Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.


Unfiltered and Filtered Cathodic Arc Deposition 501
As with closed filters, open filters can have a variety of shapes and sizes. Each of the filters has
advantages and disadvantages, as discussed in greater detail in dedicated reviews ([138] and
Chapter 7 of [3]), where other geometries such as the Venetian blind filter or large area filters
for elongated cathodes are also considered.
10.5 Energetic Deposition
10.5.1 Energy Considerations
Film growth mechanisms are dealt with in Chapter 2 of this handbook and many other
publications [139–141]. Therefore, it is sufficient here to point out some special effects and
properties associated with the high degree of ionization and relatively high energy of the
species in the condensing plasma.
Energetic deposition may be defined as a process in which most film-forming ions or atoms
exceed the displacement energy [142], E
d
, allowing them to penetrate the surface and come to
rest under the surface. Film growth can therefore occur under the surface rather than on the
surface. Such a process can be considered as ultra-shallow implantation or subplantation
[143–145]. As a result, films are often highly defective and hard, and show high intrinsic
stress. Yet, owing to film–substrate intermixing and with the formation of chemical bonds, the
films can be well adherent unless the intrinsic stress is excessive, which leads to catastrophic
failure by delamination. Therefore, special attention must be given to stress control, as
discussed below.
At the low-energy end of energetic deposition, when the kinetic energy of condensing particles
is in the range 5–25 eV and therefore not quite enough for subplantation, defects are greatly
reduced and growth occurs on the surface with high adatom mobility, leading to dense, often
well-textured films. The kinetic and potential energies of the condensing particles assist in the
growth process and, to some limited degree, those particle energies are equivalent to substrate
heating. This allows us to obtain films structures and properties at relatively low substrate
temperature otherwise associated with higher temperatures. This is very important especially
when a substrate is used that cannot tolerate higher temperature because it would anneal,
decompose or otherwise be damaged.
Let us have a closer look at the energy contributions brought to the surface by the particles.
First, there is the kinetic energy associated with the flow velocity. Cathodic arc ions have a
considerable natural kinetic energy gained at the cathode spot, as mentioned in Section 10.2.2,
E
i0
= m
i
v
2
i0
/2 (10.29)
where v
i0
can be approximately determined by the expression (10.25). The surface of a
substrate is rarely exactly at the plasma potential, and therefore a sheath will form at the

502 Chapter 10
surface accommodating the potential difference V
s
. In most cases, the surface will be
negative with respect to the plasma potential, and the ions gain kinetic energy according to
E
i,kin
= QeV
s
(10.30)
where Q is the charge state number (see Table 10.2). This point is very important because
biasing of the substrate can be utilized to control the kinetic energy of arriving ions.
In one extreme case, when the negative bias is high, perhaps reaching the kV level, ions can be
used to sputter the substrate and to produce buried layers and mixed interfaces. These effects
are considered in the field of plasma immersion ion implantation and deposition [146].
In another extreme case, when positive bias is applied to the substrate, large electron currents
are taken from the plasma, which leads to heating of the surface. This can be intentionally
utilized or may be considered detrimental in cases where the objective was only to decelerate
ions.
When ions arrive at the surface, they carry not only kinetic energy but also potential energy.
The latter is usually neglected in the treatment of conventional PVD or CVD methods because
the condensing species are not ionized. In cathodic arc deposition, however, the fraction of
ions is very large, and so is the effect of potential energy. The largest contribution to potential
energy is the ionization energy, E
ion
. Strictly speaking, since we generally deal with multiply
charged ions, it is the cumulative ionization energy,
E
ion
= E
sum
Q+
=
Q−1
Q
=0
E
Q
(10.31)
where the ionization energy E
Q
is defined as the energy needed to remove a bound electron
from an ion of charge state Q, forming an ion of charge state Q +1[147, 148]. Therefore, the
greatest effect is from the more highly charged ions. The ionization energy should be reduced
by the work function times the charge state number to account for the uptake of electrons from
the solid.
There are other forms of potential energy, too. For example, the arriving ion may have a bound
electron in an excited state, or if molecules are present, rotational and vibrational energy could
be considered. Furthermore, as the condensing particle becomes bonded to the substrate, the
cohesive energy is released. Therefore, the total energy brought to the surface by an ion may
be written as
E
total
(
Q
)
= E
kin,0
+ QeV
sheath
+ E
ion
− Qeφ + E
c
+ E
else
(10.32)
where E
else
summarizes all other small contributions including the energy gained by image
charge acceleration.

Unfiltered and Filtered Cathodic Arc Deposition 503
A numerical evaluation of (10.32) shows that the kinetic energy readily exceeds the
displacement energy in most cases, and that the contribution of potential energy is substantial,
too, especially for multiply charged ions. Such large energy input could be described as atomic
scale heating (ASH) [149, 150]. Considering film growth based on the arrival of large fluxes of
condensable (film-forming) ions, each atom of the film was subject to ASH several times,
namely, once when it arrived, and again when neighboring atoms arrived. Therefore, ASH is
equivalent – to some degree – to conventional heating, resulting in dense films via enhanced
surface mobility at generally low bulk temperature [149]. ASH eventually gives rise to
temperature elevation of the substrate and the growing film as a whole [141].
10.5.2 Stress Generation and Relief
The effect of the high-energy input is significant for the properties of the resulting films. At
high kinetic energy, facilitated by high negative bias, many processes occur, including the
sputtering of surface atoms (ion etching), formation of short collision cascades as they are
usually known from ion implantation [146, 151], and related rearranging of atoms and their
bonds. Depending on the energy level and other factors, this allows us to influence the stress of
the material, and in particular to produce thicker coatings at relatively low stress.
Following Bilek and co-workers [152–154], let us consider subsurface processes at high and
moderate energies (those terms should always be seen in relation to the material’s
displacement energy, which lies generally between 20 and 40 eV). As the energy of arriving
ions exceeds the displacement energy, they are inserted into the subsurface region where they
cause densification and compressive stress. If the material is a metal it will yield to high stress
by plastic deformation. However, if the material is covalently bonded it will longer resist
plastic deformation. The material is metastable and tends to prefer a high coordination of
bonds if more than one phase exists. For example, carbon will preferentially establish sp
3
bonds, diamond bonds, as opposed to the sp
2
bonds of the less dense graphitic material.
The evolution of stress can be understood by a competition of stress generation by
subplantation (insertion) and atomic-scale annealing in a thermal spike volume [155]. For a
thin film the stress σ is related to the strain ε by [156]
σ =
Y
1 − ν
ε (10.33)
where Y and ν are the elastic (Young’s) modulus and Poisson’s ratio of the coating material.
According to Davis [155],
σ(E
i
) ∝=
Y
1 − ν
E
1/2
i
J/j +κE
5/3
i
(10.34)
504 Chapter 10
where E
i
is the energy of arriving ions, J is the net condensing flux, j is the ion bombarding
flux, and κ is a material-dependent parameter. The more recent model by Bilek and McKenzie
[152, 153] gives an improved fit to experimental data. Stress is determined by whether or not
atoms have time to rearrange their bonding structure within the thermal spike volume. At low
energy (e.g. ∼100 eV), the energy is removed quickly from the spike volume, i.e. the quench
time is very short and the material densified, hence the compressive stress increases. At higher
energies (e.g. ∼1 keV or greater), the atoms in the heated volume can rearrange, the volume
expands toward the surface, and hence stress is relieved.
This concept can be quantified, and because of its practical importance to cathodic arc
deposition, it is briefly summarized here. One assumes that the region of the thermal spike is
spherical, with radius R
th
. Then the quench time is proportional to the square of the thermal
spike radius or to the 2/3-power of the ion energy E
i
[157],
t
quench
∝ R
2
th
∝ E
2/3
i
(10.35)
For E
i
∼ 100 eV, the quench time is less than one picosecond, which is too short for
significant rearrangement of atoms. The atoms will use a high bond coordination with the
available neighbors to minimize the total system energy under constraints. The volume of the
thermal spike is proportional to κ
(
E
i
− E
B
)
=
(
2/3
)
πR
3
th
, where E
B
is the energy barrier for
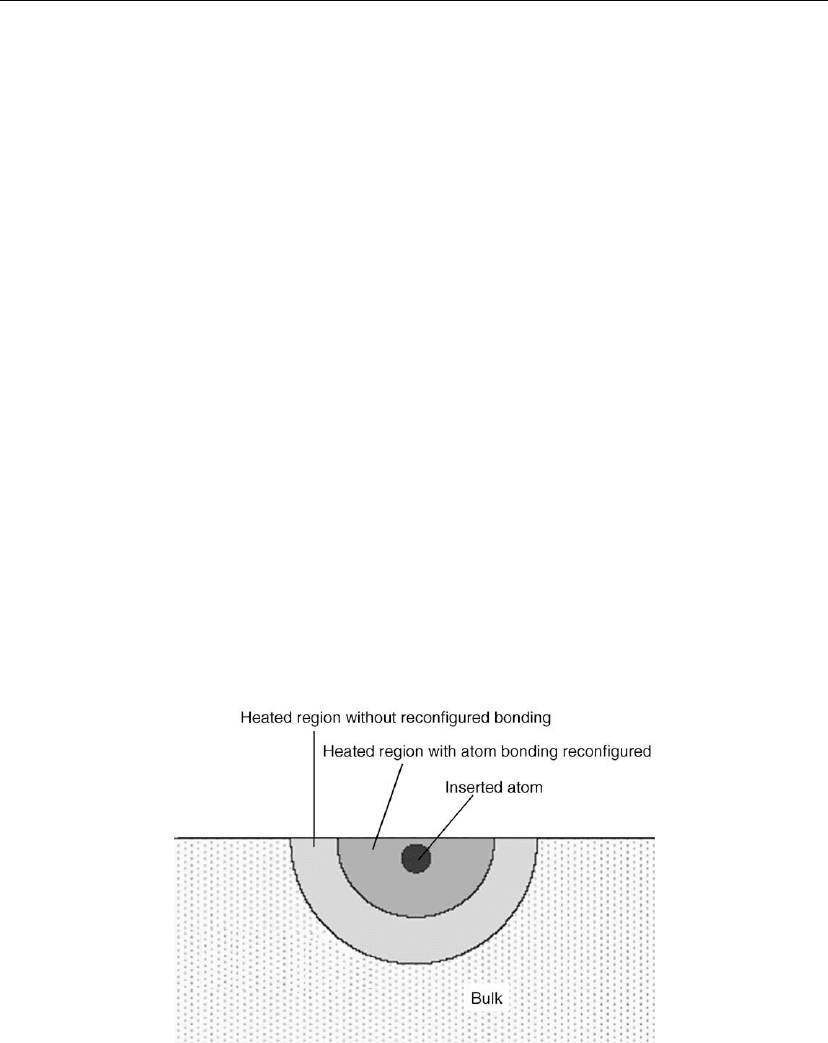
the ion to be inserted into the surface. The volume of the light gray region (Figure 10.19) can
Figure 10.19: Geometry for a model of stress generation and relaxation: the thermal spike volume
is assumed to be spherical with the inserted atom in the center. The stress is accumulated in the
light gray region, the size of which is proportional to E
2/3
i
. (Adapted from [152].)
Unfiltered and Filtered Cathodic Arc Deposition 505
be calculated as
V
stressed
= 2 πR
2
th
dτ = 2π
κ
2π
2/3
E
2/3
i
dτ (10.36)
where dτ is the thickness of the region where atom movement is restrained by bonds to
unaffected atoms in the bulk. The thickness dτ is about one bond length regardless of the
thermal spike volume or ion energy. The number of stressed sites is proportional to the volume
V
stressed
and
σ = C(E
i
− E
B
)
2/3
(10.37)
where C and E
B
are material constants.
When the kinetic ion energy clearly exceeds 100 eV, the effect on stress is quite different
because the impacting ion creates a much larger volume of a thermal spike. The dark gray
zone of Figure 10.19 is a transient melt where atoms are able to access many possible bonding
configurations. Those atoms remain in a thermodynamically preferred configuration, i.e. one
that minimizes bond and strain energy. The thermal spike volume can expand and raise the
level of the free surface. The possibility of raising the surface, thereby minimizing the strain
energy without constraint, is the basis for ion-induced stress relief by energetic ion
bombardment.
Experimentally it has been observed that only a small fraction of high energy ions is needed to
relieve stress, and that saturation of such stress relief occurs when the percentage of the
energetic ions is still small. Saturation of stress relief occurs when the volume regions of
unconstrained rearrangement start to overlap. The volume V still untreated by a thermal spike
is reduced exponentially [152] as the surface is bombarded with sufficiently energetic ions,
V
V
0
= exp
−
V
spike
N
V
0
(10.38)
where V
0
is the volume of a film slab under consideration, V
spike
= E
i
/
(
E
A
n
a
)
is the volume of
each thermal spike, E
A
is the average energy given to atoms of the thermal spike volume,
N = n
a
Vδ is the number of high-energy impacts before the slab is covered by more
condensing atoms, n
a
is the atom density of the slab, δ = wf is the duty cycle of high-energy
ions, produced by pulsed biasing, for example, with w being the pulse width of the bias and f
the bias pulse repetition frequency. Alternatively, δ could be the fraction of energetic ions
produced by other means such as an external ion source. While these considerations were
developed with the plasma immersion technique [146] in mind, the underlying physics and
derivations are rather general. Using the above expressions, Eq. (10.38) becomes
V
V
0
= exp
−
E
i
fw
E
A
(10.39)
506 Chapter 10
The stress can be assumed to vary with the volume fraction treated by high-energy ions, from
the as-deposited value, σ
0
, to the relaxed, residual value after ion treatment, σ
res
, leading to
σ = σ
0
V
V
0
+ σ
res
(10.40)
Using (10.39) gives
σ = σ
0
exp
−
E
i
fw
E
A
+ σ
res
(10.41)
We can use Eq. (10.37) to express the stress generated by ions with energy E
G
, inserted into or
just below the surface, and replace the stress-relieving ion with energy E
i
= E
R
, one obtains
σ = C
(
E
G
− E
B
)
χ
exp
−
E
R
δ
E
A
+ σ
res
(10.42)
where the exponent χ could be 2/3, as suggested by (10.37), but other exponents such as χ =1
or χ = 1/2 also give a good fit [152] to experimental data for a number of materials such as TiN
[158], AlN [155],BN[159], a-SiH [160], SiBCN [161], and hard amorphous carbon [162].
The different exponents can be interpreted to deviations from the idealized spherical shape of
the thermal spike; for example, one could consider a cylindrical geometry of the thermal spike
volume [163].
10.5.3 Preferred Orientation and Adhesion
The energy flux brought by the condensing ions can have a profound effect on nucleation,
growth, and the resulting film microstructure. In 1983, Krohn et al. [164] found epitaxial
relations of ultrathin gold islands deposited by pulsed cathodic arc on NaCl at 150
◦
C, namely
(001)Au||(001) NaCl and [100]Au||[100]NaCl. The islands were smaller than those obtained
by evaporation and coalescence occurred at smaller nominal film thickness.
In situ conductivity measurements of cathodic arc silver films showed that the contribution of
kinetic energy promotes the formation of smaller, more frequent islands, as compared to
sputtering [165]. Also here, the coalescence occurred at smaller nominal film thickness, which
can be readily detected by a sharp increase in electrical conductivity. Investigating ultrathin
titanium films made by a pulsed cathodic arc using in situ ellipsometry, Oates et al. [166]
determined the percolation threshold for titanium on silicon to be 2.7–3.1 (±0.1 nm) using a
method by Arwin and Aspnes [167] which is based on finding the moment when the real part
of the dielectric function becomes negative.
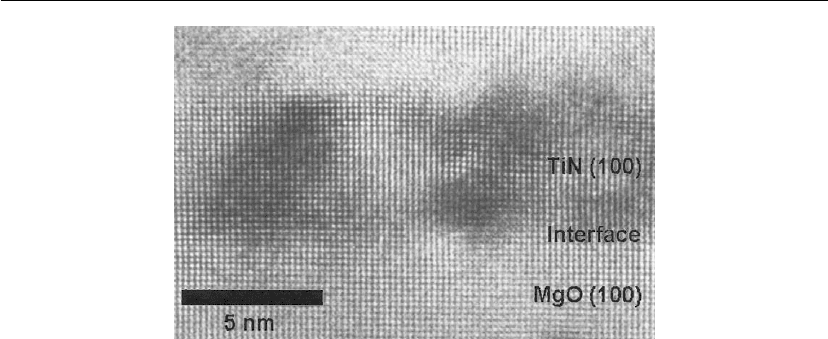
Unfiltered and Filtered Cathodic Arc Deposition 507
Figure 10.20: High-resolution TEM image showing the epitaxial growth of TiN on MgO(100); the
titanium arc was operated in nitrogen at 7 ×10
−2
Pa with the substrate at room temperature.
(Courtesy of J. Gerlach (experiment) and T. H
¨
oche (TEM), both IOM Leipzig.)
Films made by energetic deposition tend to grow with a preferred orientation, where the
specifics depend on the material and ion bombardment parameters; the film tends to prefer an
orientation that is thermodynamically stable at low levels of biaxial stress [153]. The
microstructure is directly linked to the ion-generated stress, which is a thermodynamic driving
force toward a preferred orientation thereby minimizing the system’s energy [158]. In some
cases, the preferred orientation matches the substrate’s crystal orientation and one obtains
epitaxial growth (Figure 10.20).
The adhesion of a coating is greatly affected by the chemistry of the film and substrate, as well
as by stress. A critical factor is whether or not the film material forms chemical bonds with the
substrate material. For example, an amorphous carbon film made by a cathodic arc tends to
adhere well to carbide-forming substrates such as silicon or titanium. The other important
factor is stress. The related strain energy limits the thickness of the coating because it may be
greater than the interface energy: the film cracks and delaminates. The energy related to strain
grows as the film grows in thickness, which explains why in some cases one is able to grow a
thin film (e.g. tens of nm) but completely fails to make thick films (e.g. ∼1 m or thicker).
10.5.4 Metal Ion Etching
A special case of processing occurs when a high negative bias is applied in either DC or pulsed
mode with a high duty cycle because the result can be a net removal of material. Sometimes
this is called metal ion etching – a sputtering process caused by the impact of energetic metal
ions. This can indeed be an important first process step in the formation of a well-adherent
coating. Since metal ions are deposited on or under the surface, we deal with a combined
deposition and etching situation where the etching rate exceeds the deposition rate, hence the

508 Chapter 10
net removal by sputtering. For the process to work, the energy of ions needs to be about 1 keV
or larger in order to obtain a sputtering yield that exceeds unity. Energy-dependent sputtering
yields can be readily calculated for arbitrary material combinations using a Monte Carlo code
such as TRIM or SRIM [168].
Metal ion etching by sputtering with typical voltages of −1000 V or slightly greater can be
beneficial compared to sputter etching with argon ions because some argon will remain below
the surface and cause a defective, weak interface to the coating that is subsequently deposited.
In contrast, the interface can be beneficially engineered when metal ions remain in the
thus-sputtered cleaned surface. For example, cleaning of stainless steel with chromium ions
results in a surface layer with enhanced chromium content. Chromium can become part of the
substrate because, according to the equilibrium phase diagram [169], it has unlimited
solubility in Fe below 512
◦
C. Towards the coating, chromium can readily form bonds to other
metals or to nitrogen or oxygen.
In the 1970s, Aksenov et al. [170] used this cleaning effect obtained by metal ion etching. The
cleaning of parts via energetic metal ions was used in the early 1980s, for example Bergman
applied Ti ions etching with a bias of −1000 V prior to the cathodic arc deposition of TiN
[171]. One should keep in mind that a bias of −1000 V translates into energies of at least
1 keV, 2 keV, and 3 keV, as the ions have a CSD including Q = 1, 2 and 3 (see Eq. 10.30).
The idea of replacing argon etching by metal ion etching prior to sputter deposition was later
utilized in a patented process called arc bond sputtering (ABS), by M
¨
unz et al. [172]. This
process was optimized using steered arcs for etching and unbalanced magnetrons for sputter
deposition of coatings with very high critical loads [173–175].
10.6 Deposition of Coatings Using Unfiltered Cathodic Arc Plasma
10.6.1 Hard Coatings
10.6.1.1 Binary Coatings
Perhaps the most important coating made by (unfiltered) cathodic arcs is TiN because it
combines decorative gold color with excellent wear and corrosion properties. The original
widespread use of arc-deposited TiN coatings was in the former USSR and later, since the
1980s, also in Western Europe and the USA [171, 176–180]. Large batch coaters were
developed to mainly deposit TiN at elevated temperatures (typically 450
◦
C) on cutting tools
(drills, cutting inserts, etc.).
Other binary nitrides such as CrN, ZrN, and HfN [181, 182] have advantages over TiN in some
applications. For example, CrN exhibits a lower coefficient of friction than TiN [181], and ZrN
is superior to TiN for cutting of titanium alloys [183].

Unfiltered and Filtered Cathodic Arc Deposition 509
For high-speed cutting, where the coating of the cutting edge is subject to very high
temperature (∼1000
◦
C) in an oxidizing environment, TiN fails owing to oxidation. There was
(and still is) a need for more advanced materials.
10.6.1.2 Ternary Coatings
By the late 1980s, Ti
1−x
Al
x
N, sometimes labeled (TiAl)N, and often simply abbreviated as
TiAlN, emerged as a superior tool coating because it has a better oxidation resistance and
hardness at high temperature compared to TiN [184]. Even though its wear properties at high
temperature are superior, TiAlN has not completely replaced TiN because of its grayish color:
the decorative character of the coating remains important, especially in the marketing phase of
a product.
As with other ternary compounds, the ratio of the constituents can be tuned to affect the
desired microstructure and properties. In the case of TiAlN, Ti and Al can be obtained from
one TiAl alloy cathode, which is convenient and usually done. However, one may opt to utilize
two separate cathodes to fine-tune the Ti–Al ratio in the film. In case of the alloy cathode, little
can be done to adjust the Ti–Al ratio in the film, which is usually not exactly the same as the
cathode owing to preferential sputtering from the substrate in the energetic deposition process.
The substrates are slightly biased in the film growth phase, and especially the multiply charged
ions have enough energy to cause sputtering of the growing film. The yields of sputtering Al
and Ti are not exactly equal, and hence this preferential sputtering shifts the composition of
the film [185].
The as-deposited films show a dense and columnar microstructure for various Ti
1−x
Al
x
cathodes. When the aluminum content is x ≤0.66 one finds a metastable cubic phase but for
higher Al content, x = 0.74, a second hexagonal phase appears [186]. Higher Al content
promoted the (200) orientation and had a large influence on the hardness of the as-deposited
coatings with up to 32 GPa. The TiAlN films were stable with respect to phase composition
and grain size when annealed at 900
◦
C; however, when the temperature was increased to
1100
◦
C, films deposited from the 67 at.% Al cathodes showed phase separation forming
c-TiN and h-AlN via spinodal decomposition [187] to c-TiN and c-AlN [186, 188]. The
formation of two or more phases increases the strength of the material by impeding the motion
of dislocations (spinodal hardening). Therefore, the nanostructure that forms at high
temperature increases the hardness of the coating and contributes to the superior performance
of such a coating under load [189].H
¨
orling et al. [186] argued that softening by residual stress
relaxation through lattice defect annihilation is approximately balanced by hardening by the
formation of a coherent nanocomposite structure of c-TiN and c-AlN domains by spinodal
decomposition, and therefore the hardness is approximately constant upon annealing.
The above conclusion was realized using a systematic set of single cathodes containing
different ratios of the two metals. More flexibility in terms of research and development is

510 Chapter 10
given by systems with different cathodes such as Ti, Al, and Cr cathodes, leading to coatings
in the Ti–Al–N, Ti–Cr–N, and Cr–Al–N range, and eventually even in the quaternary system
where all three metals are used in the compound [190]. The Ti–Cr–N system showed only one
phase, c-(TiCr)N, as long as the Cr content was low (10 at.%) [191]. Two phases, cubic
(TiCr)N and hexagonal β-(CrTi)
2
N, appeared with higher Cr content (17–58 at.%). The Cr
content can be adjusted not only by the Cr arc current but also by biasing: the Cr content
decreased with increasing substrate bias due to preferential sputtering of Cr. The two-phase
coatings were nanocrystalline and showed high hardness with a maximum in the range
3700–3900 Vickers at a chromium contents of about 25–30 at.% and a load of 0.5 N [191].
Other examples of arc-deposited ternary nitrides are Ti
0.94
Hf
0.06
N and Ti
0.92
Nb
0.08
N, which
have been deposited on cemented carbide inserts using random and steered arc sources [192].
These coatings showed less flank and crater wear than conventional TiN and CVD-deposited
TiC–TiN coatings.
Ternary compounds do not necessarily need two metals; rather, the process gas can be the
source of two components, and the cathode delivers only one. Carbo-nitrides are prominent
examples where changes in the gas composition between N
2
and CH
4
can be used to obtain
TiC
x
N
1−x
, for example, with x ranging from zero (TiN) to unity (TiC) [193, 194]. The
composition is determined not only by the gas supply variations but also by substrate bias. One
should keep in mind that bias is also a means of supplying energy, and hence the substrate
temperature increases. In this example, bias of −400 V led to a temperature of 550
◦
Cofthe
cemented carbide inserts (WC-6 wt.% Co). The resulting films were dense and single-phased
with a NaCl-type structure, highly stressed (up to −5.9 GPa) and superhard (up to 45 GPa).
10.6.1.3 Quaternary Coatings
The idea of using the gas as a source of two components can readily be applied to ternary
coating systems, like TiCrN, to obtain quaternary compounds like TiCr(C,N). It should be
emphasized that those multielement coatings tend to decompose into different phases, and in
some cases one should speak of nanocomposites. The Ti–Cr–C–N system produces a
nanocomposite of nanocrystalline TiCr(C,N) in an amorphous carbon (a-C) matrix when the
carbon content is in the range 9–27 at.% [195]. This is not surprising because the carbon
concentration exceeds the solubility of carbon in TiCrN. The nanocomposite exhibits higher
hardness (about 30 GPa) compared to monolithic TiCrN (about 26 GPa).
Quaternary coatings can also be obtained from several cathodes or corresponding alloy
cathodes. For example, the Ti–Al–N system can be expanded by adding Cr with the idea that
protective chromium oxide might form on the surface (similar to stainless steel). As a
side-effect, it was found that cathodes with several elements tend to produce fewer
macroparticles [196]. The orientation of films of (Ti
x
Cr
y
Al
z
)N, with x:y ≈1:2, z = 0.63–0.73,
x + y + z = 1, changed from hexagonal to cubic when the negative substrate bias was increased
