Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

T
2
1000 K and p
2
3 bar. Using Eq. 6.21a and data from Table A-22
If a table giving s (or ) is not available for a particular gas of interest, the integrals of
Eqs. 6.18 and 6.19 can be performed analytically or numerically using specific heat data such
as provided in Tables A-20 and A-21.
ASSUMING CONSTANT SPECIFIC HEATS. When the specific heats c
v
and c
p
are taken as
constants, Eqs. 6.18 and 6.19 reduce, respectively, to
(6.22)
(6.23)
These equations, along with Eqs. 3.50 and 3.51 giving u and h, respectively, are applica-
ble when assuming the ideal gas model with constant specific heats.
for example. . . let us determine the change in specific entropy, in of air
as an ideal gas undergoing a process from T
1
300 K, p
1
1 bar to T
2
400 K, p
2
5 bar.
Because of the relatively small temperature range, we assume a constant value of c
p
evaluated
at 350 K. Using Eq. 6.23 and c
p
1.008 from Table A-20
COMPUTER RETRIEVAL. For air and other gases modeled as ideal gases, IT directly returns
s(T, p) based upon the following form of Eq. 6.19
and the following choice of reference state and reference value: T
ref
0 K (0R), p
ref
1 atm, and s(T
ref
, p
ref
) 0, giving
Changes in specific entropy evaluated using IT agree with entropy changes evaluated
using ideal gas tables.
for example. . . consider a process of air as an ideal gas from
T
1
300 K, p
1
1 bar to T
2
1000 K, p
2
3 bar. The change in specific entropy, denoted
s1T, p2
T
0
c
p
1T 2
T
dT R ln
p
p
ref
s1T, p2 s1T
ref
, p
ref
2
T
T
ref
c
p
1T 2
T
dT R ln
p
p
ref
0.1719 kJ/kg
#
K
a1.008
kJ
kg
#
K
b ln a
400 K
300 K
b a
8.314
28.97
kJ
kg
#
K
b ln a
5 bar
1 bar
b
¢s c
p
ln
T
2
T
1
R ln
p
2
p
1
kJ/kg
#
K
kJ/kg
#
K,
s1T
2
, p
2
2 s1T
1
, p
1
2 c
p
ln
T
2
T
1
R ln
p
2
p
1
s1T
2
, v
2
2 s1T
1
, v
1
2 c
v
ln
T
2
T
1
R ln
v
2
v
1
s °
0.9504 kJ/kg
#
K
12.96770 1.702032
kJ
kg
#
K
8.314
28.97
kJ
kg
#
K
ln
3 bar
1 bar
s
2
s
1
s°1T
2
2 s°1T
1
2 R ln
p
2
p
1
216 Chapter 6 Using Entropy
6.4 Entropy Change in Internally Reversible Processes 217
as dels, is determined in SI units using IT as follows:
p1 = 1 // bar
T1 = 300 // K
p2 = 3
T2 = 1000
s1 = s_TP(“Air”,T1,p1)
s2 = s_TP(“Air”,T2,p2)
dels = s2 – s1
The software returns values of s
1
1.706, s
2
2.656, and dels 0.9501, all in units of
This value for s agrees with the value obtained using Table A-22 in the example
following Eqs. 6.21. Note that IT returns specific entropy directly and does not use the
special function s.
6.3.3 Entropy Change of an Incompressible Substance
The incompressible substance model introduced in Sec. 3.3.6 assumes that the specific vol-
ume (density) is constant and the specific heat depends solely on temperature, c
v
c(T). Ac-
cordingly, the differential change in specific internal energy is du c(T ) dT and Eq. 6.15
reduces to
On integration, the change in specific entropy is
When the specific heat is assumed constant, this becomes
(6.24)
Equation 6.24, along with Eqs. 3.20 giving u and h, respectively, are applicable to liquids
and solids modeled as incompressible. Specific heats of some common liquids and solids are
given in Table A-19.
s
2
s
1
c ln
T
2
T
1
1incompressible, constant c2
s
2
s
1
T
2
T
1
c1T
2
T
dT
1incompressible2
ds
c1T
2 dT
T
p dv
T
0
c1T 2 dT
T
kJ/kg
#
K.
6.4 Entropy Change in Internally
Reversible Processes
In this section the relationship between entropy change and heat transfer for internally re-
versible processes is considered. The concepts introduced have important applications in sub-
sequent sections of the book. The present discussion is limited to the case of closed systems.
Similar considerations for control volumes are presented in Sec. 6.9.
As a closed system undergoes an internally reversible process, its entropy can increase,
decrease, or remain constant. This can be brought out using Eq. 6.4b
dS a
dQ
T
b
rev
int
which indicates that when a closed system undergoing an internally reversible process receives
energy by heat transfer, the system experiences an increase in entropy. Conversely, when en-
ergy is removed from the system by heat transfer, the entropy of the system decreases. This
can be interpreted to mean that an entropy transfer accompanies heat transfer. The direction
of the entropy transfer is the same as that of the heat transfer. In an adiabatic internally
reversible process, the entropy would remain constant. A constant-entropy process is called
an isentropic process.
On rearrangement, the above expression gives
Integrating from an initial state 1 to a final state 2
(6.25)
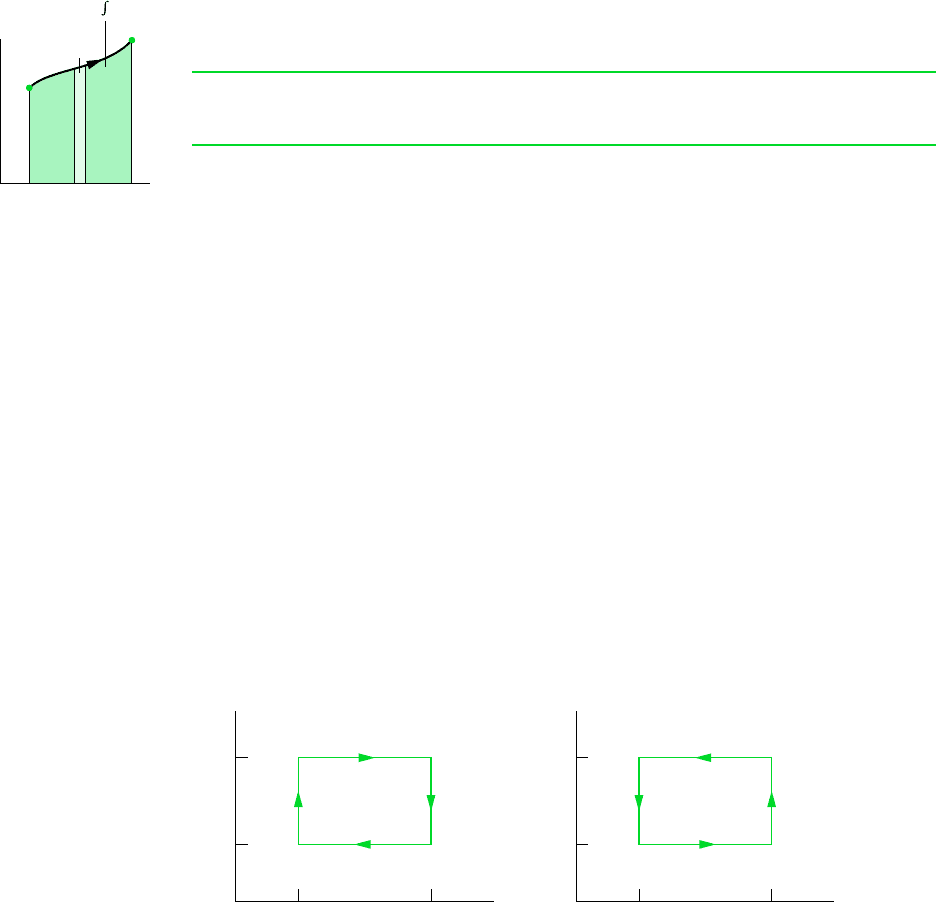
From Eq. 6.25 it can be concluded that an energy transfer by heat to a closed system dur-
ing an internally reversible process can be represented as an area on a temperature–entropy
diagram. Figure 6.5 illustrates the area interpretation of heat transfer for an arbitrary inter-
nally reversible process in which temperature varies. Carefully note that temperature must
be in kelvins or degrees Rankine, and the area is the entire area under the curve (shown
shaded). Also note that the area interpretation of heat transfer is not valid for irreversible
processes, as will be demonstrated later.
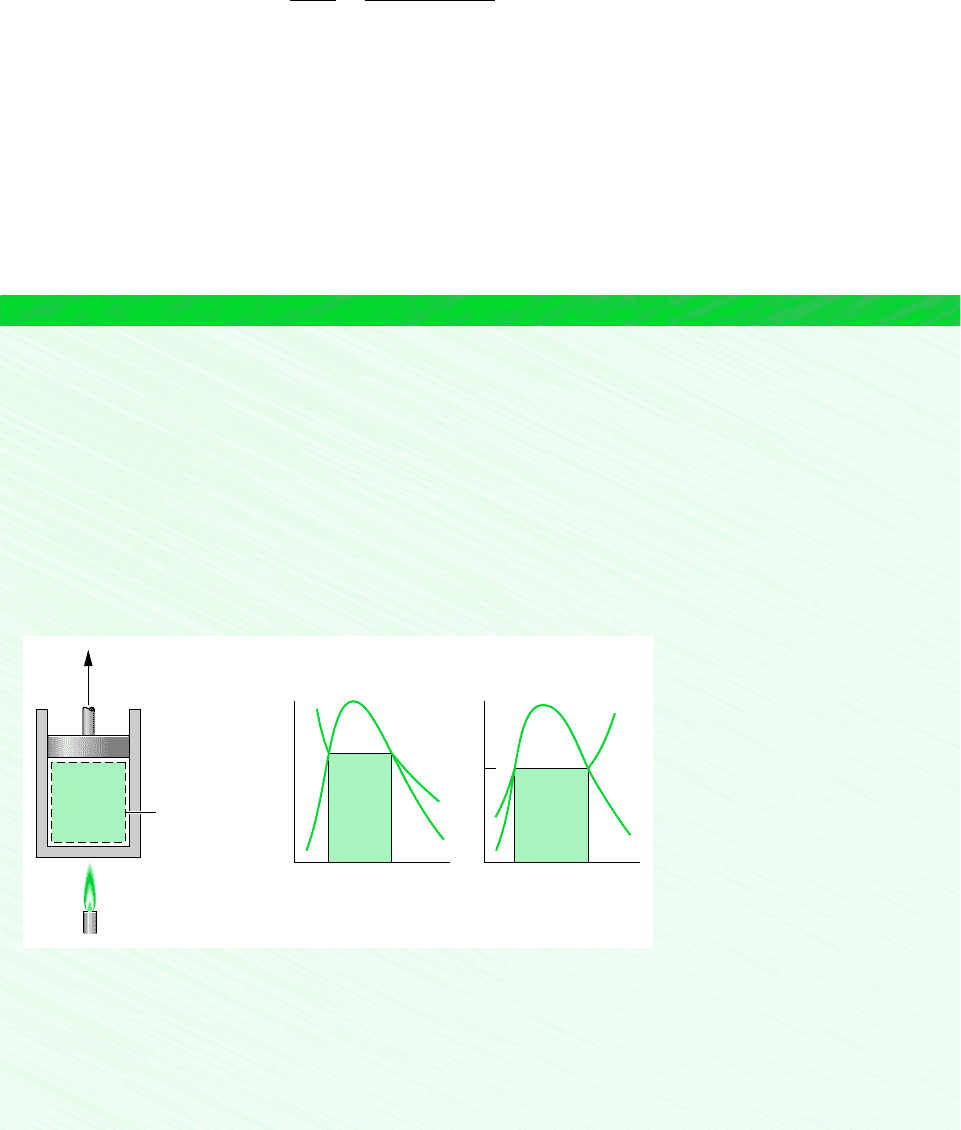
To provide an example illustrating both the entropy change that accompanies heat transfer
and the area interpretation of heat transfer, consider Fig. 6.6a, which shows a Carnot power
cycle (Sec. 5.6). The cycle consists of four internally reversible processes in series: two
isothermal processes alternated with two adiabatic processes. In Process 2–3, heat transfer
to the system occurs while the temperature of the system remains constant at T
H
. The sys-
tem entropy increases due to the accompanying entropy transfer. For this process, Eq. 6.25
gives Q
23
T
H
(S
3
S
2
), so area 2–3–a–b–2 on Fig. 6.6a represents the heat transfer during
the process. Process 3–4 is an adiabatic and internally reversible process and thus is an isen-
tropic (constant-entropy) process. Process 4–1 is an isothermal process at T
C
during which
heat is transferred from the system. Since entropy transfer accompanies the heat transfer, sys-
tem entropy decreases. For this process, Eq. 6.25 gives Q
41
T
C
(S
1
S
4
), which is nega-
tive in value. Area 4–1–b–a–4 on Fig. 6.6a represents the magnitude of the heat transfer Q
41
.
Process 1–2, which completes the cycle, is adiabatic and internally reversible (isentropic).
Q
int
rev
2
1
T dS
1dQ2
int
rev
T dS
218 Chapter 6 Using Entropy
1
2
S
T
(δQ) = T dS
Q =
2
1
T dS
int
rev
int
rev
Figure 6.6 Carnot cycles on the temperature–entropy diagram. (a) Power
cycle. (b) Refrigeration or heat pump cycle.
Figure 6.5 Area
representation of heat
transfer for an internally
reversible process of a
closed system.
isentropic process
Carnot cycle
23
14
T
H
T
C
T
ba
S
43
12
T
H
T
C
T
ba
S
(a)(b)
6.4 Entropy Change in Internally Reversible Processes 219
The net work of any cycle is equal to the net heat transfer, so enclosed area 1–2–3–4–1
represents the net work of the cycle. The thermal efficiency of the cycle may also be ex-
pressed in terms of areas:
The numerator of this expression is (T
H
T
C
)(S
3
S
2
) and the denominator is T
H
(S
3
S
2
),
so the thermal efficiency can be given in terms of temperatures only as 1 T
C
T
H
. If
the cycle were executed as shown in Fig. 6.6b, the result would be a Carnot refrigeration or
heat pump cycle. In such a cycle, heat is transferred to the system while its temperature re-
mains at T
C
, so entropy increases during Process 1–2. In Process 3–4 heat is transferred from
the system while the temperature remains constant at T
H
and entropy decreases.
To further illustrate concepts introduced in this section, the next example considers water
undergoing an internally reversible process while contained in a piston–cylinder assembly.
h
W
cycle
Q
23
area 1–2–3–4–1
area 2–3–a–b–2
EXAMPLE 6.1 Internally Reversible Process of Water
Water, initially a saturated liquid at 100C, is contained in a piston–cylinder assembly. The water undergoes a process to the
corresponding saturated vapor state, during which the piston moves freely in the cylinder. If the change of state is brought
about by heating the water as it undergoes an internally reversible process at constant pressure and temperature, determine the
work and heat transfer per unit of mass, each in kJ/kg.
SOLUTION
Known: Water contained in a piston–cylinder assembly undergoes an internally reversible process at 100C from saturated
liquid to saturated vapor.
Find: Determine the work and heat transfer per unit mass.
Schematic and Given Data:
p
fg
v
100°C
100°C
W
––
m
T
f
g
s
Q
––
m
System boundary
Water
Figure E6.1
Assumptions:
1. The water in the piston–cylinder assembly is a closed system.
2. The process is internally reversible.
3. Temperature and pressure are constant during the process.
4. There is no change in kinetic or potential energy between the two end states.

In this section, the Clausius inequality expressed by Eq. 6.2 and the defining equation for
entropy change are used to develop the entropy balance for closed systems. The entropy bal-
ance is an expression of the second law that is particularly convenient for thermodynamic
analysis. The current presentation is limited to closed systems. The entropy balance is ex-
tended to control volumes in Sec. 6.6.
6.5.1 Developing the Entropy Balance
Shown in Fig. 6.7 is a cycle executed by a closed system. The cycle consists of process I,
during which internal irreversibilities are present, followed by internally reversible process
R. For this cycle, Eq. 6.2 takes the form
(6.26)
2
1
a
dQ
T
b
b
1
2
a
dQ
T
b
rev
int
s
220 Chapter 6 Using Entropy
6.5 Entropy Balance for Closed Systems
❶
Analysis: At constant pressure the work is
With values from Table A-2
Since the process is internally reversible and at constant temperature, Eq. 6.25 gives
or
With values from Table A-2
As shown in the accompanying figure, the work and heat transfer can be represented as areas on p–v and T–s diagrams,
respectively.
The heat transfer can be evaluated alternatively from an energy balance written on a unit mass basis as
Introducing Wm p(v
g
v
f
) and solving
From Table A-2 at 100C, h
g
h
f
2257 kJ/kg, which is the same value for Qm as obtained in the solution.
h
g
h
f
1u
g
pv
g
2 1u
f
pv
f
2
Q
m
1u
g
u
f
2 p1v
g
v
f
2
u
g
u
f
Q
m
W
m
Q
m
1373.15 K217.3549 1.30692 kJ/kg
#
K 2257 kJ/kg
Q
m
T 1s
g
s
f
2
Q
g
f
T dS m
g
f
T ds
170 kJ/kg
W
m
11.014
bar2 11.673 1.0435 10
3
2 a
m
3
kg
b
`
10
5
N/m
2
1 bar
``
1
kJ
10
3
N
#
m
`
W
m
g
f
p dv p1v
g
v
f
2
1
R
I
2
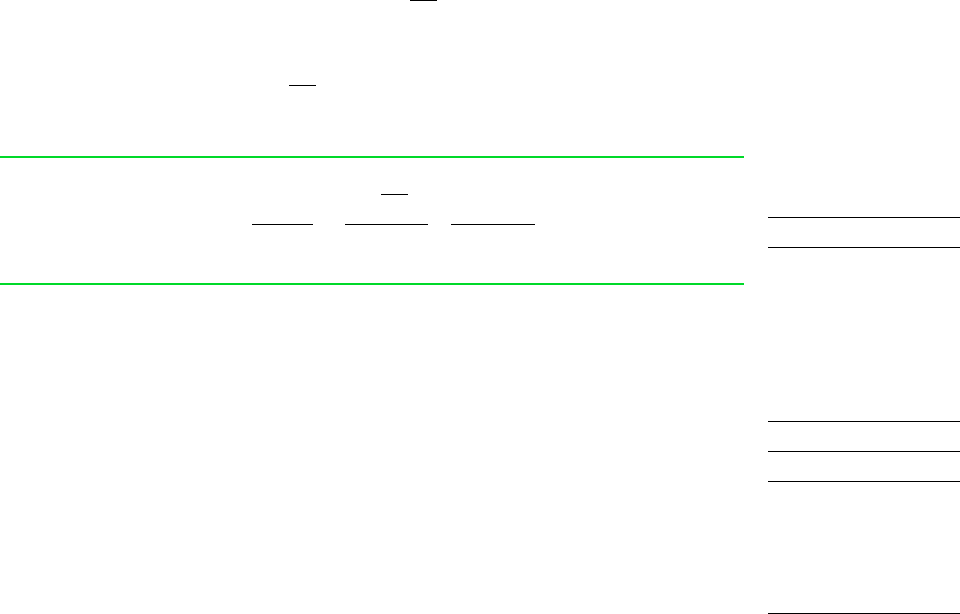
Figure 6.7 Cycle
used to develop the
entropy balance.
❶
6.5 Entropy Balance for Closed Systems 221
where the first integral is for process I and the second is for process R. The subscript b in the
first integral serves as a reminder that the integrand is evaluated at the system boundary. The sub-
script is not required in the second integral because temperature is uniform throughout the system
at each intermediate state of an internally reversible process. Since no irreversibilities are
associated with process R, the term
cycle
of Eq. 6.2, which accounts for the effect of
irreversibilities during the cycle, refers only to process I and is shown in Eq. 6.26 simply as .
Applying the definition of entropy change, we can express the second integral of Eq. 6.26 as
With this, Eq. 6.26 becomes
Finally, on rearranging the last equation, the closed system entropy balance results
(6.27)
entropy entropy entropy
change transfer production
If the end states are fixed, the entropy change on the left side of Eq. 6.27 can be evaluated
independently of the details of the process. However, the two terms on the right side depend
explicitly on the nature of the process and cannot be determined solely from knowledge of
the end states. The first term on the right side of Eq. 6.27 is associated with heat transfer to
or from the system during the process. This term can be interpreted as the entropy transfer
accompanying heat transfer. The direction of entropy transfer is the same as the direction of
the heat transfer, and the same sign convention applies as for heat transfer: A positive value
means that entropy is transferred into the system, and a negative value means that entropy is
transferred out. When there is no heat transfer, there is no entropy transfer.
The entropy change of a system is not accounted for solely by the entropy transfer, but is
due in part to the second term on the right side of Eq. 6.27 denoted by . The term is pos-
itive when internal irreversibilities are present during the process and vanishes when no in-
ternal irreversibilities are present. This can be described by saying that entropy is produced
within the system by the action of irreversibilities. The second law of thermodynamics can
be interpreted as requiring that entropy is produced by irreversibilities and conserved only
in the limit as irreversibilities are reduced to zero. Since measures the effect of irre-
versibilities present within the system during a process, its value depends on the nature of
the process and not solely on the end states. It is not a property.
When applying the entropy balance to a closed system, it is essential to remember the re-
quirements imposed by the second law on entropy production: The second law requires that
entropy production be positive, or zero, in value
(6.28)
The value of the entropy production cannot be negative. By contrast, the change in entropy
of the system may be positive, negative, or zero:
(6.29)
Like other properties, entropy change can be determined without knowledge of the details
of the process.
S
2
S
1
: •
7 0
0
6 0
s: e
7 0
0
irreversibilities present within the system
no irreversibilities present within the system
S
2
S
1
2
1
a
dQ
T
b
b
s
2
1
a
dQ
T
b
b
1S
1
S
2
2s
S
1
S
2
1
2
a
dQ
T
b
rev
int
closed system entropy
balance
entropy transfer
accompanying heat
transfer
entropy production
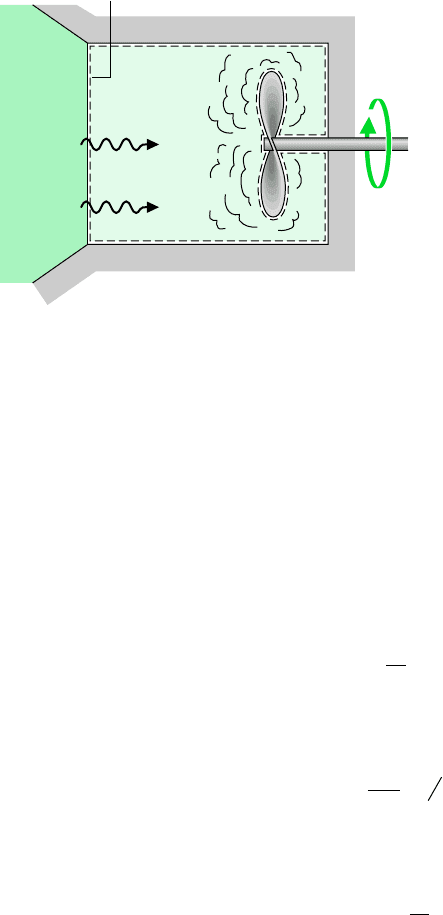
for example. . . to illustrate the entropy transfer and entropy production concepts,
as well as the accounting nature of entropy balance, consider Fig. 6.8. The figure shows a
system consisting of a gas or liquid in a rigid container stirred by a paddle wheel while re-
ceiving a heat transfer Q from a reservoir. The temperature at the portion of the boundary
where heat transfer occurs is the same as the constant temperature of the reservoir, T
b
. By
definition, the reservoir is free of irreversibilities; however, the system is not without irre-
versibilities, for fluid friction is evidently present, and there may be other irreversibilities
within the system.
Let us now apply the entropy balance to the system and to the reservoir. Since T
b
is con-
stant, the integral in Eq. 6.27 is readily evaluated, and the entropy balance for the system
reduces to
(6.30)
where QT
b
accounts for entropy transfer into the system accompanying heat transfer Q. The
entropy balance for the reservoir takes the form
where the entropy production term is set equal to zero because the reservoir is without irre-
versibilities. Since Q
res
Q, the last equation becomes
The minus sign signals that entropy is carried out of the reservoir accompanying heat transfer.
Hence, the entropy of the reservoir decreases by an amount equal to the entropy transferred from
it to the system. However, as shown by Eq. 6.30, the entropy change of the system exceeds the
amount of entropy transferred to it because of entropy production within the system.
If the heat transfer were oppositely directed in the above example, passing instead from
the system to the reservoir, the magnitude of the entropy transfer would remain the same,
but its direction would be reversed. In such a case, the entropy of the system would decrease
if the amount of entropy transferred from the system to the reservoir exceeded the amount
of entropy produced within the system due to irreversibilities. Finally, observe that there is
no entropy transfer associated with work.
¢S 4
res
Q
T
b
¢S 4
res
Q
res
T
b
s
0
res
S
2
S
1
Q
T
b
s
222 Chapter 6 Using Entropy
Gas or liquid
Reservoir
at T
b
Q/T
b
Q
This portion of the
boundary is at temperature T
b
Figure 6.8 Illustration of the
entropy transfer and entropy production
concepts.

6.5 Entropy Balance for Closed Systems 223
6.5.2 Other Forms of the Entropy Balance
The entropy balance can be expressed in various forms convenient for particular analyses.
For example, if heat transfer takes place at several locations on the boundary of a system
where the temperatures do not vary with position or time, the entropy transfer term can be
expressed as a sum, so Eq. 6.27 takes the form
(6.31)
where Q
j
T
j
is the amount of entropy transferred through the portion of the boundary at tem-
perature T
j
.
On a time rate basis, the closed system entropy rate balance is
(6.32)
where dSdt is the time rate of change of entropy of the system. The term represents
the time rate of entropy transfer through the portion of the boundary whose instantaneous
temperature is T
j
. The term accounts for the time rate of entropy production due to irre-
versibilities within the system.
It is sometimes convenient to use the entropy balance expressed in differential form
(6.33)
Note that the differentials of the nonproperties Q and are shown, respectively, as Q and .
When there are no internal irreversibilities, vanishes and Eq. 6.33 reduces to Eq. 6.4b.
6.5.3 Evaluating Entropy Production and Transfer
Regardless of the form taken by the entropy balance, the objective in many applications is
to evaluate the entropy production term. However, the value of the entropy production for a
given process of a system often does not have much significance by itself. The significance
is normally determined through comparison. For example, the entropy production within a
given component might be compared to the entropy production values of the other compo-
nents included in an overall system formed by these components. By comparing entropy pro-
duction values, the components where appreciable irreversibilities occur can be identified
and rank ordered. This allows attention to be focused on the components that contribute most
to inefficient operation of the overall system.
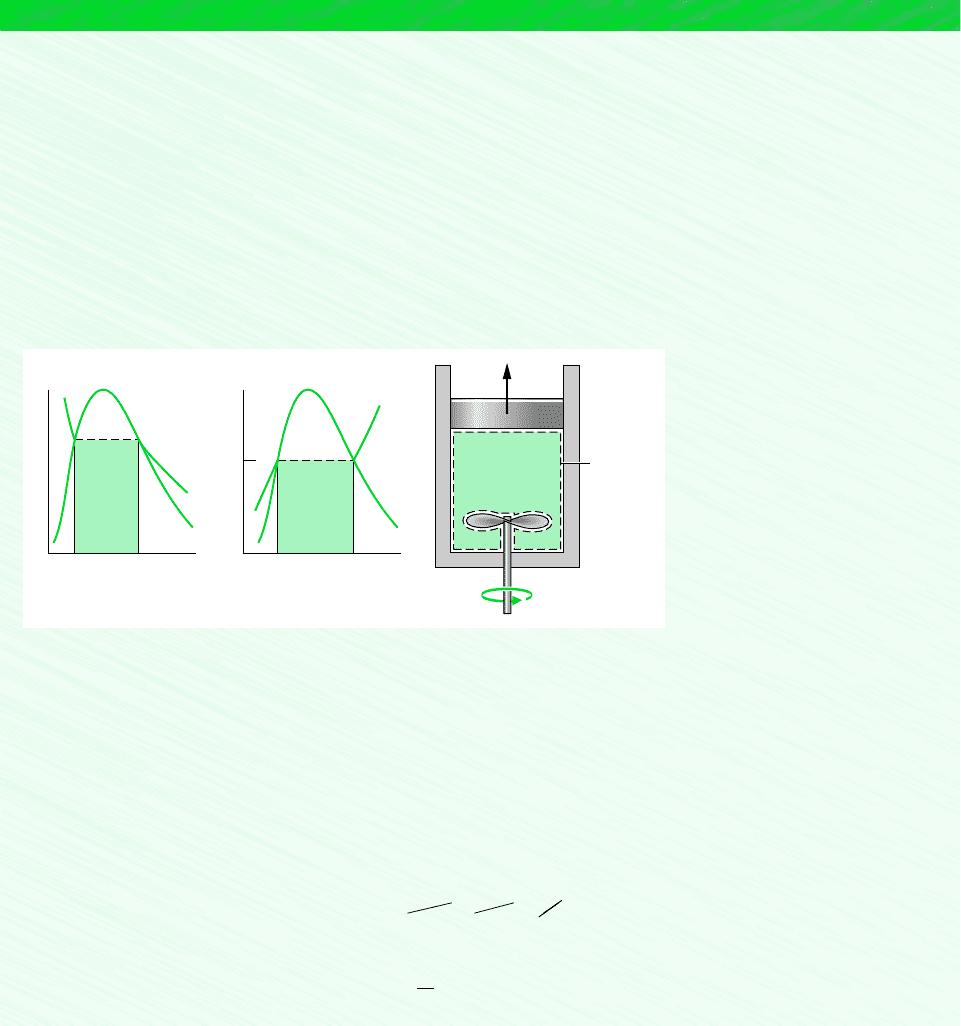
To evaluate the entropy transfer term of the entropy balance requires information re-
garding both the heat transfer and the temperature on the boundary where the heat transfer
occurs. The entropy transfer term is not always subject to direct evaluation, however, be-
cause the required information is either unknown or not defined, such as when the system
passes through states sufficiently far from equilibrium. In such applications, it may be con-
venient, therefore, to enlarge the system to include enough of the immediate surroundings
that the temperature on the boundary of the enlarged system corresponds to the tempera-
ture of the surroundings away from the immediate vicinity of the system, T
f
. The entropy
transfer term is then simply QT
f
. However, as the irreversibilities present would not be just
for the system of interest but for the enlarged system, the entropy production term would
account for the effects of internal irreversibilities within the original system and external
irreversibilities present within that portion of the surroundings included within the enlarged
system.
dS a
dQ
T
b
b
ds
s
#
Q
#
j
T
j
dS
dt
a
j
Q
#
j
T
j
s
#
S
2
S
1
a
j
Q
j
T
j
s
T > T
f
Temperature
variation
T
f
Boundary of
enlarged system
closed system entropy
rate balance
224 Chapter 6 Using Entropy
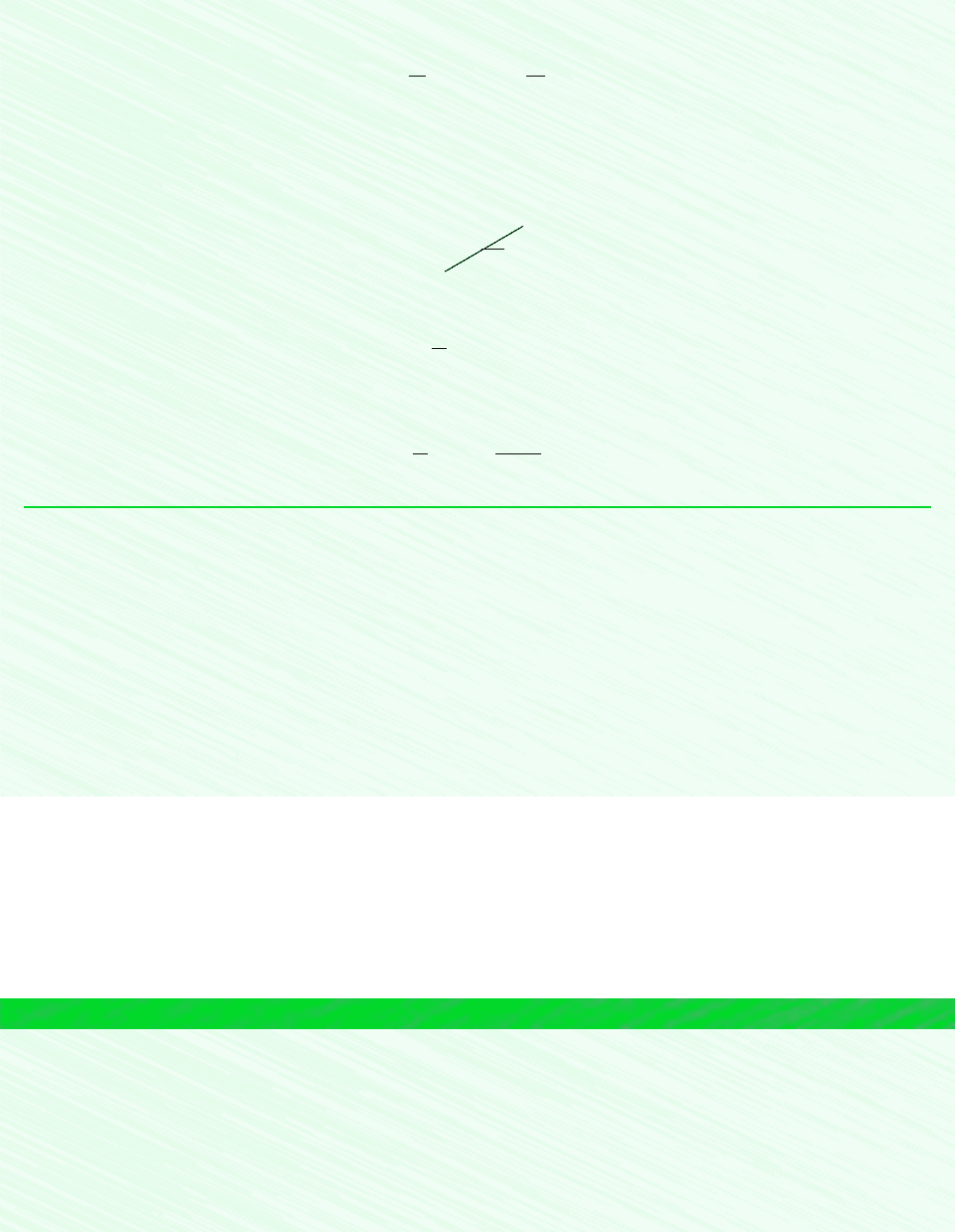
EXAMPLE 6.2 Irreversible Process of Water
Water initially a saturated liquid at 100C is contained within a piston–cylinder assembly. The water undergoes a process to
the corresponding saturated vapor state, during which the piston moves freely in the cylinder. There is no heat transfer with
the surroundings. If the change of state is brought about by the action of a paddle wheel, determine the net work per unit
mass, in kJ/kg, and the amount of entropy produced per unit mass, in
SOLUTION
Known: Water contained in a piston–cylinder assembly undergoes an adiabatic process from saturated liquid to saturated
vapor at 100C. During the process, the piston moves freely, and the water is rapidly stirred by a paddle wheel.
Find: Determine the net work per unit mass and the entropy produced per unit mass.
Schematic and Given Data:
kJ/kg
#
K.
p
v
100°C
100°C
g
f
Area is
not work
T
s
g
f
Area is
not heat
Water
System
boundary
Figure E6.2
Assumptions:
1. The water in the piston–cylinder assembly is a closed system.
2. There is no heat transfer with the surroundings.
3. The system is at an equilibrium state initially and finally. There is no change in kinetic or potential energy between these
two states.
Analysis: As the volume of the system increases during the process, there is an energy transfer by work from the system
during the expansion, as well as an energy transfer by work to the system via the paddle wheel. The net work can be evalu-
ated from an energy balance, which reduces with assumptions 2 and 3 to
On a unit mass basis, the energy balance reduces to
W
m
1u
g
u
f
2
¢U ¢KE
0
¢PE
0
Q
0
W
6.5.4 Illustrations
The following examples illustrate the use of the energy and entropy balances for the analy-
sis of closed systems. Property relations and property diagrams also contribute significantly
in developing solutions. The first example reconsiders the system and end states of Exam-
ple 6.1 to demonstrate that entropy is produced when internal irreversibilities are present and
that the amount of entropy production is not a property.
❶
6.5 Entropy Balance for Closed Systems 225
As an illustration of second law reasoning, the next example uses the fact that the entropy
production term of the entropy balance cannot be negative.
With specific internal energy values from Table A-2 at 100C
The minus sign indicates that the work input by stirring is greater in magnitude than the work done by the water as it
expands.
The amount of entropy produced is evaluated by applying an entropy balance. Since there is no heat transfer, the term
accounting for entropy transfer vanishes
On a unit mass basis, this becomes on rearrangement
With specific entropy values from Table A-2 at 100C
Although each end state is an equilibrium state at the same pressure and temperature, the pressure and temperature are not
necessarily uniform throughout the system at intervening states, nor are they necessarily constant in value during the
process. Accordingly, there is no well-defined “path” for the process. This is emphasized by the use of dashed lines to
represent the process on these p–v and T–s diagrams. The dashed lines indicate only that a process has taken place, and
no “area” should be associated with them. In particular, note that the process is adiabatic, so the “area” below the dashed
line on the T–s diagram can have no significance as heat transfer. Similarly, the work cannot be associated with an area
on the p–v diagram.
The change of state is the same in the present example as in Example 6.1. However, in Example 6.1 the change of state
is brought about by heat transfer while the system undergoes an internally reversible process. Accordingly, the value of
entropy production for the process of Example 6.1 is zero. Here, fluid friction is present during the process and the en-
tropy production is positive in value. Accordingly, different values of entropy production are obtained for two processes
between the same end states. This demonstrates that entropy production is not a property.
s
m
6.048
kJ
kg
#
K
s
m
s
g
s
f
¢S
2
1
a
dQ
T
b
0
b
s
W
m
2087.56
kJ
kg
❶
❷
❷
EXAMPLE 6.3 Evaluating Minimum Theoretical Compression Work
Refrigerant 134a is compressed adiabatically in a piston–cylinder assembly from saturated vapor at 0C to a final pressure of
0.7 MPa. Determine the minimum theoretical work input required per unit mass of refrigerant, in kJ/kg.
SOLUTION
Known: Refrigerant 134a is compressed without heat transfer from a specified initial state to a specified final pressure.
Find: Determine the minimum theoretical work input required per unit of mass.
