
f
and u
g
at 0C, so the system is a two-phase liquid–vapor
mixture. The quality of the mixture can be determined from the known specific internal
energy
Then with values from Table A-10
10.5210.19702 10.5210.91902 0.5580 kJ/kg
#
K
s 11 x2s
f
xs
g
x
u u
f
u
g
u
f
138.43 49.79
227.06 49.79
0.5
s 11 x2s
f
xs
g
s
f
x1s
g
s
f
2
#
#
S
y
S
x
a
y
x
dQ
T
b
rev
int
210 Chapter 6 Using Entropy
6.3 Retrieving Entropy Data 211
LIQUID DATA. Compressed liquid data are presented for water in Tables A-5. In these tables
s, v, u, and h are tabulated versus temperature and pressure as in the superheat tables, and
the tables are used similarly. In the absence of compressed liquid data, the value of the specific
entropy can be estimated in the same way as estimates for v and u are obtained for liquid
states (Sec. 3.3.6), by using the saturated liquid value at the given temperature
(6.7)
for example. . .
suppose the value of specific entropy is required for water at 25 bar,
200C. The specific entropy is obtained directly from Table A-5 as s 2.3294 kJkg K. Using
the saturated liquid value for specific entropy at 200C from Table A-2, the specific entropy
is approximated with Eq. 6.7 as s 2.3309 kJkg K, which agrees closely with the previous
value.
The specific entropy values for water and the refrigerants given in Tables A-2 through
A-18 are relative to the following reference states and values. For water, the entropy of
saturated liquid at 0.01C is set to zero. For the refrigerants, the entropy of the saturated liq-
uid at 40C is assigned a value of zero.
COMPUTER RETRIEVAL. The software available with this text, Interactive Thermodynam-
ics: IT, provides data for the substances considered in this section. Entropy data are retrieved
by simple call statements placed in the workspace of the program. for example. . .
consider a two-phase liquid–vapor mixture of H
2
O at p 1 bar, v 0.8475 m
3
/kg. The fol-
lowing illustrates how specific entropy and quality x are obtained using IT
p = 1 // bar
v = 0.8475 // m
3
/kg
v = vsat_Px(“Water/Steam”,p,x)
s = ssat_Px(“Water/Steam”,p,x)
The software returns values of x 0.5 and s 4.331 kJ/kg K, which can be checked using
data from Table A-3. Note that quality x is implicit in the list of arguments in the expression
for specific volume, and it is not necessary to solve explicitly for x. As another example, con-
sider superheated ammonia vapor at p 1.5 bar, T 8C. Specific entropy is obtained from
IT as follows:
p = 1.5 // bar
T = 8 // C
s = s_PT(“Ammonia”,p,T)
The software returns s 5.981 kJ/kg K, which agrees closely with the value obtained by
interpolation in Table A-15.
USING GRAPHICAL ENTROPY DATA
The use of property diagrams as an adjunct to problem solving is emphasized throughout
this book. When applying the second law, it is frequently helpful to locate states and plot
processes on diagrams having entropy as a coordinate. Two commonly used figures having
entropy as one of the coordinates are the temperature–entropy diagram and the enthalpy–
entropy diagram.
TEMPERATURE–ENTROPY DIAGRAM. The main features of a temperature–entropy
diagram are shown in Fig. 6.3. For detailed figures for water in SI, see Fig. A-7. Observe
#
#
#
#
s1T, p2 s
f
1T 2
METHODOLOGY
UPDATE
Note that IT does not
provide compressed liquid
data for any substance. IT
returns liquid entropy data
using the approximation of
Eq. 6.7. Similarly, Eqs. 3.11,
3.12, and 3.14 are used to
return liquid values for v,
u, and h, respectively.
T–s diagram
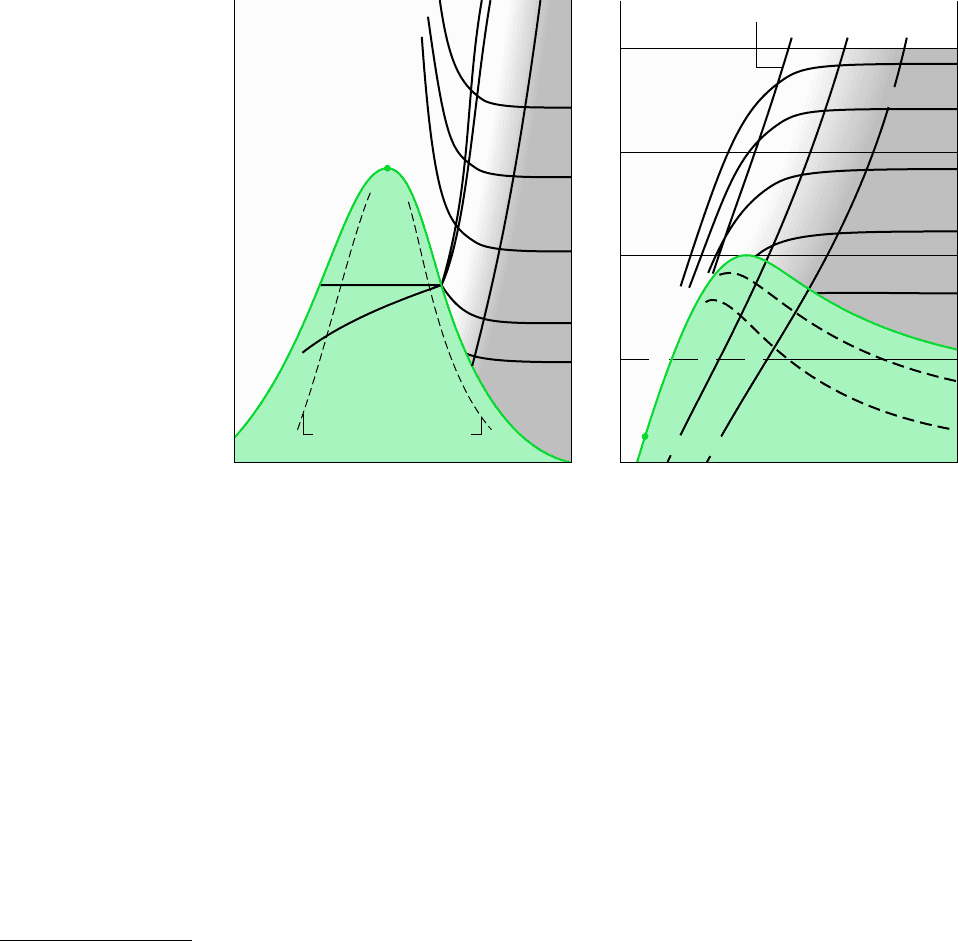
that lines of constant enthalpy are shown on these figures. Also note that in the super-
heated vapor region constant specific volume lines have a steeper slope than constant-
pressure lines. Lines of constant quality are shown in the two-phase liquid–vapor region.
On some figures, lines of constant quality are marked as percent moisture lines. The per-
cent moisture is defined as the ratio of the mass of liquid to the total mass.
In the superheated vapor region of the T–s diagram, constant specific enthalpy lines be-
come nearly horizontal as pressure is reduced. These states are shown as the shaded area on
Fig. 6.3. For states in this region of the diagram, the enthalpy is determined primarily by the
temperature: h(T, p) h(T). This is the region of the diagram where the ideal gas model
provides a reasonable approximation. For superheated vapor states outside the shaded area,
both temperature and pressure are required to evaluate enthalpy, and the ideal gas model is
not suitable.
ENTHALPY–ENTROPY DIAGRAM. The essential features of an enthalpy–entropy diagram,
commonly known as a Mollier diagram, are shown in Fig. 6.4. For detailed figures for water
in SI, see Figs. A-8. Note the location of the critical point and the appearance of lines of
constant temperature and constant pressure. Lines of constant quality are shown in the two-
phase liquid–vapor region (some figures give lines of constant percent moisture). The figure
is intended for evaluating properties at superheated vapor states and for two-phase liquid–
vapor mixtures. Liquid data are seldom shown. In the superheated vapor region, constant-
temperature lines become nearly horizontal as pressure is reduced. These states are shown,
approximately, as the shaded area on Fig. 6.4. This area corresponds to the shaded area on
the temperature–entropy diagram of Fig. 6.3, where the ideal gas model provides a reasonable
approximation.
for example. . . to illustrate the use of the Mollier diagram in SI units, consider
two states of water. At state 1, T
1
240C, p
1
0.10 MPa. The specific enthalpy and
212 Chapter 6 Using Entropy
S
a
t
u
r
a
t
e
d
v
a
p
o
r
S
a
t
u
r
a
t
e
d
l
i
q
u
i
d
Critical point
p = constant
v = constant
x = 0.2 x = 0.9
h
= constant
v = constant
p = constant
p = constant
T
s
x
=
0
.
9
0
x
=
0
.
9
6
S
a
t
u
r
a
t
e
d
v
a
p
o
r
p = constant
T = constant
T = constant
p = constant
p = constant
s
h
Critical point
Figure 6.3 Temperature–entropy diagram.
Figure 6.4 Enthalpy–entropy diagram.
Mollier diagram
6.3 Retrieving Entropy Data 213
first T dS equation
second T dS equation
quality are required at state 2, where p
2
0.01 MPa and s
2
s
1
. Turning to Fig. A-8, state 1
is located in the superheated vapor region. Dropping a vertical line into the two-phase
liquid–vapor region, state 2 is located. The quality and specific enthalpy at state 2 read
from the figure agree closely with values obtained using Tables A-3 and A-4: x
2
0.98 and
h
2
2537 kJ/kg.
USING THE T dS EQUATIONS
Although the change in entropy between two states can be determined in principle by using
Eq. 6.4a, such evaluations are generally conducted using the T dS equations developed in
this section. The T dS equations allow entropy changes to be evaluated from other more read-
ily determined property data. The use of the T dS equations to evaluate entropy changes for
ideal gases is illustrated in Sec. 6.3.2 and for incompressible substances in Sec. 6.3.3. The
importance of the T dS equations is greater than their role in assigning entropy values, how-
ever. In Chap. 11 they are used as a point of departure for deriving many important property
relations for pure, simple compressible systems, including means for constructing the prop-
erty tables giving u, h, and s.
The T dS equations are developed by considering a pure, simple compressible system un-
dergoing an internally reversible process. In the absence of overall system motion and the
effects of gravity, an energy balance in differential form is
(6.8)
By definition of simple compressible system (Sec. 3.1), the work is
(6.9a)
On rearrangement of Eq. 6.4b, the heat transfer is
(6.9b)
Substituting Eqs. 6.9 into Eq. 6.8, the first T dS equation results
(6.10)
The second T dS equation is obtained from Eq. 6.10 using H U pV. Forming the
differential
On rearrangement
Substituting this into Eq. 6.10 gives the second T dS equation
(6.11)
The T dS equations can be written on a unit mass basis as
(6.12a)
(6.12b)
T ds dh v dp
T ds du p dv
T dS dH V dp
dU p dV dH V dp
dH dU d1
pV2 dU p dV V dp
T
dS dU p dV
1dQ2
int
rev
T
dS
1dW2
int
rev
p
dV
1dQ2
int
rev
dU 1dW2
int
rev

or on a per mole basis as
(6.13a)
(6.13b)
Although the T dS equations are derived by considering an internally reversible process,
an entropy change obtained by integrating these equations is the change for any process, re-
versible or irreversible, between two equilibrium states of a system. Because entropy is a
property, the change in entropy between two states is independent of the details of the process
linking the states.
To show the use of the T dS equations, consider a change in phase from saturated liquid
to saturated vapor at constant temperature and pressure. Since pressure is constant, Eq. 6.12b
reduces to give
Then, because temperature is also constant during the phase change
(6.14)
This relationship shows how s
g
s
f
is calculated for tabulation in property tables.
for example. . . consider Refrigerant 134a at 0C. From Table A-10, h
g
h
f
197.21 kJ/kg, so with Eq. 6.14
which is the value calculated using s
f
and s
g
from the table.
6.3.2 Entropy Change of an Ideal Gas
In this section the T dS equations are used to evaluate the entropy change between two states
of an ideal gas. It is convenient to begin with Eqs. 6.12 expressed as
(6.15)
(6.16)
For an ideal gas, du c
v
(T) dT, dh c
p
(T) dT, and pv RT. With these relations,
Eqs. 6.15 and 6.16 become, respectively
(6.17)
Since R is a constant, the last terms of Eqs. 6.17 can be integrated directly. However, because
c
v
and c
p
are functions of temperature for ideal gases, it is necessary to have information about
ds c
v
1T 2
dT
T
R
dv
v
and
ds c
p
1T 2
dT
T
R
dp
p
ds
dh
T
v
T
dp
ds
du
T
p
T
dv
s
g
s
f
197.21
kJ/kg
273.15 K
0.7220
kJ
kg
#
K
s
g
s
f
h
g
h
f
T
ds
dh
T
T d s d h v dp
T d s d u p d v
214 Chapter 6 Using Entropy
6.3 Retrieving Entropy Data 215
the functional relationships before the integration of the first term in these equations can be
performed. Since the two specific heats are related by
(3.44)
where R is the gas constant, knowledge of either specific heat function suffices.
On integration, Eqs. 6.17 give, respectively
(6.18)
(6.19)
USING IDEAL GAS TABLES. As for internal energy and enthalpy changes, the evaluation
of entropy changes for ideal gases can be reduced to a convenient tabular approach. To in-
troduce this, we begin by selecting a reference state and reference value: The value of the spe-
cific entropy is set to zero at the state where the temperature is 0 K and the pressure is 1 at-
mosphere. Then, using Eq. 6.19, the specific entropy at a state where the temperature is T and
the pressure is 1 atm is determined relative to this reference state and reference value as
(6.20)
The symbol s(T ) denotes the specific entropy at temperature T and a pressure of 1 atm.
Because s depends only on temperature, it can be tabulated versus temperature, like
h and u. For air as an ideal gas, s with units of is given in Tables A-22.Values
of for several other common gases are given in Tables A-23 with units of .
Since the integral of Eq. 6.19 can be expressed in terms of s
it follows that Eq. 6.19 can be written as
(6.21a)
or on a per mole basis as
(6.21b)
Using Eqs. 6.21 and the tabulated values for s or as appropriate, entropy changes
can be determined that account explicitly for the variation of specific heat with temperature.
for example. . . let us evaluate the change in specific entropy, in kJkg K, of air
modeled as an ideal gas from a state where T
1
300 K and p
1
1 bar to a state where
#
s
°,
s
1T
2
, p
2
2 s1T
1
, p
1
2 s °1T
2
2 s °1T
1
2 R ln
p
2
p
1
s1T
2
, p
2
2 s1T
1
, p
1
2 s°1T
2
2 s°1T
1
2 R ln
p
2
p
1
s°1T
2
2 s°1T
1
2
T
2
T
1
c
p
dT
T
T
2
0
c
p
dT
T
T
1
0
c
p
dT
T
kJ/kmol
#
Ks °
kJ/kg
#
K
s°1T 2
T
0
c
p
1T 2
T
dT
s1T
2
, p
2
2 s1T
1
, p
1
2
T
2
T
1
c
p
1T 2
dT
T
R ln
p
2
p
1
s1T
2
,
v
2
2 s1T
1
, v
1
2
T
2
T
1
c
v
1T 2
dT
T
R ln
v
2
v
1
c
p
1T 2 c
v
1T 2 R
