Middleton G.V. (Ed.) Encyclopedia of Sediments and Sedimentary Rocks
Подождите немного. Документ загружается.


Cl ASSIFICATION OF SEDIMENTS AND SEDIMENTARY ROCKS
135
Fisher, R.V., 1966. MccliaiiiMii for deposition from pyroclastic flows.
.4nu'ri( till .loiirmtl
of
.Si
ience. 264: 35(1 36.^.
Flint. R.F., Satidcrs. J.E., iind Rodgcrs. J.. 1960. Diamiclitc. a
siibslitiilc Icrm for syniniictite.
Geotogicctl
Soiivlv of America Bulic-
itn.
71: 1809 1810.
Folk, R.L., 1956. The role of tcxlurc and L-omposilioii in sandstone
classilieation. Jminitilof Si'dinientarv Pctrolii^w 26:
166-171.
Folk, R.L.. 1959. Priicliciil petrographic ciassilication ol' limestones.
.Americtin
.'issmiuiiottof I'viroleunt Geolo^ist.sBulk'tin. 43: I 38.
Friedniiin, G.M., 1965. Terminology of cryslalli/iilion textures and
fabrics in sedimentary rocks. Joiirmdof Seilinienuirv Peirotogv. 35:
643-655.
Friedman. G.M.. 1985. The problem of submarine cement in
classifying reefrock: an experience in iVtistralion. In Sehneider-
mann, N., and Harris, P.M. (eds.). CurhonateCeincnis. Society of
Economic Paleontologists and Mineralogists, Special Publication.
36 pp.
117-121.
Friedman. G.M., and Sanders, J.F.., 1967. Origin and occurrence oi
dolostones. In Chilingar. G.V., Bissell, H.J., and Fairbridge, R.W.
(eds.).
Carbonate rocks, origin, oecLirrence, and classification.
Elsevier Scicntiiie Publishing Company, pp. 267-348.
Friedman. CM., and Sanders, J.E., 1978. Principles of sedimentology.
New York: John Wiley & Sons. 792 p.
Friedtnan. G.M.. Sander's. J.H.. and Kopaska-Merkel. D.C. 1992.
Principles of sedimentary deposits. New York: Macmillan Publish-
ing Co., 717p.
Fuchtbauer, H., and Leggewie. R.. 1984. Korngrossenbeziehiingen
zwisehen Silt- und Sandsteinen: Neites Jtthrhiicltf'itr Geolo^i.schc
Paliioniolosi.sche Ahluinilhtni-vii. 167: 133- 161.
Fiichtbaner. H., 1988. Svdiinetil itiid Seitimenl^esleine. Sediment-
Petiologic, Part II. Stuttgart, F. Schweizcrbart'sche Verlagsbuch-
handlung.
Ciermann. K., Bock, W.D..and Schroter. T.. 1984. Facies development
of Upper Cretaceous phosphorites in Egypt: sedimentological and
geocheniical aspects. In Klit/sch, E., Said, R., and Scbrank. E.
(eds.).
SFB 69: Results of the special research project Arid Areas.
Period 1981 1984: Berliner Geowisseusciuft. Abh. A.. Voitime 50,
pp.
354 361.
Guilbcrl. J.M.. and Park. C.F. Jr.. 1986. ThcGeolo^y of
Ore
Deposils.
W.H. Freeman and Company.
Hardie. L.A., l'-)87. Dolotniti.z;ition: a critical view of some current
views.
JournulojSediiiH-iUarvPcirolo^v. 57: 166-183.
Heling. D., 1988. ton und Siltsteine. In Ftichtbauer. H. (ed.), Sedi-
Diente und
.Sedinieiitfie.sU'iiu-.
Stuttgart: E. Schweitzerbart, pp.
185-2.32.
van Houton. F.B.. 1990. Paleozoic oolitic ironstones on Norlh
American craton (abstract):
Geoloi^iccil
Society of
.Amerivu.
Sorlli-
eti.s urn.Set
lion.
Ahslnutswilli
Pfo}ircnns.
22(2): 76 (only).
Hunt. J.M.. 1979. PclroleiiniGcoclicniisnyitittlGeology. W.H. Freeman,
.lames.
H.L.. 1954. Sedimentary facies of iron formation. Economic
Geology. 49: 235 293.
Jones,
B., and Goodbody. Q.H., 1985. Oncolites from a shallow
lagoon. Grand Cayman Island. Btillelin of Cunatliitit Peliolvtim
Geology. 32: 254-260.
Jones.
J.B.. and Segnit. F.R., 1971. The nature of opal I.
Nomenclature and constituent phases. Journal of ihc Geologieul
Socii-lyol
.•Uistralia.
18: 57 68.
Klein, G.deV.. 1963. Analysis and review of sandstone classifications
in the North American geological literature 1940 1960, Geologieal
Societvof Americii Bulletin. 74: 555-576.
Kolodny. Y.. 1981. Phosphorites. In Emiliani. C. (ed.). The Sen. 7:
Wilcx-Interscicnce, pp. 981 1023.
Krvnine. P.D.. 1948. The megascopic study and field classification of
sedimenUiry rocks. Journtilof Geology. 56: 130-165.
Lajoie, J.. 1979. Facies models 15. Voleanoclastic rocks: Geosciemc
Cunmki. 6: 129 139.
Land. L.S.. 1985. The origin of massive dolomite: Journal of
Geolov:iciil
Edticiitton.
33: 112
125.
Laschet. C. 1984. On the origin of cherts.
Fcieies.
10: 257 290.
Maynard. J.B., 1983. Geochemistry of Sedimentary Ore Depo.sits.
Springer-Vcrlag.
Mcbride. F.F.. 1962. Flysch and associated beds ofthe Martinburg
F'ormation (Ordovician) central Appalachians. JournalofSedimcn-
iiiiy Petrology. 32:
32-91.
Notholt, A.J., Sheldon. R.P.. and Davidson. D.F.. 1989. Pho.sphate
Deposils of ibf
World,
volume
2.
I'hosphiilc Rock Resources. Interna-
tional Geologieal C\irrelation Programme Project 156: Phosphor-
ites,
Cambridge University Press.
Notholt. A.J.CJ.. and Jarvis, 1. (eds.), 1990. PItosphuic Research and
Development. Geological Society of London. Special Publication.
52.
Pettijohn, F.J., 1975. Sfdimairaiv Rocks. 3rd edn. Harper & Row.
Potter. P.E.. Maynard. J.B.. and Pryor. W.A.. 1980. Seditncniologyof
Shale. Springer-Verlag.
Riggs, S.R.. 1986. Proterozoic and Cambrian phosphorites specialist
studies: phosphogenesis and its relationship to e.xploration for
Protero/oic and Cambnan phosphorites. In Cook, P.J., and
Shergold. J.H. (eds.), Proterozoic and Canihrian Phosphorites.
Cambridge University Press.
Sackett. W.M.. Poag. C.W.. and EDIE, B.J.. 1974. Kerogen recycling
in the Ross Sea, Antarctica. Science. 185: 1045-1047.
Sanders. J.E., 1978. Graywacke. In Fairbridge. R.W., and Bourgeois,
J. (eds.). The Encyclopedia of SediDienlology. Encyclopedia of earth
sciences. Volume VI: Stroudsburu. PA: Dowdeii. Huntchinson. and
Ross.
pp. 389 391.
Sanders. J.E., and Friedman. G.M., 1967. Origin and occurrence of
limestones. In Chilingar. G.V.. Bissell. H.J. and Fairbridge, R.W.
(eds.),
Cathonaic Rocks. Elsevier Scientific Publishing Company,
pp.
169 365.
Schmincke. H.U.. 1988. Pyroklastische Gc^teine. In Ilichtbauer. H..
(ed.).
.Sediment undSedimenigesleine. Sediment-Petrologie Part II:
Sluttgart. E. Schweizcrbart. pp. 731 778.
Schreiber. B.C., and Friedman, G.M., 1976. Depositional environ-
ments of Upper Miocene (Messinian) evaporites of Sicily as
determined from analysis o!' intercalated carbonates. Setlimentol-
of;y. 23: 255-270.
Scoffin. T.P.. 1987. An Iniioditetionto Cttrhonale Sedimenrs and
Rock.s.
Blackic. Glasgow.
Sheldon, R.P.. 1987. Association of phosphorites, organic-rich shales,
chert, and carbonate rocks. CurhonaivsandFvopoviles. 2: 7-14.
Shinn, E.A.. Steinen. R.P.. Lidz. B.H.. and Swart. P.K.. 1989.
Whitinus. a sedimentologic dilemma, .lourmtl of .Sedimentary
Petrology. 59:
147-161.
rada. R.. and lijima. A.. 1983. Petroioiiy and diagenetic changes of
Neogene siliceous rocks in northern Japan.
Jotirnal
of Sedimentary
Petrology. 53: 911-9.30.
Tucker. M.E.. and Wright, V.P.. 1990. Carhotiaie Seiliiiienlology.
Blackwell Scientific Publications.
USDOE. 1988. What is
Tuff.'
Office of Civilian Radioactive Waste
Management Nevada Nuclear Waste Storage Investigations. 2p.
Warren, J.K.. 1989. Evctporiu-seilintentologv. Prentice Hall.
Weaver, C.E., 1989. Clays. Mmh. and Shales. Elsevier.
Williams, L.A.. and CREAR, D.A.. 1985. Silica diagenesis II, general
mechanisms. Jouinulof Sedimentary Petrology. 55: 312 321.
Young. T.P., and Taylor. W.E.G. (eds.). 1989. PhunerozoicIronstones.
London: Geological Society of London.
Cross-references
Algal and Bacterial Carbonates Sediments
Anhydrite and Gypsum
Bioclasts
Caliche-Calcrete
Carbonate Mineralogy and Geochemistry
Cave Sediments
Dolomite Te.\tures
Dolomites and Dolomiiization
Encrinites
Fvaporites
Glaueony and Verdine
Grain Size and Shape
Ironstones and Iron Formations
Laterites
Oolite and Coated Grains
Phosphorites
Sands and Sandstoties
SedimeiUolouists

136
CLASTIC (NEPTUNIAN) DYKES AND SILLS
Silcrete
Siliceous Sediments
Speleothems
Spiculites and Spongolites
Tills and Tillites
Varves
CLASTIC (NEPTUNIAN) DYKES AND SILLS
Clastic dykes are tabular bodies of clastic material, mostly fine
sand, cutting across sedimentary formations or, more rarely,
volcanic and granitic rocks (Allen, 1984; Peterson, 1968). They
rank among the earliest described deformational sedimentary
structures. Already in 1791, Werner published his theory of
vein genesis based on the observation of marl veins in the
middle Trias of central Germany. Since then, innumerable
reports of sedimentary dykes tliroughout the world have
accumulated (see Diller (1890), Williams (1927), Strauch
(1966),
Allen (1984), and Maltman (1994) for successive
reviews). Clastic sills are also encountered, with the frequent
difficulty of distinguishing them from normal beds (Archer,
1984).
Features described as clastic dykes range from filled
centimetric crevices to several-m-wide, several-km-long struc-
tures.
A main genetic distinction is to be made between true
clastic dykes and sills, forcefully injected from below, and
passive fissure-fills, sometimes called neptunian dykes.
Intrusive clastic dykes are discordant—and sills concor-
dant—planar to irregular and often branching sheet-like bodies
which commonly occur in swarms. Their walls are generally
smooth though locally disturbed and sometimes slickensided or
erosionally grooved (Allen, 1984). The filling material is
generally fine sand or clay and includes pieces of the host
sediments. It is mostly structureless but in some cases a wall-
parallel orientation of the sediment grains or even a wall-
parallel layering is observed. Clastic dykes are often associated
with other soft-sediment deformation structures or otherwise
mobilized material (slumps). When the intrusion breaks
through to the contemporaneous sediment surface, sand
volcanoes or extruded sand sheets (often difficult to distinguish
from clastic sills) can be formed. Unless they can be traced down
to the source layer, and in spite of suggestive upward branching,
wall-parallel grain fabric and irregular or striated walls, the
intrusive origin ofthe dykes is not easily demonstrated.
Clastic dykes are formed as the result of forceful injection,
commonly from below and more rarely sideways or even from
above, after unconsolidated sediments have been liquidized (see
Liquefaction and Fluidization). Therefore, such soft-sediment
deformation features are penecontemporaneous with deposi-
tion or at least occur before complete lithification. Liquefaction
is achieved through either thixotropie behavior of some
cohesive materials (e.g., certain clays) or liquefaction of
cohesionless saturated sands, whose primary strength results
from grain interlocking and friction at particle contacts.
Liquefaction is caused by the pore fluid pressure increase to
the level ofthe lithostatie stress (thus making the effective stress
equal to zero) in a system more or less closed by overlying less
permeable, cohesive materials (Lowe, 1975). The consequence is
a dramatic loss of strength of the sand whose particles are
dispersed in the pore fluid. This will eventually result in closer
grain packing and forcing up of pore water, leading to
fluidization when escaping excess pore water drags sediment.
Fluidization occurs in an open system after overpressure has
caused hydraulic fracturing in the impermeable overlying strata.
In the case of true clastic dykes, fissuring and filling therefore
occur simultaneously. In horizontal strata, the formation of
dykes or sills respectively depends on the horizontal or vertical
orientation of the 0-3 principal stress in the fracturing layers.
Increase of pore fluid pressure and liquefaction may occur in
different ways, either static, dynamic (impulsive) or
cyclic.
Main
triggers for static liquefaction are artesian groundwater move-
ments and escape of pore water from underlying compacting
sediment. Rapid sediment deposition, slope failure, breaking
waves and flood surges may be responsible for impulsive
liquefaction. Finally, seismic shaking, pressure variations
associated with waves or with flow separation will cause cyclic
stresses and liquefaction (Owen, 1987). Unfortunately, since
clastic dykes depend primarily on overpressuring which may
occur in so many different environments, they are generally no
clear diagnostic feature of the trigger agent, and the latter has
rather to be deduced from the sedimentological context (Owen,
1987).
Indeed, clastic dykes occtir in a wide variety of
environments, ranging from deep-water turbidite associations
(Truswell, 1972) to shallow-water marine and nonmarine
sediments to subaerial mass-flow deposits (Collinson et al.,
1989).
They are also known from seismic (Obermeier, 1996) and
glacial (Amark, 1986) environments.
Though in many cases difficult to distinguish from injected
sedimentary dykes, neptunian dykes fed from above sometimes
exhibit characteristic features. Often, they will show a clear
downward tapering, while their filling may more frequently
include coarse material and is in some cases horizontally
layered. Genetically, the fissuring and filling stages are not
necessarily simultaneous and the cracks may widen progres-
sively over years, then allowing for vertical layering of the fill.
Many different causes can produce open cracks. Earthquake-
related ground cracks are common feature, while other
tectonic cracks like enlarged joints and tension gashes can
also trap sediments and develop in neptunian dykes. Subaqu-
eous slumps and subaerial landslides as well are another
frequent cause of ground cracking. Karstification ofcarbonate
rocks leads to extensive fissuring which again can and often do
catch sediments from above. Finally, desiccation and syneresis
cracks are also mentioned as features possibly evolving into
neptunian dykes. Especially when they are synsedimentary
subaqueous features, filling of the fissures may be slow witli
horizontal stratification (Strauch, 1966). However, subaerial
progressive filling has also been shown to result in vertical
layering and fining upward of the material brought in the
fissure by rainwash and clay infiltration (Demouhn, 1996).
Owing to weathering and collapse of the fissure walls, slow
fissure filling frequently displays a brecciated structure. In
other cases, rapid filling can occur, provided overlying
sediment is available. It can occasionally involve injection
from above, and yields a mostly structureless or faintly
vertically laminated infill. Neptunian dykes have been de-
scribed from as varying environments as those of true clastic
dykes.
Moreover, complex structures coupling forceful injec-
tion from below and collapse of surface sediments within the
cracks can also form, namely as the result of seismic shaking
(Thorson etal., 1986). Note however that although passive
fissure-fills of different origin have sometimes been mistakenly
interpreted as periglacial ice-wedge casts, the latter are
generally not referred to as neptunian dykes.

CLATHRATES 137
As synsedimentary features, clastic and many neptunian
dykes can experience later compaction-induced deformation
and folding. Displacement across the dykes or even across
their infill as well as slickensided walls have in some cases been
interpreted as the imprint of subsequent tectonic use of the
weakened cracked zones. They should anyway be carefully
distinguished from cataclastic shear zones which sometimes
mimic them.
A. Demoulin
Bibliography
Allen, J. R. L., 1984. Sedimentary structures. Amsterdam: Elsevier.
AmSrk, M., 1986. Clastic dykes formed beneath an active glacier.
Geologiska
Foreningens
i Stockholm Forenhandlingar, 108: 13-20.
Archer, J., 1984. Clastic intrusions in deep-sea fan deposits of the
Rosroe Formation, lower Ordovician, western Ireland. Journalof
Sedimentary Petrology, 54: 1197-1205.
Collinson, J., Bevins, R., and Clemmensen, L., 1989. Post-glacial mass
flow and associated deposits preserved in palaeovalleys: the Late
Precambrian Moraeneso Formation, North Greenland. Meddelel-
seromGronland. Geoscience, 21: 3-26.
Demoulin, A., 1996. Clastic dykes in east Belgium: evidence for upper
Pleistocene strong earthquakes west of the Lower Rhine rift
segment. Journal ofthe
Geological
Society, 153: 803-810.
Diller, G., 1890. Sandstone dykes.
Geological
Society of America Bulle-
tin,
1: 411-442.
Lowe, D., 1975. Water escape structures in coarse-grained sediments.
Sedimentology, IT. 157-204.
Maltman, A., 1994. Introduction and overview. In Maltman, A. (ed.).
The Geological Deformation of Sediments. London: Chapman &
Hall, pp. 1-35.
Obermeier, S., 1996. Using liquefaction-induced features for paleo-
seismic analysis. In MeCalpin, J. (ed.), Paleoseismology. San Diego:
Academic Press, pp. 331-396.
Owen, G., 1987. Deformation processes in unconsolidated sands. In
Jones,
M., and Preston, R. (eds.). Deformation of Sediments and
Sedimentary Rocks. London: Geological Society, Special Publica-
tion, 29, pp. 11-24.
Peterson, G., 1968. Flow structures in sandstone dikes. Sedimentary
Geoiogy, 2: 177-190.
Strauch, F., 1966. Sedimentgange von Tjornes (Nord-lsland) und ihre
geologische Bedeutung. Neues Jahrbueh fur Geologischen und
Palaontologischen Abhandlungen, 124: 259-288.
Thorson, R., Clayton, W., and Seeber, L., 1986. Geologic evidence for
a large prehistoric earthquake in eastern Connecticut. Geology, 14:
463-467.
Truswell, J., 1972. Sandstone sheets and related intrusions from Coffee
Bay, Transkei, South Africa. Journal of Sedimentary Petrology, 42:
578-583.
Werner, A., 1791. NeueTheorievon Entstehungder
Gange.
Freiberg/Sa.
Williams, W., 1927. Sandstone dikes in southeastern Alberta. Transac-
tions ofthe Royal Society of Canada, 21: 153-174.
Cross-references
Compaction (Consolidation) of Sediments
Convolute Lamination
Deformation of Sediments
Desiccation Structures (Mud Cracks, etc.)
Dish Structure
Fabric, Porosity and Permeability
Flame Structure
Fluid Escape Structures
Liquefaction and Fluidization
Pillar Strueture
Porewaters in Sediments
Slope Sediments
Storm Deposits
Syneresis
CLATHRATES
Clathrate hydrates
Clathrate hydrates (or gas hydrates) are solid ice-like
crystalline compounds of hydrogen bonded water molecules
that form a rigid lattice of cages that host small guest gas
molecules. They are stable only when the cages contain gas
molecules. They are stable at moderate to high pressures and
low temperatures (Sloan, 1998). The stability depends on the
composition of the gas molecules and of the pore fluid
(Englezos and Bishnoi, 1988). Methane and other clathrates
most likely formed in the solar nebula and thus played a role in
the accretion of planets and satellites and probably comets
(Lunine and Stevenson, 1985). On Earth, methane hydrate is
the principal clathrate hydrate.
Methane hydrate has a body centered cubic structure I,
which is one of the three hydrate structures known to occur in
the natural environment. Structure I forms with gas molecules
smaller than propane, hence with CH4, CO2, H2S. Structure II
forms with molecules greater than ethane but smaller than
propane. The two most important clathrate hydrate structures
1 and II consist of different combinations of three types of
cages;
each has two cage sizes. A third hexagonal structure H
was described from the Gulf of Mexico having three types of
cages.
The predominant natural gas hydrate on Earth, methane
hydrate, has a density of 0.93 g/cm''. Clathrate hydrates have
large storage capacity for gas molecules; for example, the ratio
of gas to water molecules in structure I methane hydrates is
1CH4:5.75 H2O, when decomposed, the volumetric ratio is
164 CH4:0.8 H2O (Kvenvolden, 1993). When hydrates form
they exclude ions and fractionate O and H isotopes; they
become enriched in '^O and D isotopes, like sea ice.
Most ofthe modern ocean seafloor is within the temperature
and pressure stability field for methane hydrate. Methane
concentration constraints restrict its occurrence to two main
environments: (I) in the oceans to the uppermost few hundred
meters of sediments on submerged continental margins slope
and rise sediments, where water depths exceed ~500 m, and (2)
in polar regions associated with permafrost. A conservative
estimate of the oceanic gas hydrate reservoir is enormous:
~10"g of methane C is stored in them (Kvenvolden, 1988).
This C reservoir is larger than the C in all other known fossil
fuel deposits. The permafrost reservoir is considerably smaller
than the oceanic one by about two orders of magnitude
(~4 X lO'^g).
The methane in hydrates originates either from bacterial
anaerobic degradation of organic matter or from thermal
breakdown of organic matter at elevated temperatures (>80 to
90° C). The conventional bacterial methane is characterized by
(5'^C values that range from about -55%o to
85%o
(PDB) and Ci/
(C2 + C3) > 200, whereas conventional thermogenic methane
typically has 5'^C values of-20%o to -50%o, and Ci/(C2+C3) <
100.
The source of methane in the hydrates is from either insitu
production of bacterial methane or upward transport of
methane-rich fluids into the hydrate stability zone. The latter
source may have a bacterial, thermogenic, or mixed origin
(Hyndman and Davis, 1992). In situ production and/or
transport of methane by fluids into the hydrate stability zone
is the limiting factor for methane hydrate formation in most
regions of the modern ocean. Geochemical studies, especially

138 CLATHRATES
of C isotope values of the methane, indicate that in methane
hydrates, the methane is largely bacterial in origin, but in some
regions, such as the Gulf of Mexico or Caspian Sea, it is
thermogenic.
Only a fraction of the marine sediment organic C is
available for methane production bacterially or thermogeni-
cally. Reasonable estimates of the efficiency for methane
generation from organic matter vary from 2-3 percent to 20
percent. Accordingly, the maximum methane hydrate that can
be generated in situ in continental margin slope and rise
sediments, assuming an average porosity of 50 percent and 1
percent organic C content is 4-6 percent ofthe void space. The
higher concentrations of 20-35 percent in convergent margins
based on geochemical indicators, such as pore fluid Cl
concentrations, or on geophysical interpretations, indicate
that transport of methane into the stability field is widespread.
Because advection of methane-rich fluids is more pervasive and
aggressive in convergent than in rifted and sheared margins,
Kastner (2001) suggested that convergent margins host 60-65
percent of the enormous oceanic methane hydrate reservoir.
Much of our knowledge about methane hydrate occurrence,
distribution, and geochemistry in marine sediments is from
recovered samples and geochemical data obtained through the
Deep Sea Drilling Project (DSDP) and Ocean Drilling
Project (ODP). All the successful recoveries were from about
100-400
mbsf,
primarily from samples in the two-phase region
of the stability field. The direct observations and inferences
obtained from geophysical and pore fiuid geochemical data
indicate that methane hydrates are distributed inhomogen-
eously in ocean sediments, irregularly disseminated in turbidite
sediments and in silty mudstones with increasing abundance
near and in the sediment at the base of the three-phase
equilibrium field of methane hydrate. A Bottom Simulating
Refiector (BSR) of reverse polarity that parallels the seafioor
often characterizes this boundary (Shipley etal., 1979). In clay-
rich sediments methane hydrate occurs in mierofractures, or in
thin platelets parallel to fissility or scaly fabric; it cements
coarser sediment horizons of higher permeability, such as sandy
silts or ash layers; and concentrates in fractures, fault zones, or
along lithologic boundaries where the occurrence is modular to
massive. Gas hydrates thus affect the sediment physical
properties, particularly the permeability, seismic velocity,
thermal conductivity, and electric resistivity. Sedimentological
processes such as compaction or cementation by silicates or
carbonates are inhibited where gas hydrate fills void space.
Because of continuous sedimentation, methane hydrate in
the sediment at the BSR dissociates. The methane released is
recycled, it migrates upward into the stability field, thus
stimulates the formation of the methane hydrate near the base
of the 3-phase stability field, and produces the generally larger
accumulations near the BSR. This overall vertical distribution
is amplified in convergent margins where upward transport of
methane-rich fiuids is widespread. The recently observed
moderate to large accumulations of methane hydrate near
the sea-fioor at the Caseadia margin. Eel River basin in
northern California, Gulf of Mexico, Okhotsk Sea, Black and
Caspian Seas thus require active focused transport of methane-
rich fiuids or methane gas to the sea-fioor.
Recent interest in natural clathrate hydrates has resulted
from recognition that global warming may destabilize some of
the vast quantities of methane hydrate in shallow marine slope
sediments (and permafrost). The potential environmental
consequences of rapid release of large quantities of methane
for both ocean and atmosphere are important questions
surrounding the very large amounts of gas hydrates in the
shallow geosphere. In the ocean, intense microbial oxidation of
methane would reduce the amount released into the atmo-
sphere, but this would result in extensive local oxygen
consumption and CO2 production, therefore in some reduction
of the capacity of the ocean to incorporate fossil fuel CO2.
Because methane gas is an important contributor to the
atmospheric radiation balance as it is a significantly more
effective greenhouse gas than CO2, any additional flux of
methane into the atmosphere beyond the present annual
growth rate of ~0.8 percent per year would accelerate global
warming.
Significant environmental stresses or geologic perturbations,
such as global warming, rapid deglaciation or glaeiation,
earthquakes, or tectonic uplift, may cause clathrate hydrate
dissociation. If at the BSR it may trigger geologic hazards such
as giant landslides that could cause tsunamis and perhaps
rapidly release large quantities of methane to the ocean and
atmosphere with complex climatic feedbacks. New evidence
exists for massive methane releases from gas hydrate and their
possible association with global warming in the geologic past,
for example in the late Paleocene, ~55.6 Ma (Dickens et al.,
1997);
this event was accompanied by a thermal maximum.
It also has been suggested that clathrate hydrates may have
played a role in past climate change. For example, during
deglaciation, an increase in atmospheric methane from hydrate
dissociation may have accelerated the retreat of continental ice
sheets (Nisbet, 1990); or during glaeiation, the decomposition
of gas hydrate resulting from lowering sea level may have
moderated the extent of glaeiation (Paull etal., 1991).
A clathrate structure of silica
Melanophlogite is a rare cubic, low-density (2.06 g/cm^)
polymorph of SiO2 having a clathrate structure. The guest
material in the cavities consists of small organic molecules
containing S that are essential structural elements stabilizing
the Melanophlogite structure. Melanophlogite occurs as
authigenic single crystals and interlocking intergrowths on S
crystals in Sicilian sulfur deposits (Skinner and Appleman,
1963;
Kamb, 1965).
Miriam Kastner
Bibliography
Dickens, G.R., and Castillo, M.M., and Walker, G., 1997. A blast of
gas in the latest paleocene: simulating first-order effects of massive
dissociation of oceanic methane hydrate. Geology, 25: 259-262.
Englezos, P., and Bishnoi, P.R., 1988. Prediction of gas hydrate
formation conditions in aqueous electrolyte solutions. AIChE
Journal, 34: 1718-1721.
Hyndman, R.D., and Davis, E.E., 1992. A mechanism for the
formation of methane hydrate and seafloor bottom-simulating
reflectors by vertical fluid expulsion. Journal of Geophysical Re-
.search,
97: 7025-7041.
Kamb, B., 1965. A clathrate crystalline form of silica. Science, 148:
232-234.
Kastner, M., 2001. Gas hydrates in convergent margins: formation,
occurrence, geochemistry, and global signiflcance. In Natural Gas
Hydrates:
Occurrence,
Distribution, and Detection, AGU (American
Geophysical Union), Geophysical Monograph, 124, pp. 67-86.
Kvenvolden, K.A., 1988. Methane hydrate—a major reservoir of
carbon in the shallow geosphere. Chemical
Geology,
71:
41-51.

CLAY MINERALOGY
139
Kvenvolden,
K.A., 1993. Gas
hydrates—geological perspectives
and
global change. Reviews of Geophysics, 31: 173-187.
Lunine,
J.I., and
Stevenson,
D.J.,
1985. Thermodynamics
of
clathrate
hydrate
at
low
and
high pressure with application
to the
outer solar
system. Astrophysical Journal Supplementary Series,
58:
493-531.
Nisbet,
E.G.,
1990.
The end
ofthe
ice age.
Canadian Journal of Earth
Sciences,
27:
148-157.
Paull,
C.K.,
Ussier,
W., and
Dillon,
W., 1991. Is the
extent
of
glaeiation limited
by
marine
gas
hydrates? Geophysical Researeh
Letters, 18: 432-434.
Shipley,
T.H.,
Houston,
M.,
Buffler,
R.
etal., 1979. Seismic reflection
evidence
for the
widespread occurrence
of
possible gas-hydrate
horizons
in
continental slopes
and
rises. American Association
of
Petroieum
Geologists
Bulletin, 63: 2204-2213.
Skinner,
B.J., and
Appleman,
D.E., 1963.
Melanophlogite,
a
cubic
polymorph
of
silica. Ameriean Mineralogist,
48:
854-867.
Sloan,
D.D. Jr., 1998.
Clathrate Hydrates
of
Natural Gases,
2nd edn.
New York: Marcel Dekker.
CLAY MINERALOGY
additionally minerals like allophane and imogolite are included.
In contrast, minerals such
as
quartz
and
feldspars, although
frequently found
in
clay size fractions, would never
be
considered "clay minerals".
A
further difficulty with
a
more
precise definition
of
"clay mineral" arises
in
part because there
are
no
meaningful boundaries
to
continuous properties such
as
particle size. Hence many hydrous layer silicates that occur
as
clay minerals
for
example, micas
and
chlorites have larger
relatives whose size flouts any such description.
For
other types,
for example, kaoiinite
and
smectite,
it is
rare indeed
to
find
specimens that have outgrown
the
description "clay mineral"
probably because their crystal structures place severe
con-
straints
on the
size
to
which they
can
grow. Clay minerals thus
occur
as
fine-grained particles with consequent high surface
to
volume ratios, further augmented
by the
platy shapes
of
many,
such that surface properties
are
accentuated.
The
historical
development
of the
modern concept
of
"clay minerals"
is
described
in
more detail
by
Grim (1968); there have long been
points
of
contention
in
relation
to
definitions
and
some will
no
doubt remain (Moore, 1996; Guggenheim
and
Martin, 1996).
Definitions
of
clay and clay mineral
The term "clay"
is
common
to
many disciplines
and
frequently
used
in two
quite different ways.
On the one
hand,
it is a
term
for
the
finest division
of
many particle size schemes;
on the
other hand
it is a
term applied
to
many naturally occurring
materials that
may
otherwise
be
classified
as
soil, sediment
or
rock.
Not
surprisingly then, there
is no
universally accepted
definition
of
the word "clay", although
in
context
its
meaning
is generally understood. Most recently Guggenheim
and
Martin (1995) offered
the
following definition
of
clay
as a
material:
"The
term clay refers
to a
naturally occurring
material composed primarily
of
fine-grained minerals, which
is
generally plastic
at
appropriate water contents
and
will harden
when dried
or
fired. Although clay usually contains phyllosi-
licates,
it may
contain other materials that impart plasticity
and harden when dried
or
fired. Associated phases
in
clay
may
include materials that
do not
impart plasticity
and
organic
matter".
In its
other context,
i.e.,
particle size, clay
is
variously
defined depending
on
discipline
or
country.
The
fraction less
than
2
micrometers (equivalent spherical diameter)
is a
common definition,
but the
boundary with silt
can be as
high
as
6
microns
in
some schemes. When dealing with natural
materials, such
as
sediments, there
is a
conceptual link between
both uses
of the
word clay, since clay materials inevitably
contain
a
large proportion
of
particles that
are
clay size.
Additionally,
the
clay size fraction, even
if
only
a
minor
fraction
of
any given soil, sediment
or
rock, will
in
most cases
be composed predominantly
of
minerals that
are
known
as
"clay minerals". Again, just like clay, there
is no
universally
accepted definition
of the
term "clay mineral". Guggenheim
and Martin (1995) define
it as
follows:
'The
term "clay
mineral" refers
to
phyllosilieate minerals
and to
minerals which
impart plasticity
to
clay
and
which harden upon drying
or
firing'. Most "clay minerals"
are
phyllosilicates (synonym:
layer silicates)
and a
correspondingly narrower
use of
the term
is common place,
but it is
important
to
realize that minerals
that
are not
phyllosilicates
are
also included
in the
definition
of
clay mineral
if
they contribute
to the
properties associated with
clay. This encompasses many oxides
of
iron
and
aluminum,
that like
the
phyllosilieate clay minerals
are
similarly predis-
posed toward
a
natural occurrence
in the
clay size fraction;
Structure and classification
of
clay minerals
The crystal structures
of
phyllosilieate clay minerals have been
elucidated mainly from
the
study
of
larger analogous speci-
mens (Brindley
and
Brown, 1980). They
are
hydrous layer
silicates consisting
of
planes
of
atoms arranged
in
layers, their
crystal habits
and
morphologies reflecting this arrangement,
so
that most
are
platy
and
have perfect cleavage. There
are two
basic modular components
of
phyllosilieate clay minerals;
sheets
of
tetrahedrally coordinated atoms
and
sheets
of
octahedrally coordinated atoms, know
as
tetrahedral
and
octahedral sheets (Figure
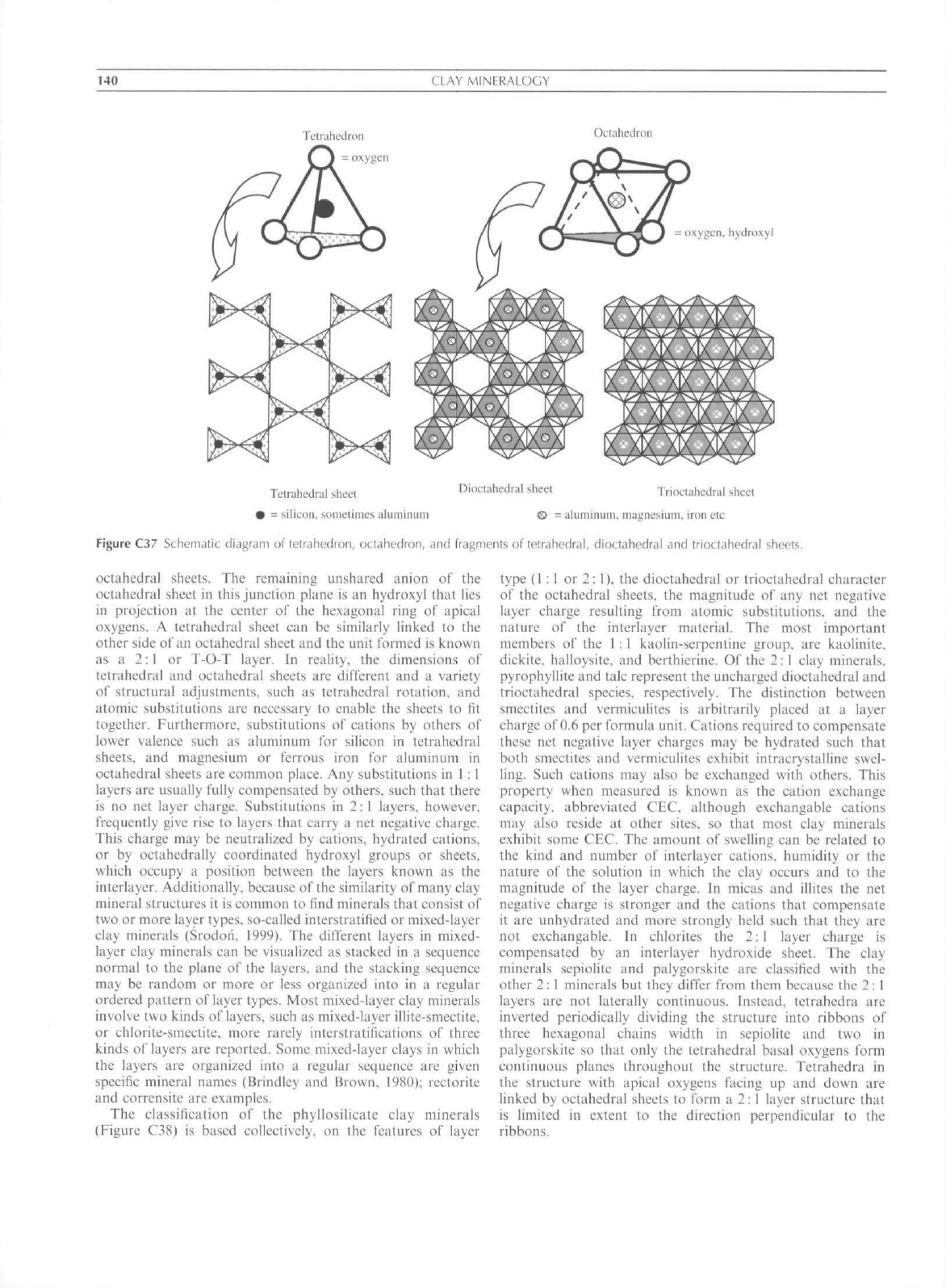
C37).
Tetrahedra
are
formed
of a
cation, usually silicon,
surrounded
by
four oxygens. By sharing three
of
these oxygens
between adjacent tetrahedra they
may be
linked together
to
form
a
continuous tetrahedral sheet,
in
which
the
unshared
oxygens
all
point
in the
same direction. Thus
one
side
of a
tetrahedral sheet consists
of an
hexagonal mesh
of
shared
oxygens, whilst
the
other side
is
formed
by the
remaining
so-
called "apical" oxygens.
An octahedral sheet
is
formed from
two
planes
of
close
packed oxygens and/or hydroxyls.
In
the center
of
such
a
sheet,
and adjacent
to
every anion, there
are
three octahedral sites
which
may be
occupied
by
cations such
as
aluminum, iron
and
magnesium, each cation being surrounded
by six
anions. There
are
two
kinds
of
common octahedral sheets distinguished
by
different cation
to
anion ratios
as
required
for
electrical
neutrality.
If
divalent cations fill
all
three sites
the
octahedral
sheet
is
know
as
trioctahedral. Trivalent cations need only
occupy
two out of
every three sites
to
maintain neutrality
and
the sheet
is
then ealled dioctahedral. Octahedral sites
are
linked together
by
sharing
of
edges.
The
vacancies
in the
dioctahedral sheet lead
to a
more distorted structure than
the
completely occupied trioctahedral sheet.
By joining tetrahedral
and
octahedral sheets together,
two
basic clay mineral units known
as
layers
can be
formed.
The
unit formed
by
linking
one
tetrahedral sheet
and one
octahedral sheet together
is
called
a
1
:
1
layer, sometimes also
designated
T-0. The
linkage
is
achieved
by
replacing
two out
of every three anions
of an
octahedral sheet
by the
apical
oxygens
of a
tetrahedral sheet,
so
that
at the
'junction piane'
the apieal oxygens
are
shared jointly
by
tetrahedral
and
140
CI.AV MINERALOGY
Tetrahedrnn
= oxygen
Octahedron
= n.i:ygen, hydroxyl
Dioctahedral sheet Trioctahedral sheet
© = aluminum, magnesium, iron etc
Figure C37 Schemalic diagram of tetrahedron, octahednin, and fragments of letrahedral, dioctahedral and trioctahedral sheets.
Tetrahedral sheet
= silicon, sometimes aluiiiinuni
octahedral sheets. The remaining unshared anion of the
octahedral sheet in this junction plane is an hydroxyl that lies
in projection at the center of the hexagonal ring of apical
oxygens. A tetrahedral sheet can be similarly linked to the
other side of an octahedral sheet and the unit formed is known
as a 2:1 or T-O-T layer. In reality, the ditnensions of
tetrahedral and octahedral sheets are different and a variety
of structural adjustments, such as tetrahedral rotation, and
atomic substitutions are necessary to enable the sheets to fit
together. Furthermore, substitutions of cations by others of
lower valence such as aluminum for silicon in tetrahedral
sheets, and magnesium or ferrous iron for alumintim in
octahedral sheets arc common plaee. Any substitutions in I : I
layers are usually fully compensated by others, such that there
is no net layer charge. Substitutions in 2:
1
layers, however,
frequently give rise to layers that carry a net negative charge.
This charge may be neutralized by cations, hydrated cations,
or by octahedrally coordinated hydroxyl groups or sheets,
which occupy a position between the layers known as the
interlaycr. Additionally, because of the similarity of many clay
mineral structures it is common to find minerals that consist of
two or more layer types, so-called intcrstratitied or mixed-layer
clay minerals (Srodori. 1999). The dilTereiit layers in mixed-
layer clay minerals can be visualized as stacked in a sequence
normal to the plane of the layers, and the stacking sequence
may be random or tnore or less organized into in a regular
ordered pattern of layer types. Most mixed-layer clay minerals
involve two kinds of layers, such as mixed-layer illite-smectite,
or ehlorite-smectitc, more rarely interstratiftcations of three
kinds of layers are reported. Some mixed-layer clays in which
the layers are organized into a regular sequence are given
specific mineral names (Brindley and Brown, 1980); rectorite
and corrensite are examples.
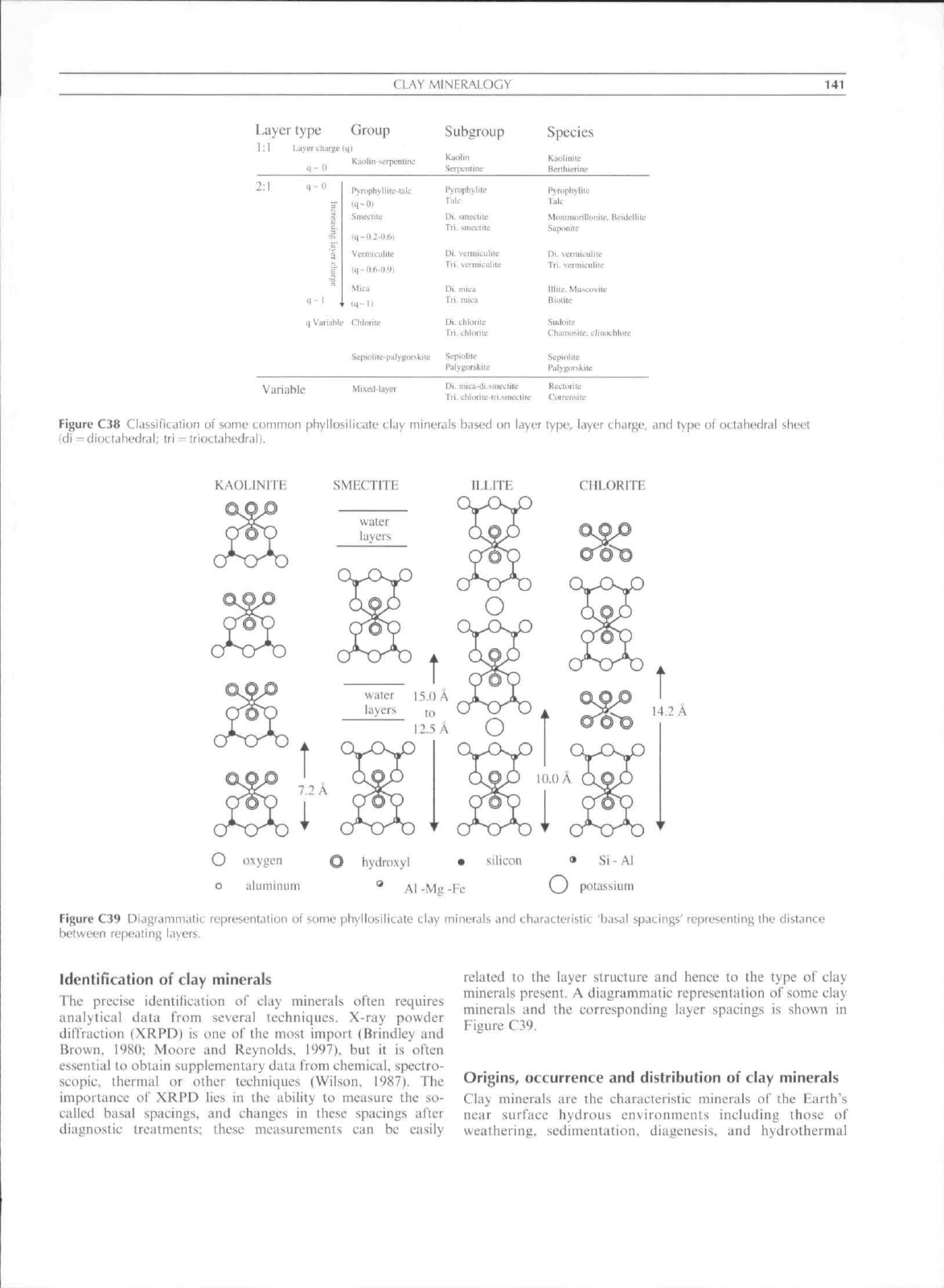
The classification of the phyllosilieate clay minerals
(Figure C38) is based collectively, on the features of layer
type (1 :
1
or 2: 1), the dioctahedral or trioctahedral character
of the octahedral sheets, the magnitude of any net negative
layer charge resulting from atomic substitutions, and the
nature of the interlayer material. The most itiiportant
members of the
1
:1 kaolin-serpentine group, are kaoiinite,
dickite. halloysite. and berthierine. Ofthe 2:1 clay minerals,
pyrophyllitc and talc represent the uncharged dioctahedral and
trioctahedral species, respectively. The distinction between
smectites and vermiculites is arbitrarily placed at a layer
charge of 0.6 per formula unit. Cations required to compensate
these net negative layer charges may be hydrated such that
both smectites and vermiculites exhibit intracrystalline swel-
ling. Such cations may also be exchanged with others. This
property when measured is known as the cation exchange
capacity, abbreviated CEC, although exchangable cations
may also reside at other sites, so that most clay minerals
exhibit some CEC. The amount of swelling ean be related to
the kind and number of interlayer cations, humidity or the
nature of the solution in which the clay occurs and to the
magnitude of the layer charge. In micas and illites the net
negative charge is stronger and the cations that compensate
it are unhydrated and more strongly held such that they arc
not exchangable. In chlorites the 2:1 layer charge is
compensated by an interlayer hydroxide sheet. The clay
minerals sepiolite and palygorskite are classified with the
other 2: I minerals but they differ from them because the 2: 1
layers are not laterally continuous. Instead, tetrahedra are
inverted periodically dividing the structure into ribbons of
three hexagonal chains width in sepiolite and two in
palygorskite so that only the tetrahedral basal oxygens form
continuous planes throughout the strueture. Tetrahedra in
the structure with apical oxygens facing up and down are
linked by octahedral sheets to form a 2: I layer structure that
is limited in extent to the direction perpendicular to the
ribbons.
CLAY MINERALOGY
141
Layer type Group
Subgroup
rf
4-1
q Va^|ah^
Kaol,n-.e,,x.n,,n.
F>>,L.phvlli(.-.ak
14-LII
SmeulUe
Vermit'ulilc
Micj
(4-1)
<-h.or,,e
Sepiolite-pal VHcirski It
Kaulin
Scrpenlme
Py,,,phylit.
Talf
Dl.
tmeclile
Tri.
smeclil
Di.
verniitu
Dl.
mi>.;i
Tn. mil a
Dl.
chl.irile
Sepiohie
Species
Katiliniie
Btnhifrmo
2:1
Palygmskile
Monlmorllloiiiie. Beidellilc
Sajioniie
ItliiL'.
Mu'covili-
ChamoiilL-, Llin(K.hl,.re
Stpi.ilile
Palygorskile
VariiihlL-
. miL-a-di.smeutiie
Figure C38 Classification of some common phyllosilieate clay minerals based on layer type, layer charge, and type of octahedral sheet
(di = dioctahedral; tri = trioctahedral).
KAOLINITE SMECTITE ILLITE
CHLORITE
7.2 A
14.2 A
ID.O
A
O oxygeti
o alitmititim
hydroxyl
silicon
Al -Mg -Fe
•> Si - Al
potassitim
Figure C39 Diagrammalic representation of some phyllosilieate clay minerals and characteristic 'basal spacings' representing the distance
between repealing layers.
Identification of clay minerals
The precise identificcition of" clay minerals often requires
analytical data from several techniques. X-ray powder
diffraction (XRPD) is one ofthe most import (Brindley and
Brown. 1980; Moore and Reynolds. 1997). but it is often
essential to obtain sttpplementary data from chemical, spectro-
scopie. thermal or other techniques (Wilson. 1987). The
itnportaiice of XRPD lies in the ability to measure the so-
called basal spacings, and changes in these spacings after
diagnostic treatments: these measurements can be easily
related to the layer strueture and hence to the type of clay
minerals present. A diagramtnatic representation of some clay
minerals and the corresponding layer spacings is shown in
figure C39.
Origins, occurrence and distribution of clay minerals
Clay tninerals are the characteristic minerals of the Larth"s
near surface hydrous environments including those of
weathering, sedimentation, diagenesis. and hydrothermal

142
CLIMATIC CONTROL OF SEDIMENTATION
alteration. Within nil of these environments ckty minerals may
have one of three different origins: they may be neoformed.
transfornied. or inherited (Millot. 1970; Eberl. 1984). Neofor-
mation describes the direct tbrmation by crystallization of a
clay mineral from solution or by ageing of amorphous gels.
Neoformation is limited by the constraints of time and
temperature, such that longer titnes and higher tetnperatures
promote neoformation. The sluggishness of neoformation and
the apparent stability of clay minerals at low temperatures
means that clay minerals formed in one environment are
often recycled into another. This is an origin known as
inheritance. Most clay minerals found in modern sedimentary
environments represent the redistributed products of weath-
ering and as such they are inherited (Chamley. 1989; Weaver,
1989).
The origin known as transfortned lies between these
two poles since it describes the fonnaiion of clay minerals
where some structural elements of a precursor mineral
(usually another clay mineral) are retained but new ones are
added.
As far as sediments and sedimentary roek are concerned, the
importance of weathering is as a primary and often the
ultimate source for tiiany clay minerals. Weathering and
the formation oi" various kinds of clay minerals in soils is
controlled by many variables operating at various .scales
including, climate, drainage, topography, parent rock type.
and titne. At a global scale, a broadly latitudinal climatically
controlled pattern of clay mineral distribution is recognized in
zonal soils. Thus in polar, tundra, temperate and desert
climatic zones the processes of inheritance and transformation
are dominant and the clay minerals assemblages are, very
generally speaking, characteri/cd by assemblages containing
illite and chlorite inherited from parent tnaterials and mixed-
layer clay minerals and vermieulite formed by transformation
from illite and chlorite. In tropical climates neoformation
processes are much more evident: kaolin is formed whei"e soil
solutions are thoroughly depleted of bases eations. usually as a
result of constant leaching, and smeetite is formed where
conditions allow base cations to accumulate.
The broadly latitudinal pattern of elay mineral distribution
in zonal soils is mirrored in the reeent sediments of the ocean
basitis and attests to the largely detrital origin of clay minerals
in the modern marine environment (Chatnley, 1989; Weaver.
1989).
However, neotbrmation of elay minerals in certain
sedimentary environments is cotnnionplace and in most cases
the elay minerals involved are rich in iron or magnesium and
poor in aluminum. In the marine environment examples
include the iron-rieh clay minerals glauconite. berthierine,
odinitc. and nontronite. Magnesiutn-rich clay minerals, such
as sepiolite and palygorskite. and some species of trioctahedral
stnectites. tend to be most eommonly encountered in peri-
marine evaporitic settings or in alkaline lakes.
Clay minerals are found in tnost sediments and sedimentary
roeks,
but they are most abundant in mudroeks and shales,
where they typically account for about 50 percent of the
mineral components. When sediments are buried by yet more
sediment then diagenetic processes begin to act and their
aetion is cotnmoiily recorded by the changes they induce in the
clay tninerals and clay mineral assemblages present. Probably
the best known and most widespread is the smeetite-to-illite
reaction, whereby originally deposited smectitic clays are
progressively transformed to illite via mixed-layer illite-
smectite, although there is much debate about the nature of
the reaction(s) involved. The article by Srodoti (1999) provides
a recent review, and summarizes those aspects that are still
contentious.
contentious.
Stephen Hillier
Bibliography
Biindiev, G.W., and Brown. G., 1980. Ciystal structures of cliiy
minerals and their X-ray identification. Miticialogiial .Society
Monograph No.5.
Chamley, M., 1989. Clay Setlimeniology. Springer Verlag.
Eber).
D.D.. 1984. Clay mineral Ibrmalion and transformation in
rocks and soils. Philosophical
'Fntnsactions
of
the
Roval Societv of
London. Ail \. pp. 241-251.
Grim. R.E.. 1968. Clay Mineralogy. 2nd edn. McGraw-Hill.
Guggenheim. S., and Martin, R.T.. 1995. Delinitlon (if clay and clay
mineral: joint report oi" the AIPEA nomenclature and CMS
nomenelalurc committees. Claysand Clav Minerals 43: 255-256.
Guggenheim, S., and Miirtin, R.T., 1996. Reply to the comment by
D.M. Moore on "Definition of claj and clay minenil: joinl report
of thf AIPEA nomenclattire and CMS nomenclature committees".
C/avsanil Clav Minerals. 44, pp. 7i3 715.
Millot, G..
197(1."
The
Geology
of Chivs. Paris: Masson.
Moore, D.M.. 1996. Comment on: "'Deiinition of clay and clay
mineral: joint report of the AiPFA notneticlature and CMS
nomenclature committees". Clavs and Clav Minerals. 44. pp. 710-
712.
Moore. D.M.. and Reynolds, R.C. Jr., 1997. X-ray Diffhution and the
hlcniification and Analysis of Clay Minerals. 2nd edn. Oxlotd
University Press.
Srodoti. J.. 1999. Nature ofrni.xed-layer clays and mechanisms of their
formation and alteration. .Annual Review of Fariti and Plimeiarv
Sciences. 27: 19 53.
Weaver. C.B.. 1989.
Clays,
tiiuds
and.sluiles.
Elsevier.
Wilson. M.J.. 1987. A Handbook of Delerminativc Methods in Clay
Mineralogy. New York: Blackie & Son.
Cross-references
Bentoniics and Tonsteins
Berthierine
Black Shales
Cation Exchange
Chlorite in Sediments
Classification of Sediments and Sedimentary Rocks
Colloidal Properties of Sediments
Diagenesis
Glaueony and Verdine
Grain Size and Shape
Hydroxides and Oxyhydroxide Minerals
Illite Group Clay Minerals
Kaolin Group Minerals
Mixed-Layer Clays
Mudrocks
Smectite Group
Tale
Vermieulite
Weathering, Soils and Paleosols
CLIMATIC CONTROL OF SEDIMENTATION
Introduction
Sedimentation is influenced by three extrinsic variables,
teetonies, sea level, and clitnate. with climate potentially
dependent upon the other two variables. Global clitnate has
undergone substantial changes on a variety of temporal and
geographic scales throughout earth history. Interpretitig
climates of the past (paleoclimate) is an important aspect of
CLIMATIC CONTROL OF SEDIMENTATION
143
LindcrsUindinii ciirlh history (Crowley and North, 1991;
Parrish. '
Global climate
Climate may be defined a.s the condition of ihc atmosphere at a
specific location near the earth's surface averaged over several
years or tens of years. The climatic conditions that most affect
sedimentation are mean annual temperature, mean annual
precipitation, seasonal variations in temperature and precipi-
tation, the rate of evaporation compared to precipitation, and
the direction, velocity, and seasonal variation of winds and
ocean currents.
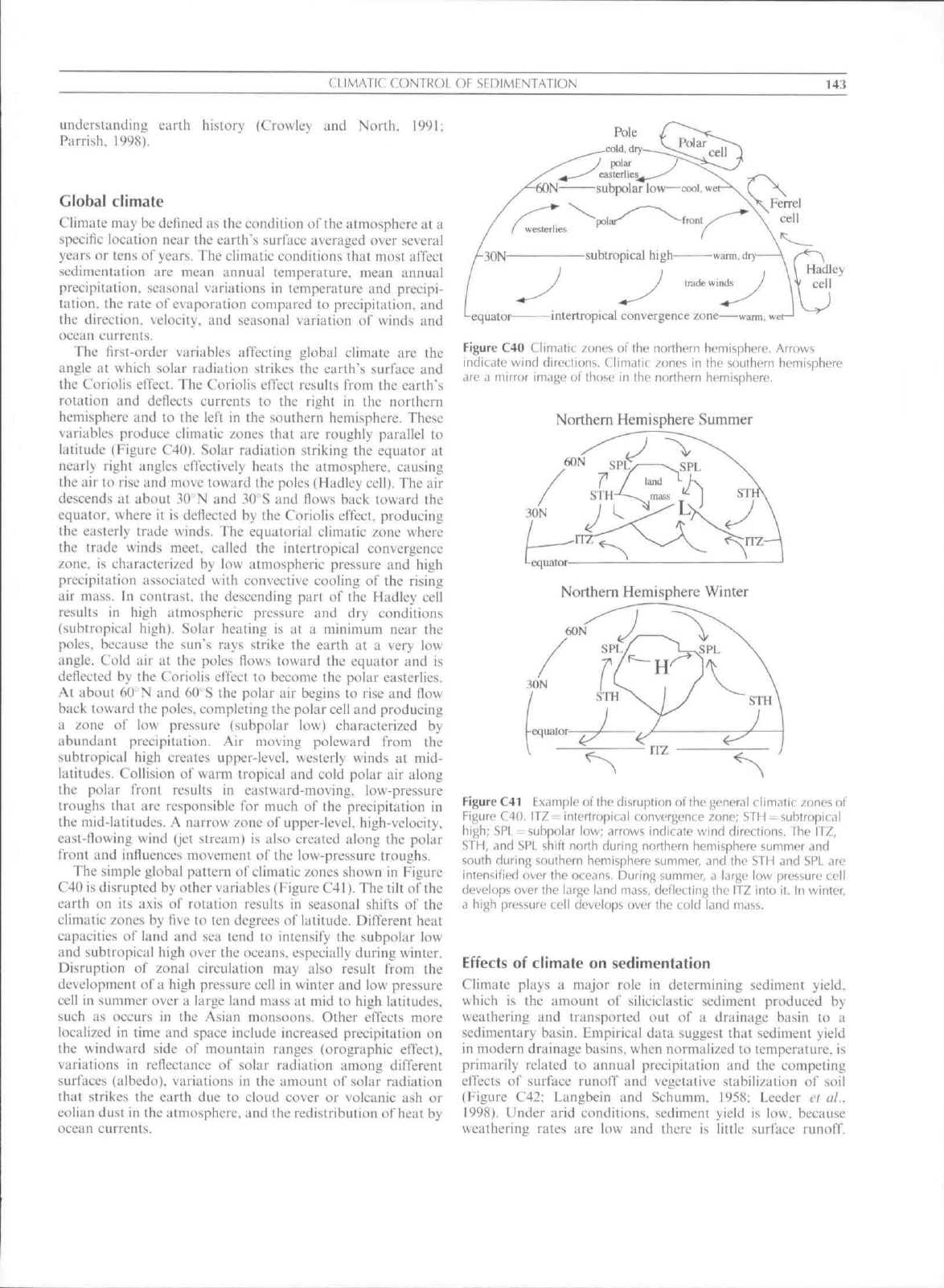
The lirst-order variables affecting global climate are the
angle at whieh solar radiation strikes the earlh's surfaee and
Ihe Coriolis effect. The Coriolis effect results from the earth's
rotation and deflects currents to the right in the northern
hemisphere and to the left in the southern hemisphere. These
variables produce ciimatie zones that arc roughly parallel to
latitude (Figure C4()). Solar radiation striking the equator at
nearly right angles effectively heats the atmosphere, causing
the air to rise and move toward the poles (f-Iudley cell). The air
descends at about 30 N and ?0"S and tlows back toward the
equator, where it is deflected by the Coriolis effect, producing
the easterly trade winds. The equatorial climatic zone where
the trade winds meet, called the intcrtropieal convergence
zone,
is characterized by low atmospheric pressure and high
precipitation associated with convective cooling of the rising
air mass. In contrast, the descending part of the Hadley cell
results in high atmospheric pressure and dry conditions
(subtropical high). Solar heating is at a minimum near the
poles,
beeause the sun's rays strike the earth at a very low
angle. Cold air at the poles flows toward the equator and is
deflected by the Coriolis effect to become the polar easterlies.
At about 6U' N and 60 S the polar air begins to rise and flow
back toward the poles, completing the polar eell and producing
a zone of low pressure (subpolar low) characterized by
abundant precipitation. Air moving poleward from the
subtropical high creates upper-level, westerly winds at mid-
kilittides. Collision of warm tropical and cold polar air along
the polar front results in easlward-moving. low-pressure
troughs that are responsible for much of the precipitation in
the mid-latitudes. A narrow zone of upper-level, high-velocity,
east-flowing wind (jet stream) is also created along the polar
front and influences movement of the low-pressure troughs.
The simple global pattern of climatic zones shown in Figure
C41) is disrupted by other variables (Figure C4!). The tilt of the
earth on its axis of rotation results in seasonal shifts of the
climatic zones by five to ten degrees of latitude. Diflerent heat
capacities o\' land and sea tend to intensify the subpolar low
and subtropical high over the oceans, especially during winter.
Disruption of zonal circulation may also result from the
development of a high pressure cell in winter and low pressure
cell in summer over a large land mass at mid to high latitudes,
sueh as occurs in tbe Asian mons<tons. Other effeets more
localized in time and space include increased precipitation on
the windward side of mountain ranges (orographic etTect),
variations in reflectance of solar radiation among different
surfaces (albedo), variations in the amount of solar radiation
that strikes the earth due to cloud cover or volcanic ash or
eolian dust in the atmosphere, and the redistribution of heat by
ocean currents.
Pole
cold, dry
polar
easterlies
subpolar low—coo!, we
wesferlies
-30N- subtropical high
-equator
trade winds
-intertropical convergence zone—-warm, wei-
Figure C40 Climatic zones of the northern hemisphere. Arrows
indicdle wind directions. Climatic zones in ihe southern hemisphere
are ti mirror image of those in the northern hemisphere.
Northern Hemisphere Summer
Northern Hemisphere Winter
Figure
C41
Example of the disruption of the general climatic zones of
Figure C40. ITZ^ interlropical convergence zone; STH - subtropical
high;
SPL
= subpolar low; arrows indicate wind directions. The ITZ,
STH,
and SPL shift north during northern hemisphere summer and
south during southern hemisphere summer, and the STH and SPL are
intensified over (he oceans. During summer, a large low pressure tell
develops over the large land mass, deflecting the ITZ into it. In winter,
a high pressure ceil develops over the cold land mass.
Effects of climate on sedimentation
Climate plays a major role in determining sediment yield,
whieh is the amount of siliciclastie sediment produced by
weathering and transported out of a drainage basin to a
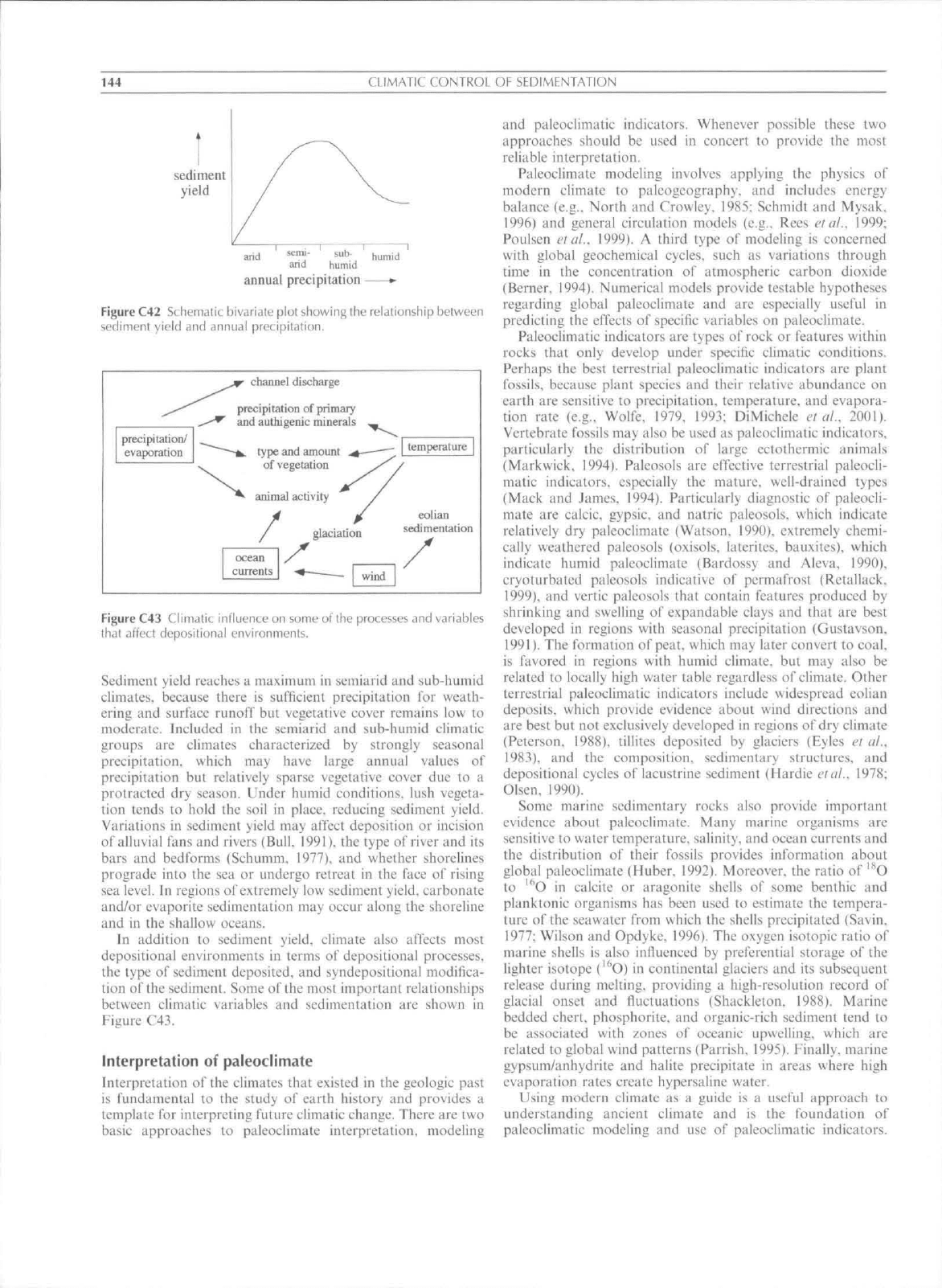
sedimentary basin. Empirical data suggest that sediment yield
in modern drainage basins, when normalized to temperature, is
primarily related to annual precipitation and the competing
effects of surfaee runoff and vegetative stabilization of soil
(Figure C42; Langbein and Schumm. 1958; Leeder et af.
1998).
Under arid conditions, sediment yield is low, because
weathering rates are low and there is little surface
runoff.
144
CLIMATIC CONTROL OH SHDIMENTATION
sediment
yield
arid
senu- sub- humid
and humid
annual precipitation ^
Figure C42 Schematic bivjriale plot showing the relationship between
sediment yield and annual preti|)ilation.
precipitalion/
evaporation
chaimel discharge
precipitation of primary
and authigenic minerals
type and amount
of vegetation
animal activity
/ glaciation
ocean
currents
temperature
eolian
sedimentation
wind
Figure C43 Ciimtitic influence on some of the processes and variables
that aflect depositional environments.
Sediment yield reaehes a maximum in semiarid and sub-humid
climates, because there is sufficient precipitation for weath-
ering and surface runoff but vegetative cover remains low to
moderate. Ineluded in the semiarid and sub-humid climatic
groups are climates characterized by strongly seasonal
precipitation, which may have large annual values o{
precipitation but relatively sparse vegetative cover due to a
protracted dry season. Under humid conditions, lush vegeta-
tion tends to hold the soil in plaee. reducing sediment yield.
Variations in sediment yield may affect deposition or incision
of alluvial fans and rivers (Bull. 1991), the type of river and its
bars and bedforms (Schumm, 1977), and whether shorelines
prograde into the sea or undergo retreat in the face of rising
sea level. In regions of extremely low sediment yield, carbonate
and/or evaporite sedimentation may occur along the shoreline
and in the shallow oceans.
In addition to sediment yield, climate also affects most
depositional environments in terms of depositional processes,
the type of sediment deposited, and syndepositional modifica-
tion of the sediment. Some of the most important relationships
between climatic variables and sedimentation are shown in
Figure C43.
Interpretation of paleoclimate
Interpretation of the climates that existed in the geologic past
is fundamental to the study of earth history and provides a
template for interpreting future climatic change. There are two
basie approaches to paleoelimate interpretation, modeling
and paleocliinatic indicators. Whenever possible these two
approaches should be used in concert to provide the most
reliable inlerprelation.
Paleoclitnate modeling involves applying the physics of
modern climate to pateogeography. and includes energy
balance (e.g.. North and Crowley, 1985; Schmidt and Mysak.
1996) and general circulation models (e.g.. Rees etal.. 1999;
Poulsen etal.. 1999). A third type of modeling is concerned
with global geoehemical cycles, sueh as variations through
time in the concentration of atmospheric carbon dioxide
(Berner, 1994). Numerieal models provide testable hypotheses
regarding global paleoclimate and are especially useful in
predicting the effeets of specific variables on paleoclimate.
Paleoclitnatic indicators are types of rock or features within
rocks that only develop under specKie climatic conditions.
Perhaps the best terrestrial paleoelimatie indicators are plant
fossils, because plant species and their relative abundance on
earth are sensitive to precipitation, temperature, and evapora-
tion rate (e.g., Wolfe, 1979, 1993; DiMichele etal., 2001).
Vertebrate fossils may also be used as paleoelimatie indicators,
particularly the distribution of large eetothermic animals
(Markwick, 1994). Paleosols are effective terrestrial paleoeli-
matie indicators, especially the mature, well-drained types
(Mack and James. 1994). Particularly diagnostic of paleocli-
mate are calcic, gypsic, and natric paleosols. which indicate
relatively dry paleoelimate (Watson, 1990), extremely chemi-
cally weathered paleosols (oxisols, laterites, bauxites), which
indicate humid paleoelimate (Bardossy and Aleva, 1990),
cryoturbated paleosols indicative of permafrost (Retallack,
1999).
and vertic paleosols that contain features produced by
shrinking and swelling of expandable clays and that are best
developed in regions with seasonal preeipitation (Gustavson.
1991).
The formation of peat, which may later convert to coal,
is favored in regions with humid climate, but may also be
related to locally high water table regardless of climate. Other
terrestrial paleoelimatie indicators include widespread eolian
deposits, which provide evidence about wind directions and
are best but not exclusively developed in regions of dry climate
(Peterson. 1988), tillites deposited by glaciers (Eyles et al.,
1983),
and the composition, sedimentary structures, and
depositional eyeles of laeustrine sediment (Hardie ctuL. 1978;
Olsen, 1990).
Some marine sedimentary roeks also provide important
evidence about paleoclitnate. Many marine organisms are
sensitive to water temperature, salinity, and ocean eurrents and
the distribution of their fossils provides information about
global paleoelimate (Huber, 1992). Moreover, the ratio of '^O
to "'O in ealcite or aragonite shells of some benthic and
planktonic organisms has been used to estimate the tempera-
ture of the seawater frotn which the shells precipitated (Savin.
1977;
Wilson and Opdyke. 1996). The oxygen isotopic ratio of
marine shells is also influenced by preferential storage of the
lighter isotope
('*'O)
in continental giaciers and its subsequent
release during melting, providing a high-resolution record of
glacial onset and fluctuations (Shaekleton. 1988). Marine
bedded chert, phosphorite, and ofganic-rich seditiient tend to
be associated with zones ol" oceanic upwelling, which are
related to global wind patterns (Parrish, 1995). Finally, marine
gypsum/anhydrite and halite precipitate in areas where high
evaporation rates create hypersaline water.
Using modern climate as a guide is a useful approach to
understanding ancient elimate and is the foundation of
paleoclitnatic modeling and use of paleoelimatie indicators.
