Middleton G.V. (Ed.) Encyclopedia of Sediments and Sedimentary Rocks
Подождите немного. Документ загружается.


LUNAR SEDIMENTS
415
LUNAR SEDIMENTS
Introduction
The surface and surficial deposits on the Moon are radically
different from Earth's, The Moon is completely dry and does
not have an atmosphere; it is devoid of an internally driven
magnetic field and plate tectonics is absent; large and small
impacts through time have produced a thick regolith, and
sediments so produced are transported ballistically, Regolith
may be shock-indurated by fresh impacts, or may be buried
under fresh ejecta that may be hot and may sinter unconso-
lidated grains together, to produce regolith breccias. All lunar
samples are from the regolith; and, all remote sensing signals
(albedo, UV-Vis-IR reflectance [of sunlight], natural
y-ray,
and
X-ray
fluorescence [by solar X-rays] spectra) of the Moon are
from its regolith. Most of the signals come from the uppermost
0,5 |im layer, that is, almost entirely from the dusty veneer of
the Moon, Because the composition of the finest fractions of
lunar soils is different from the bulk composition of any lunar
soil, it is essential that all calibration be corrected back to the
compositions of bulk soils. The remote sensing techniques and
their lunar calibrations are used to explore all atmosphere-free
rocky bodies in the solar system; hence the extreme importance
of precise and accurate characterization of lunar soils in
planetary exploration.
In the initial days of lunar sample return by Apollo
missions, the sub-centimeter fraction of the samples was
informally termed "fines" and then "lunar soil" came in
vogue. In lunar science literature and in the community, "soil"
is now entrenched with an informal size connotation; the word
has no significance vis a vis its use for Earth materials in
agricultural, engineering, geological, and hydrological
sciences. In general, lunar soils are gray in color and consist
of angular and irregularly shaped grains with a mean size of
about 60|im, Grains cemented with fresh glass produced by
micrometeorite impacts on lunar soils produce a type of
constructional particle called agglutinate (a term adapted
from terrestrial pyroclastic vocabulary). Except for a minor
(<2 percent) meteoritic component, lunar soil composition
varies between anorthositic and basaltic, McKay etal. (1991)
summarize lunar soil data gathered up to about 1990,
Petrographic composition
Two principal terrains configure the lunar surface. One is a
high mountainous terrain (highlands) consisting of light
colored anorthositic rocks that are 4,4 billion years or older
in age. Flat lowlands, essentially impact basins (mare) filled
with dark Fe-rich basalt flows ranging in age from about 3
billion years to 4 billion years in age, comprise the other.
Impacts have mechanically disintegrated these rocks and have
also indurated some of the clasts together. The regolith on the
surfaces of these units generally reflects the composition and
the color of the rock units below although there has been some
mixing of distant impact ejecta in all local soils. The majority of
lunar soils consist of five major grain types: mineral fragments
(10-40 percent), igneous and metamorphic rock fragments
(5-20 percent), breccias with various degrees of coherence
(10-40 percent), and agglutinates (20-50 percent). Glass
spherules of either impact or volcanic fire fountain origin
make up a minor component (usually <5 percent) except in a
few unusual soils and in some restricted size ranges. Genetic-
ally, lunar soil components may also be classified into two
groups: bedrock derived and regolith derived. Agglutinates,
regolith breccias, and glass spherules are regolith-derived
components.
Orange soil
An orange layer in the regolith near the Shorty Crater in Mare
Tranqullitatis consists of more than 90 percent high-titanium
(up to 16 percent TiO2) glass beads ranging in color from
orange and red to black in hand specimen. At the bottom of a
nearby drill core the soils consist of nearly 100 percent black
beads in which skeletal crystals of olivine and ilmenite abound.
These beads are products of volcanic fire fountaining with
remnants of condensed volcanic gases on their surface. Beads
of high magnesium iron-poor green volcanic glass are also
found in significant amounts a few soils from the Apennine
Mountains but not in layers.
Chemical composition
Chemical compositions of lunar soils also range from basaltic
to anorthositic. Mixing of mare and highland components in
different proportions give rise to diverse soil compositions.
Differential comminution and differential agglutination of
lunar soils have rendered different size fractions to be
compositionally different from each other. The extent of mare
ejecta mixing with highland soils and vice versa can be
estimated better from the chemical compositions of soils than
from modal compositions, partly because a few minor and
trace element concentrations are characteristic of certain
pristine igneous rocks. Average compositions (wt percent) of
a few typical mare and highland soils are given below.
SiO2
TiO2
AI2O3
Cr2O3
FeO
MnO
MgO
CaO
Na20
K,0
Mare
40,6
8,4
12,0
0,45
16,7
0,23
9,9
10,9
0,16
0,14
Highland
45,0
0,54
27,3
0,33
5,1
0,30
5,7
15,7
0,46
0,17
Crustal composition of the moon
With no bedrock sampled so far, direct evidence of rock types
in the crust of the Moon comes from regolith samples. Rock
fragments from boulder sizes to sand sizes have been analyzed
for chemical and mineralogic compositions, crystal chemistry,
isotopic systematics, radiometric age dating, experimental
petrology, and nuclear and ferromagnetic resonance measure-
ments, to name a few. It is a tale of incredible provenance
sleuthing. The crust of the Moon is inferred to be made mostly
of ferroan and gabbroic anorthosites, norites enriched in K,
REE, P, U, and Th, various kinds of Fe-rich (~20 percent
FeO) basalts that have large ranges of K2O (0,06-0,36 percent)
and TiO2 (0,4-13,0 percent), and a low-Fe basalt (~10 percent

416 LUNAR SEDIMENTS
FeO) with higher concentrations of K2O (~0,5 percent or
more),
P (~2500ng/g), and REE,
Grain size
Lunar regolith samples are not plentiful, NASA allocated
approximately 0,5 g or 0,25 g of the submillimeter fraction of
lunar soils for determining their grain size distribution. Sieving
with minimal loss required more than 20 hours spanned in
about 4 to 5 workdays per sample. The results were then
combined with those of the subcentimeter fraction of the
rather hastily sieved lunar regolith at NASA's curatorial
facilities
(Graf,
1993), The mean grain size (Mz) of lunar soils
range between
34>
and 4(^ (125-62,5 fim) and their inclusive
graphic sorting
((TIG)
generally vary between
2(j>
and
3.0(j>,
that
is,
they are poorly sorted by terrestrial standards. Many soils
are coarsely skewed; much of the fine dust is locked up in
agglutinates.
Vertical profile
Several trenches, drive tubes, and drill cores have penetrated
and sampled the lunar regolith up to a depth of about
3
m.
Understandably none has reached bedrock. Each section of the
regolith shows evidence of layering, by way of color (shades of
gray),
grain size, chemical and mineralogical composition, and
maturity. However, layers in closely spaced drill cores and in
adjacent trenches (within a few meters) do not correlate
indicating that impacts have moved the regolith considerably.
Nearly all vertical profiles of soils show that the topmost layer
is more mature (see below for the concept) than those below.
equal. In this steady state, continued exposure of lunar soils at
the surface increases the abundance of agglutinates but does
not reduce the mean grain size any more. However, slightly
larger impacts excavate material from below, which may not
be as mature, and mix the two together. Such events disturb
the steady state. Most lunar soils presumably are not in a
steady state but may be close to one.
Because the Moon does not have any magnetosphere or
any atmosphere, energetic particles of the solar wind freely
bombard the lunar surface and implant solar wind elements
(SWE), especially hydrogen ions, in the outer rinds (~150nm)
of exposed soil grains. Abundance of SWE in lunar soils
increases with maturity. Apart from melting a small fraction of
lunar soil grains, micrometeoritic impacts also vaporize a part
of the target and much of
itself.
Elemental dissociation takes
place in the high temperature vapor from which nanophase
Fe° metal condenses and is deposited on surfaces of soil grains.
Additionally, the melt itself may undergo reduction reactions
with implanted hydrogen and produce nanophase Fe" globules
that are strewn in agglutinitic glass. The two processes not
only darken lunar soils but also increase the abundance of
nanophase Fe" with increasing maturity (Hapke, 2001; McKay
etal., 1991),
A robust correlation between the abundance of agglutinates,
SWE, nanophase Fe" (measured by ferromagnetic resonance),
inverse grain size, and to some extent with soil color strongly
supports the inferred processes of lunar soil evolution,
Abhijit Basu
Origin and evolution
Large meteorite impacts up to about 1 b,y, ago have produced
most of the regolith of the Moon, Impacts by micrometeorites
(<1 mm) have continuously comminuted the regolith and are
largely responsible for the production of lunar soils, Micro-
meteoritic impacts on lunar soils melt a miniscule amount,
mostly the finest dust, at the point of impact. The melt
incorporates soil grains as clasts and quenches quickly to
produce agglutinates, that is, glass bonded aggregates, Micro-
meteoritic impacts affect only the topmost millimeters of the
lunar regolith. With continued exposure, that is, increasing
maturity, the soil in this layer becomes finer and finer
simultaneously producing agglutinates that are larger. Over
time the rate of comminution and agglutination becomes
Bibliography
Graf,
J,C,, 1993,
Lunar
soils
grain
size
catalog,
NASA Reference
Pub-
lication
1265.
Houston:
NASA,
406pp,
Hapke,
B,, 2001,
Space
weathering
from
Mercury
to the
asteroid
belt.
Journal
of
Geophysieal
Research-Planets,
106: 10039-10073,
McKay,
D,S,,
Heiken,
G,H,,
Basu,
A,,
Blanford,
G,,
Simon,
S,,
Reedy,
R,,
French,
B,M,, and
Papike,
J,J,, 1991, The
lunar
regolith.
In
Heiken,
G,H,,
Vaniman,
D,, and
French,
B,M, (eds,).
Lunar
Soureebook.
Cambridge
University
Press, pp,
285-356,
Cross-reference
Sediments
Produced
by
Impact

M
MAGADIITE
Magadiite is a hydrous sodium silicate mineral that was
discovered by Hans Eugstcr (1967) in late Pleistocene lake
sediments at Lake Mttgadi iti the southern Kenya Rifl Valley,
Although rare, magadiite is a well-knoun precursor of non-
niariiic chert. Qiiartzose "Magadi-lype cherts"", which have
fortncd by the diagenetic alteration of tnagadiite. have been
reported frotii rocks of Precambrian to Holocene
age,
Magadi-
type chert is generally considered diagnostic of
saline,
alkaline
lacustrine environments.
Magadiite [NaSi7Oi,(OH)v4H:O] and the telated sodium
silicates, kenyaitc Na^Sin.OjitOHls •6HO]. tnakalitc [Na^.
Si4O^,(OHb 4H.O]. and kancmite [NallSi.OiOHb 2H2O].
form mainly in hypersaline. sodiutn carbonate-rich brines
that have u pH > 9 atid consequently have high concentrations
of dissolved silica (up to 2,71)0 ppm). Sodiutn silicate tninerals
arc present at Lakes Magadi and Bogoria in Kenya. Lake
Chad,
and in some alkaline lakes in California and Oregon.
When fresh, magadiite is white, soft, putlylike. and easily
deformable. but it dehydrates rapidly on exposure to air. and
hardctis irreversibly to (brm a tine chalky aggregate. At high
magtiification. magadiite typically displays minute aggregates
of thin platy monoclinic crystals (<l()|,im) that may be equant
or have a sphcrulitic habit (Icole and Peritict. 1984; Scbag
etal..
2001),
At Lake Magadi, magadiite tbrni beds a few decimeters
thick, lenses, and concretions with irregular protrusions, and
veins that Iratiscct bedding in zeolitic lacustrine silts
(Figure Ml), Hlsewhcrc. magadiite also tills cracks iu large
(tnctcr-scale) polygotis
(e.g,.
Alkali Lake, Oregon), or replaces
plants (Sebag(7<//.. 2001),
Formation of magadiite
Two geticral pathways have been proposed to explain the
formation of magadiite in silica-rich NaCO^ brines: a de-
crease in pH and evaporative concentration. At Magadi.
subhorizontal beds have been explained by seasotial precipita-
tion in a stratified saline, alkaline lake. Magadiite has been
inferred to precipitate when dilute inflow waters flow across a
dense,
sodium carbonate brine rich in dissolved silica and
lower the pH at the fluid interface (chemociine). At Lake Chad
and in some Americati examples, magadiite may have
precipitated by eviipotative concetitration or by capillary
evaporation of saline, alkaline brines ul a shallow subsurface
water table. Other inferred mechanisms include subsurface
mixitig of dilute and saline, alkaline groundwalers, a reduction
in pH of
an
alkaline brine resulting from an influx of biogenic
or geothermally sourced CO;, and precipitation from inter-
stitial brines. Different sodium silicate tninerals may fonn
according to the concentrations of Na and SiO- in the brine.
Conversion to chert
At Magadi, beds of tnagadiite pass laterally into quartzose
cherts confirming their genetic relationship, Magadi-type
cherts form beds, plates, and lobate nodules, commonly with
features indicating soft-sediment deformation upon compac-
tion.
Reticulate line eracks, salt crystal pscudomorphs or
molds, and relict spherulites are also cotnmon.
The conversion of magadiite to quartz is rapid and involves
a loss of sodium, water, and volume. Leaching by percolating
meteoric water, dehydration, and spontaneous reerystalliza-
tion due to sorption of silica by clays, have been proposed.
Intermediate diagenetic products, including kcnyaite. amor-
phous silica and tnoganite. may form during the transforma-
tion (kole and Perinet, 1984: Sheppard and Gudc. 1986). Both
magadiite and the associated cherts have a distinctive trace
element signature unlike other cherts.
Although the genetic models are well established and
magadiite is easily synthesized in the laboratory, few natural
examples of modern precipitation have been documented.
Recently Behr (2002) has shown that many of the cherts at
Lake Magadi may have been precipitated as amorphous silica
by bacteria and may not have had a sodium silicate precursor.
Similarly, many ancient continental cherts resemble Magadi-
type cherts in morphology and fabric, but are utilikely to have
had a magadiite precursor. The discovery of magadiite in
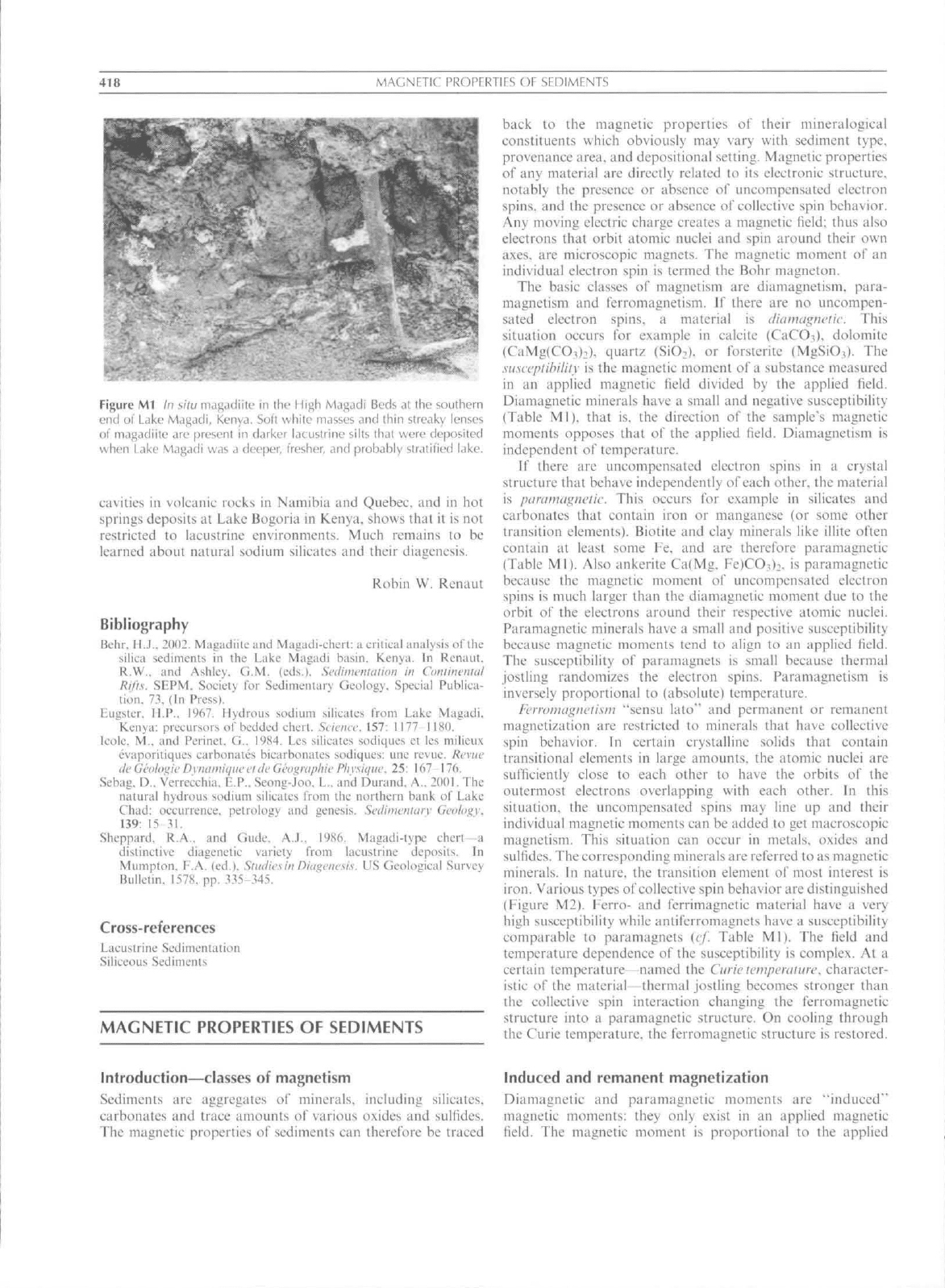
418
MAGNETIC PROPERTIES OE SEDIMENTS
Figure
Ml In
situ nuij^adiilc
iii
ilic
I
li^li Maj;Lidi Beds
Jl ihe
southern
end
of
Lake Mdgadi, Kenya. Soft while mtisses and thin streaky lenses
of m.igadiite Arc present
in
darker lacustrine silts that were deposited
when Lake Magadi
was
a deeper, fresher, and probably stratified lake.
cavities
in
volcanic rocks
in
Namibia
and
Quebec,
and in hot
springs deposits
at
Lake Bogoria
in
Kenya, shows that
it is not
restricted
to
lacustrine environments. Much remains
to be
learned about natural sodium silicates
and
their diagenesis,
Robin
W.
Renaut
Bibliography
Bell!', H,J,. 2002, Magadiiieand Magadi-chcrt:
a
critical analysis oflhe
silica sediments
in the
Lake Magadi basin. Kenya,
In
Rcnaiil.
R,W,.
and
Ashley.
G,M.
(eds,), Sedinictitaticn
in
Continental
Rifts. SEPM. Society
for
ScdimciiUiry Geology, Special Publica-
tion, 7,1,
(In
Press),
Eugster,
H.P.. 1967,
Hydrnus sodium ?,ilicalL's from L;ike Magadi,
Kenya; precursors
of
bedded chert, Scienee.
157: 1177 1180,
lcolc,
M,, and
Perinet,
G,, 1984, Les
silieates .sodiqucs
et les
milieux
cvLiporitiques carbonates bicarbotiates sodiques:
une
revue, Reyiic
de
Gi'alogie
Dynamiqueet dc Geographic
Physiquf.
25;
I f>7
176.
Sebag.
D,,
Verreecliia. F.,P,. Seong-Joo,
L,. and
Durand.
A,,
2(101,
The
natural hydrous sodium silicates from
the
northern bimk
of
Lake
Chad: occuirence. petrology
and
genesis. Sedimentary Geology.
139:
15 31,
Sbeppttrd,
R,A,. and
Gude,
A,J,,
I9S6, Magadi-type cherl
a
distinctive diagenetic variety from lacusirine deposits.
In
Mumpton.
F,A.
(ed.). Studies
in
Diagenesis,
US
Geological Survey
Bulletin, 1578,
pp,
335-345,
Cross-references
Lactistrine SedimentiUioii
Siliceous Sedimenls
MAGNETIC PROPERTIES
OF
SEDIMENTS
back
to the
tiiagnetic properties
of
their mineralogical
constituents which obviously tnay vary with sediment type,
provenance area,
and
depositional setting. Magnetic properties
ot"
any
material
are
directly related
to its
electronic structure,
notably
the
presence
or
absence
of
uncompensated electron
spins,
and the
presence
or
absence
of
collective spin behavior.
Any tnovitig electric charge creates
a
magnetic field: thus aiso
electrons that orbit atotnic nuclei
and
spin around their
own
axes,
are
microscopic tnajznets.
The
magnetic tnotncnt
of an
individual electron spin
is
termed
the
Bohr magneton.
The basic classes
of
magnetistn
are
diamagnetism. pata-
magnetism
and
ferroniagnetism.
If
there
are no
uncompen-
sated electron spins,
a
material
is
ilianmgnetic. This
situation occurs
for
example
in
calcite (CaCOi), dolomite
(CaMgiCO;,):). quartz (SiO.),
or
fbrsteritc (MgSiO.,)-
The
susecptibility
is the
magnetic moment
ofa
substance measured
in
an
applied tiiagnetic lield divided
by the
applied field,
Diamagnetic tninerals have
a
stnall
and
negati\e susceptibility
(Table
Ml),
that
is. the
direction
of the
sample's magnetic
moments opposes that
of the
applied field. Diamagnetism
is
independent
of
temperature.
If there
are
uncompensated electron spins
in a
crystal
structure that behave independently
of
each other,
the
material
is paramagnetic. This occurs
for
example
in
silicates
and
carbonates that contain iron
or
manganese
(or
sotne other
transition elements), Biotite
and
clay minerals like illite often
contaiti
at
least some
Fe. and are
therefore paramagnetic
(Table
Ml).
Also ankerite Ca(Mg, FejCOi)^.
is
paramagnetic
because
the
magnetic motnent
of
uncompensated electron
spins
is
much larger than
the
diamagnetic moment
due to the
orbit
of the
electrons around (heir tespective atomic nuclei.
Paramagnetic tninerals have
a
stnall
and
positive susceptibility
because magtietic moments tend
to
align
to an
applied field.
The susceptibility
of
paramagncts
is
stnall because thermal
jostling randomizes
the
electron spins. Paramagnetism
is
inversely proportional
to
(absolute) temperature.
Forromagtu'lism "sensu lato"
and
pertiianent
or
retnancnt
magnetization
are
restricted
to
minerals that have collective
spin behavior.
In
certain crystallitie solids that contain
tratisitional eletnents
in
large amounts,
the
atomic nuclei
arc
sufficiently close
to
each other
to
have
the
orbits
of the
outet"most electrons overlapping with each other.
In
this
situation,
the
uncotnpensated spins tnay line
up and
their
individual magnetic moments ean
be
added
to get
macroscopic
magnetism. This situation
can
occur
in
metals, oxides
and
sullidcs. The corresponding minerals are referred to as magnetic
minerals.
In
nature,
the
transition element
of
most interest
is
iron. Various types of collective spin behavior are distinguished
(Figure
M2),
Ferro-
and
ferrimagnetic material have
a
very
high susceptibility while antiferrotnagnets have
a
susceptibility
cotnparable
to
paratnagnets
(cf.
Table
Ml), The
field
and
temperature dependence
of (he
susceptibility
is
complex.
At a
certain temperature—natned
the
Curie temperature, character-
istic
of the
material —thermal jostling becotnes stronger than
the collective spin interaction changing
the
ferromagtietic
structure into
a
paramagnetic structure.
On
cooling through
the Curie temperature,
the
ferromagnetic structure
is
restored.
Introduction—classes of magnetism
Seditnents
are
aggregates
of
minerals, including silicates,
carbonates
and
trace amounts
of
various oxides
and
sulfides.
The tnagnetic properties
of
seditnents
can
therefore
be
traced
Induced
and
remanent magnetization
Diamagnetic
and
paratnagnetic moments
are
"induced"
magnetic moments: they only exist
in an
applied magnetic
field.
The
magnetic moment
is
proportional
to the
applied
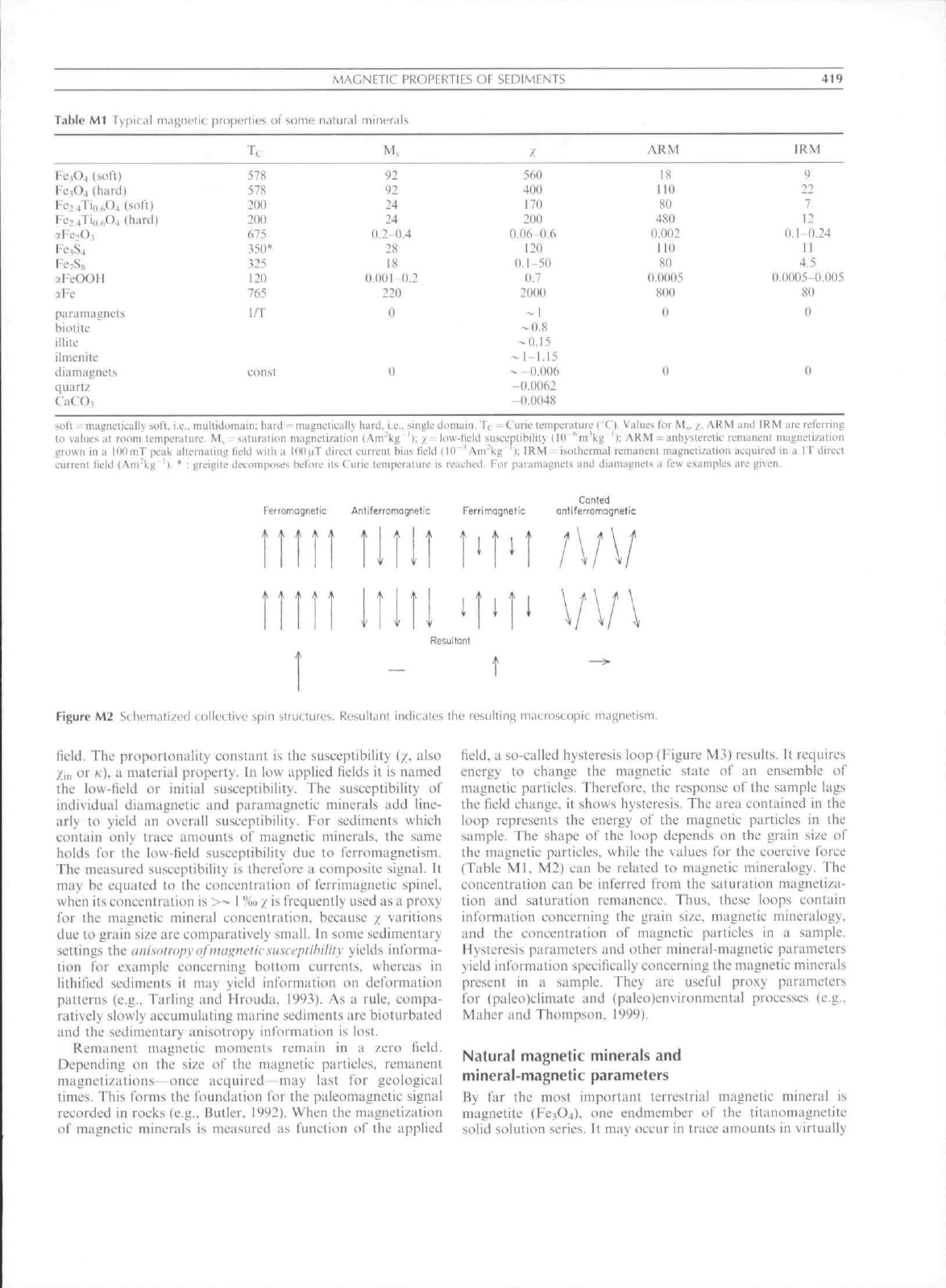
Table Ml Tv|ilic.il magnetic
MAGNETIC
pruficrties of some natural niinfra
Tt M,
PROPERTIES OF SEDIMENTS
/
ARM
419
IRM
Fc,04 (solt)
Fi;,04 (hard)
Fc:^4Ti,,hO4 (soft)
Fc:4Ti,i.,,O4 (hard)
3:Fe-.O,
Fc,S4
FciSs
JY-COOU
>Fc
pa Til magnets
biotite
illite
ilincnitc
tlia magnets
quartz
CaCO,
soil mai;ni.'iiL.allv ^o^l,
578
578
200
200
675
350
325
120
765
I/T
eon
i.e,. mullidoniaiii: h
92
92
24
24
0,2 0,4
28
18
0,00! 0,2
220
0
0
560
400
170
200
0,06-0,6
120
0.1-50
0,7
2000
-"
1
-0.8
-0.15
-1-1,15
- -O.(X)6
-0,0062
-0.0048
18
110
80
480
0.002
110
80
0,0005
800
0
0
9
22
7
12
0,1-0,24
11
4,5
0.0005-0.005
80
0
0
, mullidoniaiii: hard = magnetically hard, i,e,, single domain, T, - Cune lempeniturc t C). Values tor M,, /;, AKM and
I
KM are referring
til values :it room temix-raturc, M, ^Liturjtioii magnetization (Anrkg '): / = liiw-rn;ld susueptibility (IU "m'kg 'l: ARM = anhystcrctic remanent magnetization
jiroxvn in a ItlOmT peak alternating lield wiih a lOO^iT direct current bias tield 110 ' Am"kg ): IRM = isothermal rananent magnetization acquired in a IT direct
current tield
(,'\m"ka
' I, * ; grcigitc dfco in poses hefore its Curie temperature is reached. For paramagncts and diamagneis a (ew examples are given.
Ferfomagnetic
Antiferromagnetic
Ferrimagnelic
Conted
•ntiferromagnetic
Re
sul
to
ni
Figure M2 S( hem.ilized tollective spin structures, KosulLint inrlicates thf resulting macroscopic magnetism.
(icld.
The proporionality constant is the susceptibility (/, also
/jn or
K).
a material property. In low applied lields it is named
the low-field or initial stisceptibility. The susceptibility of
itidividual diatnagnetie and paratnagnetie minerals add line-
arly to yield an overall sitseeptibility. For sedimetits which
contain only trace amounts of magnetic minerals, the same
holds lor the low-field susceptibility due to ferrotnagnetisni.
The tneasured susceptibility is therefore a cotnposite signal. It
may be equated to the concentration of ferrimagnetic spinel,
when its concentration is >-•
1 %o /_
is frequently used
as a
proxy
for the magnetic mineral concentration, because y varitions
due lo grain size are comparatively stnall- In some sedimentary
settings the
attisotropy
of
ma^itwticsusceptibility
yields infortiia-
tion for example concerning bottom currents, whereas in
lithified sediments it may yield infonnation on deformation
patterns
le.g,,
Tarling and Hrotida, 1993). As a rule, conipa-
raiively slowly accumulating marine sediments are bioturbated
and the seditnenlary anisotropy infonnation is lost,
Retnanent tnagnetie moments remain in a zero
fteld.
Depending on the size of the magnetic partieles, remanent
magnetizations onee acquired may last for geological
times.
This forms the foundation for the paleomagnetie signal
recorded in rocks
(e,g,,
Butler, 1992), When the magnetization
of tiiagnetic minerals is measured as function of the applied
tield,
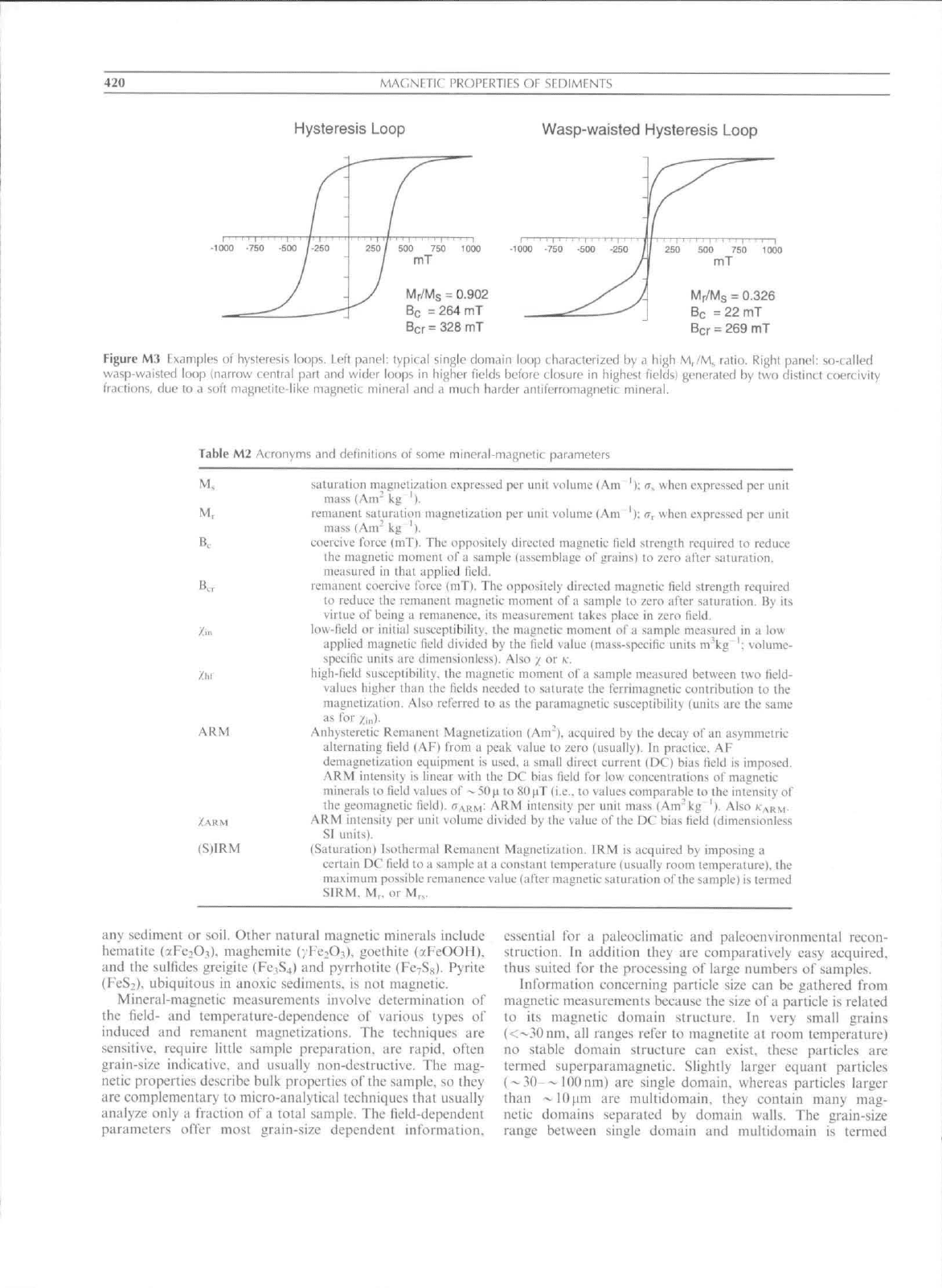
a so-called hysteresis loop (Figure M3) results. It requires
energy to change the magnetic state of an ensemble of
magnetic particles. Therefore, the response of the sample lags
the fteld ehange. it shows hysteresis, T"he area contained in the
loop represents the energy ol' the magnetic particles in the
sample. The shape of the loop depends on the grain size of
the tnagnetie particles, while Ihe values for the coercive force
(Table Ml. M2) can be related to magnetic tnineralogy. The
concentration can be inferred from the saturation magnetiza-
tion and saturation retnanence. Thus, these loops contain
infotmation concerning the grain size, magnetic mineralogy,
and the concentration of magnetic particles in a sample.
Hysteresis paratneters and other tnineral-magnetic parameters
yield information specifically concerning the magnetic minerals
present in a sample. They are usel'ul proxy parameters
for (paleo)cliinate and (palco)cnviromnental processes
(e.g..
Maher and Thompson, 1999).
Natural magnetic minerals and
mineral-magnetic parameters
By tar the nn)st itnportant terrestrial tnagnetic mineral is
tnagtietite (FeiO4). one endtnetnber of the titanotnagnetite
solid solution series. It may occur in trace amounts in virtually
420
MAGNFTIC
PROPERTIES
Or
SEDIMENTS
Hysteresis Loop
Wasp-waisted Hysteresis Loop
r/Ms = 0.902
e = 264 mT
cr
=
328 mT
Mr/Ms = 0.326
Be =22mT
Ber = 269 mT
Figure M3 Examples of hysteresis loops. Left panel: typical single domain loop characterized by a high M,/M^ ratio. Right panel: so-called
wasp-waisted loop (narrow central part and wider loops in higher fields before closure in highest fields) generated by two distincl coercivity
fractions, due to a soft magnetite-like magnetic mineral and a much harder antiferromagnetic mineral.
Table M2 Acronyms and definitions of some mineral-magnetic parameters
M^ sattiration tnagnctization expressed per unit volume (Am '):
n..
when expressed per unit
mass (Am~ kg ').
Mr remanenl saturation magnetization per unit volume (Am '); n, when expressed per unit
mass (Am" kg '),
Be
coercive foree (mT), The oppositely directed magnetic field sirenjith required to icditec
the
magnctie moment of a sample (assemblage of grains) to zero after
saturation,
measured
iti ihat applied
field,
Bcr
remanetit eocreive force
fniT),
The oppositely directed magnctie lield strength required
to
reduce the remanent magnetic tnomcnt of ;i sample to /ero after
saturation,
B\ its
virtue
of being ;i remanence. its measurement takes place in zero
field,
Xm
low-iidd or initial susceptibility, the magnetic moment ofa sample trteasurcd in a low
applied
magnetic field divided by the field vaiue (niiiss-specific units m'kg ': vo!ume-
specific
units are dimeiisionlcss). Also
/_
or K.
XM
high-fteld susceptibility, the magtietic tiiometit ofa sample measured between two
iield-
values
higher than the fields needed to saturate the ferritiiagnetic contribution to the
magnetizalion.
Also relerted to as the paramagnetic susceptibility (units are the same
as
for
•/,„).
ARM Anhysteretie Remanent Magnetization (Am'), acquired b> ihe decay of an asymttietrie
alternating lield (AF) from a peak value to zero (usually). In praeliec, AF
demagnetization equipment is used, a small direct current (DC) bias lield is imposed,
ARM intensity is linear v\ith ihe DG bias iieid for low concentrations o)'tnagnetie
minerals to fteld values of ^50fi to 80|,iT(i,e,. to values comparable to the intensity of
the geomagnetic lieid),
RARM:
ARM inlensily per unit mus.s (Am"kg '). Also
KARM-
ZARM
ARM
intensity per unit volume divided by the value of the DC bias iieici (dimensionless
Si units),
(S)IRM (Saturation) Isothermal Remanent Magnetization, IRM is acquired by imposing a
certain DC tield to a sample at a constant temperature (usually room temperature), the
maximum possible remanenee value (after magnetic saturation of
the
sample) is lermcd
.SiRM.
M,. or M,,,
any sediment or
soil.
Other nalural magnetic tiiitierals include
hetnatite (3:Fe2O3), maghetnite (•/Fe^Oi), goethite (aFeOOH).
and the sulfides greigite (Fe.^S4) and pyrrhotitc (FeySw). Pyrite
(FeS:). ubiquitous in anoxic sediments, is not magnetic.
Mineral-magnetic measurements involve determination of
the tield- and temperature-dependence of various types of
induced and remanent tnagnetizations. The techniques are
sensitive, require little sample preparation, are rapid, often
grain-size indicative, and usually non-destructive. The mag-
netic properties describe bulk properties of the satnple, so they
arc cotnplementary to micro-analytical techniques that itsually
analyze only a Traction of a total sample. The fteld-dependcnt
parameters oiTer most grain-size dependent inlbrination.
essential for a paleoclimatie and paleoenvironmental recon-
struction.
In addition they are comparatively easy acquired,
thus suited for the processing of large numbers of satnples.
Information concerning particle size can be gathered from
magnetic measurements because the size ofa particle is related
to its magnetic domain structure. In very small grains
{<--30nm,
all ranges refer to magnetite al room tetnperature)
no stable domain structure can exist, these particles are
termed superparamagnetic. Slightly larger equant particles
( - 30-"-lOOnm) are single domain, whereas particles larger
than -• l()|,tm are multidomain, they contain many mag-
netic domains separated by domain walls. The grain-size
range between single domain and multidomain is tenned

MAGNETIC PROPERTIES OF SEDIMENTS
421
pseudo-single-domain. Single dotnain grains respond to a
changing magnetic field by rotation of iheir magnetic
motnents. while muitidomain grains basically respond by
tnovement of domain walls, iwo processes that are character-
ized by a difTerent field dependence. This very fact allows
deduction of grain size from Held-dependent measurements.
The tnore comtnonly used mineral-magnetic paratnelers are
sutnmarized in Table M2.
For low concentrations
(i.e..
in the absence of magnetic
interactions) mass-dependent parameters are linear with
concentration at least to a first-order approximation which is
applicable to the situation in sediments. By dividing two mass-
depetident paratneters. a concentration-independent variable is
obtained uhich allows interpretation ol'grain-size trends. Field
values, such as the coercivity paratneters. are concentration-
independent lo a tirsi-order approxitnation.
Large tnultidotnain particles acquire comparatively low
ARMs and IRMs. IRM, by its nature, is a tnuch stronger
remanence than ARM (Table Ml), Single domain magnetite
particles are particularly prone to aequire ARM, so ihe ARM/
IRM ratio may be used to irace grain size if magnetite is
magnetically the dominant mineral. Different coercivity
fracliotis in a single sample may be quantified utilizing ihe
IRM cotnponent analysis proccditre that fits an optimal
number of coercivity components to a measured IRM
acquisition curve (Kruiver
etal...
2001),
Sediment magnetism
In sediments, the magnetic mineral suite is a tnixture of
particles with two main origins: detrilal particles and particles
that were formed ittsitii. The detrital particles are eroded and
subsequently transported from a source area by eolian.
riverine, lacustritie or marine processes (only in very slowly
accumulating abyssal plain sediments eosmogenic particles
may constitute a significant source). Iron oxides are ehemiealiy
sufficiently stable during transport to survive, although the
panicles abrase and surficially oxidize. Iron sultides do not
survive transport cotiditions, they arc fortned in the sediment
eoiumti at ihe deposilional site.
In situ fortned attthigenic particles are a consequence of
diagenetic processes. These processes may also change the
detrital input to some extent. Biological factors often are
important. Bioniineraiization is a widespread phenomenon; for
exatnple a tnajor part of the global calcium carbonate mass is
of bi{)genic origin. In virtually all sedimentary environments
and water-logged soils, bacteria whieh use iron in their
tnelabolism are known to occtir. The bacteria may use iron
as a tertninal electton acceptor: magnetite is (brnied outside
the cells (extraeellular magnetite) as a consequence of its low
solubility product. The crystals are very fine-grained and
usually are poorly crystalline. Alternatively, other bacteria
may produce chains of highly crystalline SD magnetite grains
(magnetosomes) inside their cells; these are referred to as
magnctotaclic bacteria
(e.g..
Hesse and Stolz, 1999).
The distinction betweeii authigencsis and biogenesis is
arbitrary. Authigenesis refers to inorganic chemical, rather
thati biological processes. However, usually at least one of the
reactants in an "inorganic" chemical reaction process is
bactcrially produced or tnediated. The sulfide in the pyrite
fot-malion process, which usually has a profound impact on
the assemblage of magnetic minerals in sediments, is produced
bv sulfate-reduciniz bacteria. The sull'ate reacts with available
ferrous iron to
i'orm
intermediate monosullides. which usually
read completely to form pyrite, Greigite and pyrrhotite have
been reported as intertnediate products in these reactions.
Fspecially greigite is increasingly docutnented in a wide variety
of (lake and tnarine) sediments
(e.g..
Snowball, 1997; Roberts
el al.., 1996). The reactioti pathway which leads to the
formation of pyrrhotite, is nol yel clearly established. Both
sulfides may fortn authigenically in sediments. Greigile in
particular is documetited in a variety of lake sediments that are
characterized by much lower sulfate contents than marine
conditions, Sulfate reduction under anoxie conditions usually
does not lead to the formation of pyrite. the final product in
marine sediments. Instead greigite or pyrrhotite. intermediate
products, often retnain. The hysteresis properties are very
similar to those of tnagnetite and thermal methods are often
required to unambigitously diagnoze greigite (and pyrrhotite)
with magnetic means. Because greigite may occur in magne-
lotactic bacteria as well its occurrence can be used as a
biomarker (Posfai
etal..
1998).
From the many examples that show the usefulness of
magnetic properties to document paleoclimate and paleoenvir-
onmental processes two are shown here: (1) ihe influence of
diageneiic processes due to cyclic redox conditions in
seditnenls from the Mediterranean Sea; and (2) the observation
of climate variability in the North Atlantic including Heinrich
evenis. Noteworthy as well are the well-known loess-paleosol
records from the Chinese Loess Plateau that have been
shown to be a continuous recorder of paleoclimate throughout
the Quaternary
(e.g,,
Maher and Thotnpson, 1999) with
the paleosols having higher magnetic sucepiibility than the
intercalating loess. Although il has been known for a long time
that the paleosols are correlated to wartner and moister
climatic periods, only recently a robust orbital chronology
could be formulated for the loess/paleosol sequence (Hcsiop
etal..
2000).
The Mediterranean Sea
Apart iVom sulfide formation, ihe influence of diagenesis on
the mineral-tnagnetic record can be substantial beeausc iron
through its two redox states is an important element in many
diagenetic reactions, iticluding biologically mediated ones.
Frotn the Miocene into the Quaternary many Meditcrratiean
sediments are characterized by astronomically controlled
sequences of anoxic and suboxic sediments
(e.g.,
Hilgen.
1991). The dominant source of the original magnetic minerals
is thought be dust from the Sahara wilh tninor riverine input
depending on the location in the Mediterranean Sea. The role
of biogenic magnetite in oxic sediment seems to be tninor.
Under anoxic conditions, layers etiriehed in organic material
may be deposited, so-called sapropels. During sapropel
deposition,
below each sapropel there is a zone where the
formerly existing magnetic minerals have been by and large
reductively dissolved; the iron is precipitated as pyrite
(Figure M4). When oxic conditions are restored in the water
column these sapropels are (partially) reoxidized starling Irom
their top parts. During this oxidative diagenesis. pyrite and
other sulfides are oxidized; the iron precipitates amongsl
others as magnetic oxides in the zone of the sapropel thai is
slowly oxidizing away. Compared to "aveiagc" sediment, the
zones straddling sapropels are thus characterized by low
magnetic intensities below the visible sapropel and higher
magnetic intensities in the zone directly above the sapropel. In
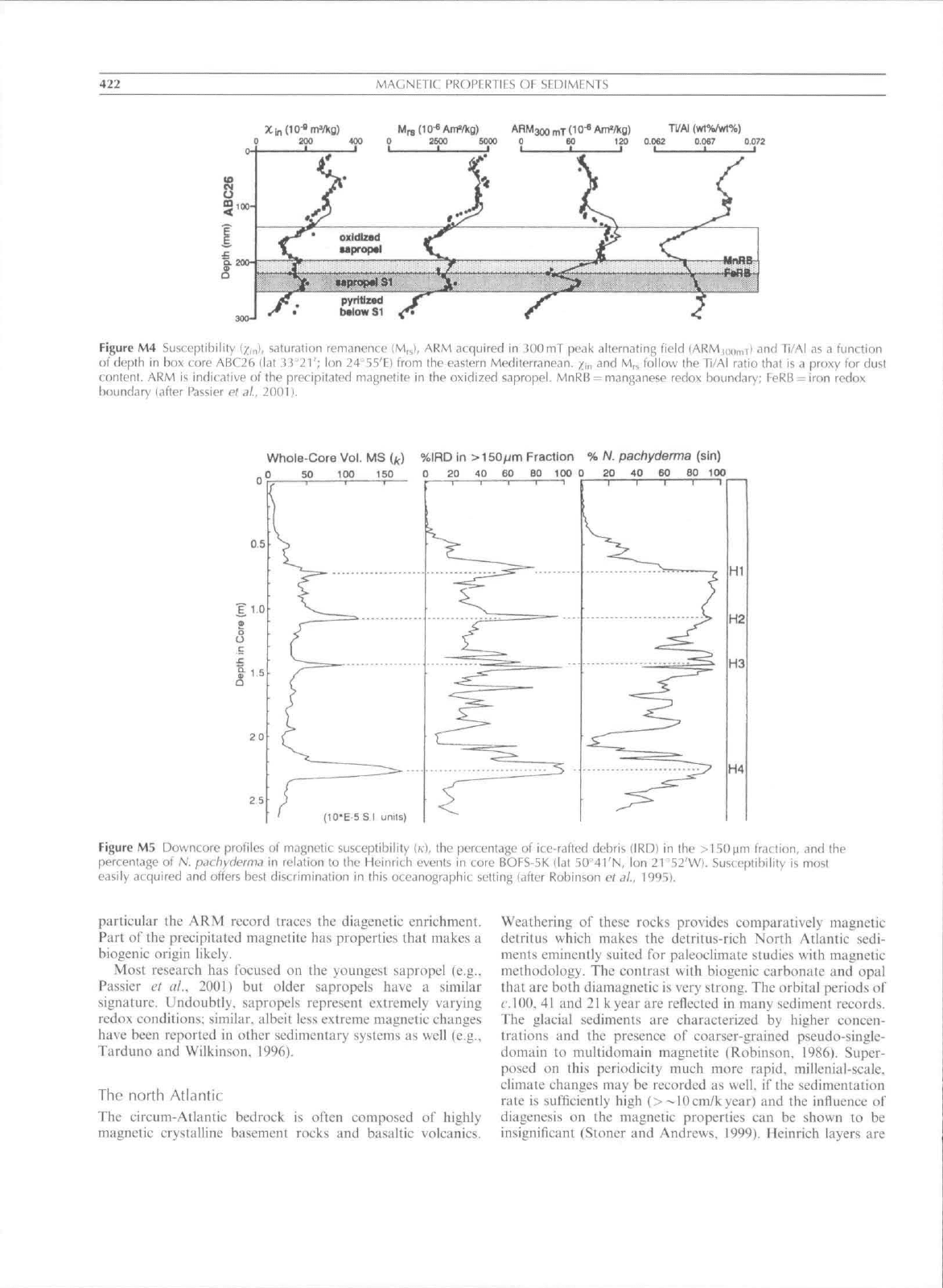
422
MAGNETIC PROPERTIES OF SEDIMENTS
Ti/AI (wt%/¥rt%)
Figure M4 Susceptibility
(/;,„),
saturation remanence (M,^), ARM acquired in JOOmT peak alternating field (ARMi,,,,,,,!) and Ti/Al as a function
of depth in box core ABC26 (!at J.i
'21';
Ion 24^55'E) from the eastern Mediterranean. /„, and M,., follow the Ti/Al ratio that is a proxy for dust
content, ARM is indicative of (he precipitated majinetite in the oxidized sapropel, MnRB = manganese redox boundary; FeRB = iron redox
boundary (after Passier e( ^/., 2001).
Whole-Core Vol. MS (if)
,0 50 100 150
25
%IRD in >150|jm Fraction % N.
pachyderma
(sin)
0 30 40 60 eo 100 0 20 40 60 60 100
H4
Figure M5 Downcore profiles of magnetic susceptibility
IK),
the percentage of ice-rafted debris (IRD) in the >150Mm fraction, and the
percentage of N. pachyderma in relation lo the Heinrirh events in core BOES-5K (lat 50MrN, Ion 21 52'W). Susceptibility is most
easily acquired and offers best discriminaticin in this oceanographic setting (after Kobinsoti ef JI
particular the ARM record traces the diagenetic enrichment.
Part of the precipitated magnetite has properties that makes a
biogenic origin likely.
Most research has focused on the youngest sapropel (e,g..
Passier et it!,, 2001) but older sapropels have a similar
signature. Undoubtly. sapropels represent extremely varying
redox conditions; similar, albeit less extreme magnetic changes
have been reported in other sedimentary systems as well (e.g..
Tarduno and Wilkinson. 1996).
The north Atlantic
The circum-Atlantic bedrock is often composed of highly
magnetie crystalline basement rocks and basaltic volcanics.
Weathering of these rocks provides comparatively magnetic
detritus which makes the detritus-rich North Atlantic sedi-
ments eminently suited for paleoclimate studies with magnetic
methodology. The contrast with biogenic carbonate and opal
that are both diamagnetic is very strong. The orbital periods of
(,1(K), 41 and 21 kyear are refleeted in many sediment records.
The glacial sediments are eharaelerized by higher concen-
trations and the presence of coarser-grained pseudo-single-
domain to multidotnain tnagnetite (Robinson. 1986). Super-
posed on this periodicity mueh more rapid, milleniat-scalc.
climate changes may be recorded as well, if the sedimentation
rale is sufficiently high (>-IOcm/kyear) and the influence of
diagenesis on the magnetic properties can be shown to be
insigtiificanl (Stoner and Andrews. 1999). Heinrich layers are
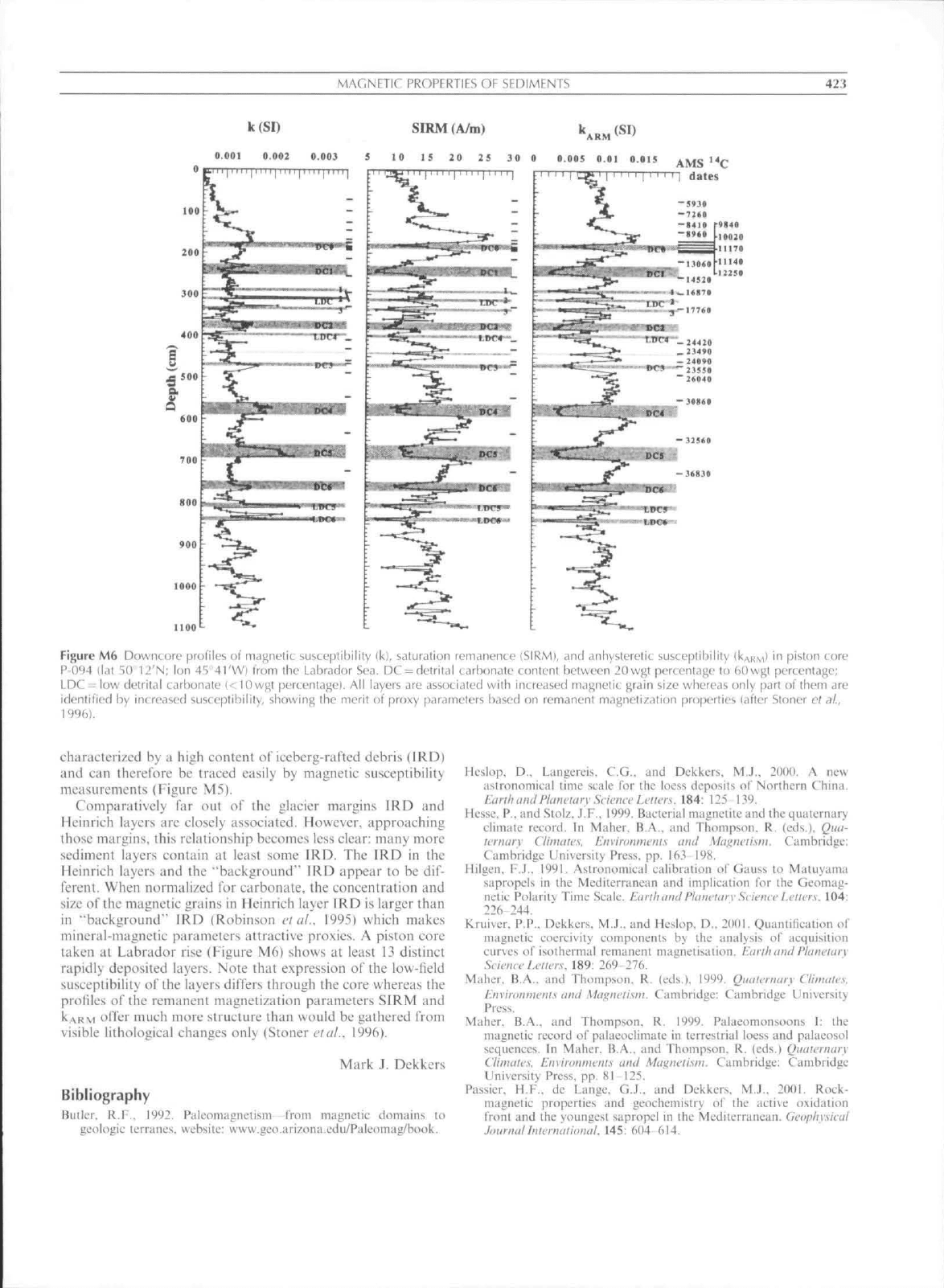
MAGNFTir PROPERTIES
OE
SEDIMENTS 423
k(SI)
D.OOI
0.002
SIRM (A/m)
IS
20 2S
30
0
0.005
0.01
O.OIS
200
300
a.
600
700
800
Figure
M6
Uowncore profiles
of
magnetic susceptibility (k), Stituration remanence (SIRM), and anhysterelic susceptibility
(k^^M'
in
piston core
P-094
ildt
50
12'N;
Ion 43
41'W) from
the
Labrador Sea, DC
=
detrital Larbonate content between 20w^t percentage
to
(>t)wgt percentage;
LDC
- low
detrital carbonate
(<;1G
wj;!
pfrcentagei.
All
layers are associated wilh increased magnetic grain size whereas only part
of
them
are
identified
by
increased susceptibility, showing
the
merit
of
proxy parameters based
on
remanent magnetization properties (after Sioner et
^^1.,
i;har;it:tcri/i:d by ;t high cotitcnt of" iccbcrg-raftcd debris (IRD)
;ttid
c;tti
ihererorc be traced easily by magtietic susceptibility
measitrctiictils (Figure M5).
ConiparLitivcly far out of the glacier margins IRD atid
Heitirich layers are closely associated. However, approaching
those margins, this relatiotiship becomes less clear: many more
sediment layers contain at least some IRD. The IRD in the
Heinrich layers atid the "background" IRD appear to be dif-
ferenl. When normalized for carbonate, the concentralion and
si/e of the magnetic grains in Heinrich layer IRD is larger than
in "background" IRD (Robinson etal.. 1995) which makes
mineral-tnagnctic paratneters attractive proxies. A piston core
taken at Labrador rise (higiire M6) shows at least 13 distinct
rapidly deposited layers. Note that expression of the low-field
susceptibility of the layers differs through the core whereas the
profiles of the remanent tnagnetization paratneters SIRM and
l^AKM offer tnuch more structure than would be gathered from
visible lithological changes only (Stoner et al.. 1996).
Mark J, Dekkers
Bibliography
Huller.
R.F,. 1992,
Paleoniagnetistn—from magnetic domiiins
to
geologic terranes. website: wu'\v,geo,ari7ona,cdu/Pa!eomag/book.
Hoslop,
D,.
Langereis,
C,G,, and
Dekkers.
M,J,,
2000.
A new
iistronomical time scale
for the
loess deposits
of
Northern
C
hina.
Earth and Planetary Science Letters. 184:
125 139.
Hosse. P,.
and
Stolz, J,F.. 1999, Bacterial mugnetite
and
the quaternary
climate record.
In
Maher.
B,A,. and
Thompson,
R,
(eds,).
Qua-
ternary Climates. Enyironnu-nts
and
Magnetism. Cambridge:
Cambridge University Press,
pp, 163 198,
Hilgen.
h,J,.
1991, Astronomical calibration
of
Gauss
to
Matiiyama
sapropels
in the
MedUerraiiOLiii
and
implication
for the
Geomag-
netic Polarity Titne Scale. Earth ami
I'linu-tary
Science Letters.
104:
226-244.
Kruiver. P,P.. Dckkcrs.
MJ.. and
Hcsiop.
D..
2111)1.
Oi'antitication
oi
magnetic coereivity components
by Ihe
analysis
of
acqtiisition
curves
of
isothermal remanent magnetisation. Earth and Phmctary
Science Letters. 189:
2(i9 276,
Maher.
B,A,, and
Thompson.
R,
(eds,),
1999,
Quaternary Climates.
Enyironments
and
.Magnetism, Cambridge: Cambridge liniversity
Press,
Maher.
B.A,. and
Thompson.
R, 1999.
Palaeomonsoons
I: the
magnetic record
of
palaeoclimatc
in
terrestrial loess
and
palaeosol
sequences.
In
Maher. B,A,.
and
Thompson.
R,
(eds,) Quaternary
Climates. Environments
and
.Magnetism. Cambridge: Cambridge
University Press,
pp,
81-125,
Passier.
H.F.. de
Unge,
G.J,, and
Dekkers.
M.J,, 2001.
Rock-
magnetic properties
and
geochemistry
of the
aclive oxidatioti
front
and the
youngest sapropel
in the
Mediterranean, Geophysical
JournalInlcrnational. 145:
604 614,

424 MASS MOVEMENT
Posfai,
M,,
Buseck,
P,R,,
Bazylinski,
D.A., and
Franket,
R.B.,
t998.
Reaction sequences
of
iron sultide minerals
in
bacteria
and
their
use
as
biomarkers. Scienee, 280: 880-883.
Roberts, A,P,, Reynolds,
R,L,,
Verosub, K,L,,
and
Adam, D,P,,
1996,
Environmental magnetic implications
of
greigite (Fe3S4) formation
in
a
3 million years lake sediment record from Butte Valley,
northern California.
Geophysieal
Researeh Letters, 23: 2859-2862.
Robinson,
S.G,, 1986, The
late Pleistocene palaeoclimate record
of
North Atlantic deep-sea sediments revealed
by
mineral-magnetic
measurements, Physiesofthe Earthand Planetary Interiors, 42: 22-47,
Robinson,
S,G,,
Maslin,
M.A., and
McCave,
N., 1995.
Magnetic
susceptibility variations
in
upper Pleistocene deep-sea sediments
of
the N.E. Atlantic: implications
for ice
rafting
and
palaeocirculation
at
the
Last Glacial Maximum. Paleoeeanography,
10:
221-250,
Snowball,
t.F.,
1997. Gyroremanent magnetization
and the
magnetic
properties
of
greigite-bearing clays
in
Southern Sweden. Geophysi-
eal Journal International, 129: 624-636.
Stoner,
J,S,, and
Andrews,
J.T., 1999, The
North Atlantic
as a
Quaternary magnetic archive,
tn
Maher,
B.A,, and
Thompson,
R,
(eds.) Quaternary Climates, Environments
and
Magnetism.
Cambridge: Cambridge University Press,
pp.
49-80.
Stoner,
J.S,,
Channell, J.E.T.,
and
Hillaire-Marcd,
C, 1996, The
magnetic signature
of
rapidly deposited detrital layers from
the
deep Labrador
Sea:
relationship
to
North Atlantic Heinrich
Layers, Paleoeeanography, 11: 309-325.
Tarduno,
J,A,, and
Wilkinson,
S.L.,
1996. Non-steady state magnetic
mineral reduction, chemical lock-in,
and
delayed remanence
acquisition
in
pelagic sediments. Earth
and
Planetary Science
Letters, 144: 315-326,
Tarling, D.t-t.,
and
Hrouda,
F.,
1993, Magnetic Anisotropy. Cambridge:
Cambridge University t'ress.
Cross-references
Authigenesis
Bacteria
in
Sediments
Bedding
and
Internal Structures
Biogenic Sedimentary Structures
Carbonate Mineralogy
and
Geochemistry
Cyclic Sedimentation
Diagenesis
Diagenetic Structures
Eolian Transport
and
Deposition
Fabric, Porosity,
and
Permeability
Geophysical Properties
of
Sediments
Flydroxides
and
Oxyhydroxide Minerals
Iron-Manganese Nodules
Milankovitch Cycles
Neritie Carbonate Depositional Environments
Paleoeurrent Analysis
Red Beds
Sapropel
Sulfide Minerals
in
Sediments
Weathering, Soils,
and
Paleosols
MASS MOVEMENT
Introduction
Mass movemetit refers
to
processes
by
which soil
and
rock
materials move downslope under their
own
weight (Selby,
1993;
Turner
and
Schuster, 1996),
The six
principal movement
mechanisms
are
creep, sliding, flow, toppling, free-fall,
and
rolling. Given
the
spectrum
of
possible processes, mass
movement deposits exhibit
a
wide range
of
sedimentological
features
and
surface morphology. Most deposits
are
typically
poorly sorted, unstratified clastic aggregates (i,e,, diamictons)
with predominantly angular clasts
of
local provenance.
Deposits associated with rapidly flowing events, such
as
debris tlows
and
rock avalanches, often exhibit crude
stratification
and
inverse grading.
The
typical morphologies
of mass movement deposits
are
colluvial cones
and
fans,
planar colluvial blankets, linear ridges
and
lobes,
and com-
plex hummocky forms.
The
latter have often been confused
with glacial drift deposits. Given
the
shear strength
of
many
landslide materials, some
are
emplaced
at
relatively steep
angles (5°-l5°)
and
therefore violate
the
Principle
of
Original
Horizontality, Such deposits require cautious structural inter-
pretation when encountered
in
ancient lithitied assemblages.
Strength
of
hillslope materials
Detachment
of
material from
a
slope
as a
slide
or
fall requires
that shear stress,
due to the
downslope component
of
the bulk
weight
of the
material, equals shear strength along
a
plane
of
detachment,
as
described
by the
Mohr-Coulomb equation:
s
= c'
-\-
{(7
— u)tan (
(Eq,
1)
where
s is
shear strength,
c' is
cohesion,
a is
total normal
stress,
u is
pore water pressure,
and
cj)'
the
angle
of
shearing
resistance. Term (a—u)
= a' is
effective normal stress (Craig,
1997),
Cohesion
is
associated with either clay
or
cementing
material. Clay cohesion
is a
function
of the
load history
of a
material, being relatively
low (<
10
kPa)
in
recently deposited
muddy deposits,
and
relatively high (>100kPa)
in
over-
consoiidated clay-shale formations (Selby,
1993;
Craig,
1997),
Angle
of
shearing resistance,
(/>',
controls
the
steepest
stable slope
for a dry
frictional material,
and
depends
on
particle angularity, mineralogy,
and
packing density.
It is
highest
in
dense deposits
of
angular quartz-feldspathic grains
(35°-45°),
and
lowest
in
low-density deposits
of
clay (5°-15°)
(Kenney, 1984), Clay mineral species also controls frictional
strength, being highest
in
kaolinite, intermediate
in
illite,
and
lowest
in
montmorillonite. Shear strength also depends
on
material displacement history. First-time failures
in
soil
and
rock mobilize peak strength, comprising in situ cohesion
and
friction
due to
maximum grain
or
rock-joint interlocking
(Craig, 1997), Small shear displacements,
of the
order 0,1
m,
cause substantial loss
of
cohesion
and a
reduction
of
frictional
resistance
due to
shear dilatancy
and the
development
of
preferred grain alignments.
The
resultant ultimate
or
residual
strength
is
appreciably lower than
the
original peak value
(Craig, 1997), This means that surfaces which have experi-
enced past movements
are
predisposed
to
continued move-
ment. Similar reduced strength commonly occurs along
tectonically sheared rock surfaces.
On slopes where soil
is
significantly reinforced
by
tree roots,
a third strength component termed root reinforcement,
c'r,
exists.
It is
significant
on
slopes with relatively thin
(<2m)
mantles
of
colluvial material
or
weathered glacial drift. Since
most mountain soils
are
relatively thin, landslide events,
and
hence regional sediment tlux
to
depositional areas,
are
strongly
regulated
by
plant cover
and its
degree
of
anthropogenic
disturbance (Sidle etal., 1985),
Causes
of
mass movement events
Slope failure
as a
landslide
may be
induced
by an
increase
in
shear stress,
a
decrease
in
shear strength
or
both. Shear stress
increase, coupled with shear strength decrease, typically occur
when materials
are
water saturated
by
rainfall
or
snowmelt.
