Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.


Unfiltered and Filtered Cathodic Arc Deposition 481
The fractal approach to cathodic modeling implies that a number of conventional assumptions
are not applicable at all times; this includes a simplified layer structure of the cathode zone (it
may well be used for the postexplosion stage), the assumption of a typical current density, size
of the spot, etc. Instead, those properties are self-similar down to a physical cutoff size or time.
One may consider the parameters associated with ectons as the lower physical cutoff limits.
However, until now, no convincing lower limit has been demonstrated: ever smaller and faster
properties have been detected as the spatial and temporal resolution of the instrumentation
improved.
The simple question ‘What is a cathode spot?’ does not have a simple answer, but the
definition ‘A cathode spot is an assembly of emission centers showing fractal properties in
spatial and temporal dimensions’ captures the essential elements [3].
10.2.2 Properties of Cathodic Plasmas
10.2.2.1 Plasma Density
In this section we look at the expanding, interelectrode plasma, which is much more accessible
than the plasma-producing cathode processes. The plasma properties are largely determined
by the cathode processes, and therefore plasma diagnostics far from the spots helps to uncover
the mysteries of the cathode spots [45].
As the generation of plasma is governed by the perpetual ignition, development and death of
emission centers, plasma generation occurs in bursts; and the local plasma density is therefore
strongly fluctuating or noisy. It is also clear that not all ions have the same velocity; rather,
there is a broad velocity distribution which itself is noisy. As the plasma expands and flows
away from the emission center, the fast ions from one plasma-generating burst will overtake
the slow ions of the previous burst, and therefore density and ion velocity distribution are
interrelated. In light of this, density values published in the literature have a meaning only in
the time-averaged sense.
Electrons emitted from the cathode have to find the anode to close the electric circuit. Through
electron–ion interaction (sometimes called electron–ion friction), the plasma tends to drift to
the anode. The plasma expansion can generally be approximated by a cosine distribution
n ∼ cos
α
ϑ (10.16)
where ϑ is the angle to the surface normal. The exponent α describes how much the plasma
plumes along the surface normal. In one extreme case, the anode may be large (i.e. whole
chamber) and symmetric with respect to the cathode location, and no external field is present
[46]. Then α →0 and one gets a nearly spherical distribution. In many cases, a dedicated
anode is used, and the expansion shows pluming with α >1.

482 Chapter 10
An alternative approximate description is the exponential function [47]
n ∼ F
max
exp(−ω
2
/k
2
) (10.17)
where F
max
is the maximum value (about 2.5%/sr for copper arcs of 100 A), ω is the solid
angle, and k is the shape factor (about 4.6 sr for the example of an annular anode with 11 mm
diameter and copper arc of 100 A [47]).
As the plasma expands its density drops according to the point source law
n = C
I
arc
r
2
(10.18)
along the surface normal, where C is a constant related to the ion erosion rate specific to the
cathode material, I
arc
is the arc current, and r is the distance from the spot. For copper,
C ≈10
13
A
−1
m
−1
[48]; the constants for other cathode materials can be estimated from ion
erosion data relative to copper [47, 49–52]. Taking both the angular and distance dependence
into account, a more general expression can be written as
n = C
I
arc
r
2
cos
α
ϑ (10.19)
The plasma density distribution is greatly altered in the presence of an external magnetic field.
The field is usually strong enough to magnetize electrons but not ions. With magnetization we
mean a situation in which the electrons can complete many gyration rotations before a
collisions occurs; this implies that the electron gyration radius
r
e
=
v
⊥
m
e
eB
(10.20)
is much smaller than the typical size of the system (e.g. the cathode–anode distance), where B
is the scalar value of the magnetic induction, m
e
is the electron mass, and v
⊥
is the velocity
component perpendicular to the magnetic field vector; for estimates one can use the thermal
velocity. The magnetic induction is usually not strong enough to also magnetize ions, i.e. the
ion gyration radius is usually not much smaller than the system size. Even though only the
electrons are magnetized, the plasma as a whole tends to be guided by the magnetic field lines
owing to the interaction of electrons with ions. This becomes very important when considering
macroparticle filters (Section 10.4).
10.2.2.2 Ion Charge State Distributions
The comment on the fluctuating properties of the plasma density is also applicable to all other
parameters, including the ion charge state distributions (CSDs). At a given location, fast
fluctuations occur, reflecting the fractal properties of ion generation at and near the emission
centers. Therefore, published ion CSDs always refer to time-averaged values. CSDs and the

Unfiltered and Filtered Cathodic Arc Deposition 483
derived average, or most likely, velocities have been extensively studied [51, 53–62]. Multiply
charged ions are of interest because, in contrast to most other low-temperature laboratory
plasmas, they are produced in copious amounts by the cathodic arc processes. This suggests
utilizing those ions as ion feedstock for metal ion sources, which indeed has been done in
several embodiments [61, 63, 64].
Charge states are very important to most applications, especially because they directly
influence the energy delivered to a surface in the energetic condensation process (Section
10.5). Table 10.2 provides CSDs as measured for vacuum arcs averaged over many individual
measurements.
While the data of Table 10.2 give a good indication what to expect from the various cathode
materials, one needs to realize that those data, especially the ion charge states, are the result of
complicated ionization and recombination processes, and therefore variations can occur,
especially when an external magnetic field is present, and when the expansion is impeded by
background or process gas.
In the case of an external magnetic field, the average motion of electrons perpendicular to the
magnetic field lines is greatly reduced, and the plasma is expanding at a reduced rate, which
leads to less cooling compared to the freely expanding plasma. As a result, the
ionization–recombination balance is shifted to greater ionization; higher charge states are
obtained, and the average charge states are clearly shifted [51, 59, 60, 68–71].
Collisions of metal ions with background or processing gas atoms or molecules reduce the
charge states of the metal ions [72–81], but they also contribute to activation of the collision
partner. Activation includes electronic excitation, ionization, or, if the collision partner is a
molecule, dissociation or excitation of vibrational or rotational modes. The reduction of metal
ion charge state occurs most effectively through charge exchange collisions [82, 83] of the type
Me
Q+
+ Me ⇒ Me
(Q−1)+
+ Me
+
,Q= 1, 2, 3,... (10.21)
where Me
Q+
stands for the Q-fold charged metal ion. Charge exchange reactions with multiply
charged ions will lead to ionization of the atom and to a reduction of the population of highly
charged ions. Therefore, even though the total charge is conserved in each collision of the type
(10.21), the average charge state number is not conserved because previously neutral particles
are now included in the averaging of ion charge states. Neutral particles are conventionally not
included in the averaging procedure mainly for reasons of practicality, i.e. because the
concentration of neutrals is not known.
10.2.2.3 Plasma Velocity
The random component of the electron velocity is much greater than the plasma velocity in a
laboratory frame of reference. Therefore, the electrons may be approximated with a
484 Chapter 10
Table 10.2: Average ion velocity [51], burning voltage [65], cohesive energy [66], average charge state numbers [51,61], and
electron temperature [67] for most conducting elements of the Periodic Table measured for arc currents 100–300 A at pressure
10
–4
Pa
Symbol
Atomic
no.
Arc
burning
voltage
(V)
Average
ion
velocity
(m/s)
Kinetic
ion
energy
(eV)
Ion
momentum
(10
−22
kg m/s)
Cohesive
energy
(eV/ atom)
Average
ion
charge
state no.
Temperature
of electrons
(eV)
Approx.
ion
Mach
no.
Li 3 23.523, 100 19.32.67 1.63 1.02.03.1
C 6 31 17, 300 18.73.45 7.37 1.02.03.0
Mg 12 18.619, 800 49.47.98 1.51 1.52.14.8
Al 13 22.615, 400 33.16.89 3.39 1.73.13.3
Si 14 21.015, 400 34.57.18 4.63 1.42.04.1
Ca 20 20.513, 900 39.910.21.84 1.92.24.2
Sc 21 21.614, 600 49
.610.93.90 1.82.44.5
Ti 22 22.115, 400 58.912.24.85 2.13.24.3
V2322.716, 300 70.213.85.51 2.13.44.5
Cr 24 22.716, 300 71.614.14.10 2.13.44.6
Fe 26 21.712, 600 45.911.74.28 1.83.43.7
Co 27 21.812, 100 44.411.84.39 1.73.03.8
Ni 28 21.711, 500 40
.611.24.44 1.83.03.6
Cu 29 22.713, 200 57.413.93.49 2.03.54.0
Zn 30 17.110, 300 35.711.11.35 1.42.04.2
Ge 32 20.011, 100 46.213.43.85 2.02.04.8
Sr 38 18.511, 500 60.516.81.72 2.02.54.9
Y3919.913, 200 80.319.54.37 2.32.45.8
Zr 40 22
.715, 400 112 23.36.25 2.63.75.5
Nb 41 27.916, 300 128 25.17.57 3.04.05.6
Mo 42 29.517, 300 149 27.66.82 3.14.55.8
Ru 44 23.813, 900 139 23.36.74 2.94.54.8
Rh 45 23.814, 600 142 24.95.75 3.04.55.1
(Continued)
Unfiltered and Filtered Cathodic Arc Deposition 485
Table 10.2: (Continued)
Symbol Atomic
no.
Arc
burning
voltage
(V)
Average
ion
velocity
(m/s)
Kinetic
ion
energy
(eV)
Ion
momentum
(10
−22
kg m/s)
Cohesive
energy
(eV/ atom)
Average
ion
charge
state no.
Temperature
of electrons
(eV)
Approx.
ion
Mach
no.
Pd 46 23.512, 100 80.121.43.89 1.92.06.3
Ag 47 22.811, 100 68.719.92.95 2.14.04.1
Cd 48 14.76, 800 26.612.61.16 1.32.13.6
In 49 16.06, 000 21.611.52.52 1.42.13.2
Sn 50 17.47, 000 29.513.63.13 1.52.13.7
Ba 56 16.57, 900 44.618.01.90 2.02.34.4
La 57 18
.76, 900 34.616.04.47 2.21.44.9
Ce 58 17.67, 900 45.518.44.32 2.11.75.1
Pr 59 20.58, 400 51.519.63.70 2.22.54.5
Nd 60 19.28, 100 49.719.53.40 2.21.65.6
Sm 62 18.88, 100 51.820.32.14 2.12.24.9
Gd 64 20.48, 100 54.121.34.14 2.21.75.6
Tb 65 19
.68, 400 58.122.14.05 2.22.15.3
Dy 66 19.88, 400 59.422.63.04 2.32.45.0
Ho 67 20.08, 600 64.123.73.14 2.32.45.2
Er 68 19.28, 900 69.324.83.29 2.42.05.9
Hf 72 23.310, 300 97.530.46.44 2.93.65.2
Ta 73 28.612, 000 136 36.28.10 2.93.76.0
W742
8.711, 100 117 33.68.90 3.14.35.2
Ir 77 25.510, 700 113 34.16.94 2.74.25.2
Pt 78 23.78, 100 67.226.45.84 2.14.04.1
Au 79 19.76, 900 49.022.63.81 2.04.03.5
Pb 82 17.35, 800 35.819.82.03 1.62.04.2
Bi 83 14.44, 700 23.916.32.18 1.21.83.6
The results do not noticeably depend on arc current and are valid for pressures up to about 10
−2
Pa. No external magnetic field was applied. Most data were taken for
t > 150 s after arc initiation.
486 Chapter 10
Maxwellian distribution and we can focus on the velocity distribution functions of ions. First,
we will consider the situation without an external magnetic field and later move on to consider
the influence of such an external field.
The description of ion motion can be thought of as being composed of a thermal motion and a
drift, as seen in a laboratory frame of reference. If measured in one direction, usually with the
detector facing the incoming plasma flow, the ion distribution function is a shifted Maxwellian
distribution which can be expressed as [77, 81, 84, 85]
φ
i
(E) = C
s
E exp
√
E −
√
E
dir
2
/kT
i
(10.22)
where C
s
is a scaling constant, and E
dir
is the kinetic energy of the directed motion.
Distribution functions of kinetic energy, φ(E), and directed velocity, f (v), are equivalent and
can be converted [86]
φ(E) =
v(E)
m
f
[
v(E)
]
(10.23)
with v(E) = (2E/m)
1/2
. The most likely velocity for a given cathode material, v
∗
i
, i.e. the peak
value of the velocity distribution function, can readily be converted into the most likely ion
energy according to E
∗
kin
= m
i
v
∗
i
2
/2. The most likely values of velocity and energy are not
the average values because the distribution functions are not symmetric but have a long tail
[87]. Time-of-flight (TOF) techniques give information on velocities [51, 88–92] and
electrostatic field methods provide energy distributions [54, 55, 77, 85]. For the latter, one has
to carefully take into account the acceleration or deceleration caused by the sheath at the
detector entrance – doing so allows us to reconcile apparently differing results obtained by
those methods.
Table 10.2 includes the most likely ion velocities (peak of the distribution) for a wide range
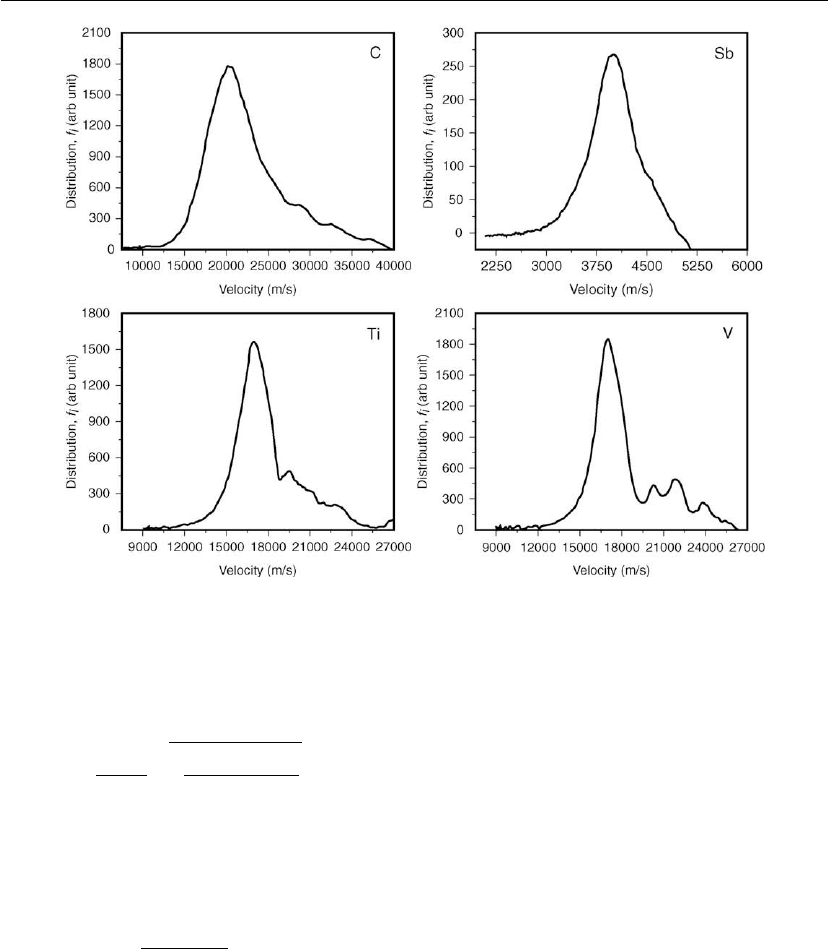
of materials. If we look for example at the distribution functions measured by Byon and
Anders [93],wefindone distinct peak in the distribution functions, even though there was
no resolution of charge states in those measurements (Figure 10.5). This shows that
most ions drift with approximately the same velocity regardless of their charge state
[91, 94].
For the case of expansion into vacuum and in the absence of significant magnetic fields, a
two-fluid plasma model (ions, electrons) works fairly well to determine the average ion
velocity. Assuming that all energy is deposited in a small volume at the cathode spot and
Unfiltered and Filtered Cathodic Arc Deposition 487
Figure 10.5: Examples of ion velocity distribution functions. The measuring principle implies
greater errors at the high-energy end and those small peaks may be artifacts caused by density
fluctuations. Note that each material has its own velocity scale. (Adapted from [93].)
considering adiabatic expansion, the expression
¯
v
i
≈
2
γ − 1
γ
kT
∗
i
+
¯
QkT
∗
e
m
i
(10.24)
can be derived for the final ion velocity [10, 95], where γ ≈5/3 is the adiabatic coefficient and
the star superscript indicates the initial temperature in the vicinity of the cathode spot.
Similarly, considering the equations of energy and motion for a multicomponent plasma, the
relatively simple approximate expression
¯
v
i
≈ 3.5
¯
QkT
∗
e
/m
i
(10.25)
can be derived, where T
∗
e
denotes the electron temperature at the point where the ion velocity
becomes supersonic [96–98]. To evaluate these expressions, one may use the temperature data
[67] that have been derived from measured ion CSDs [61]. From the results (Figures 10.6 and
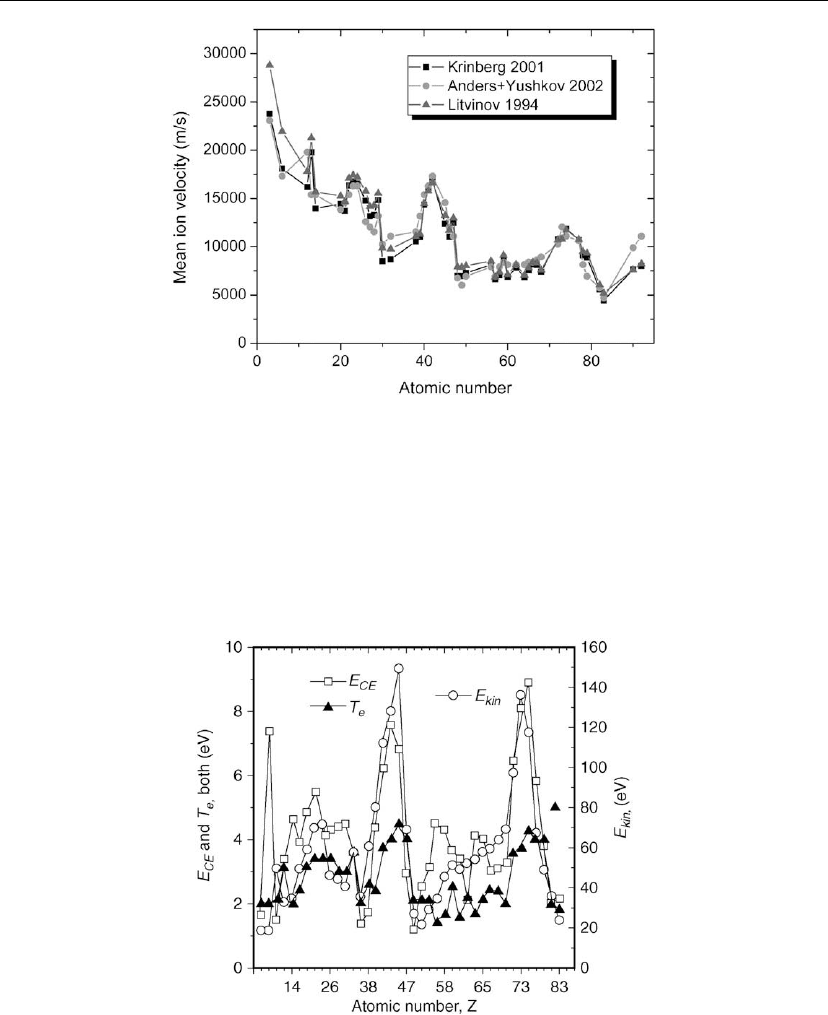
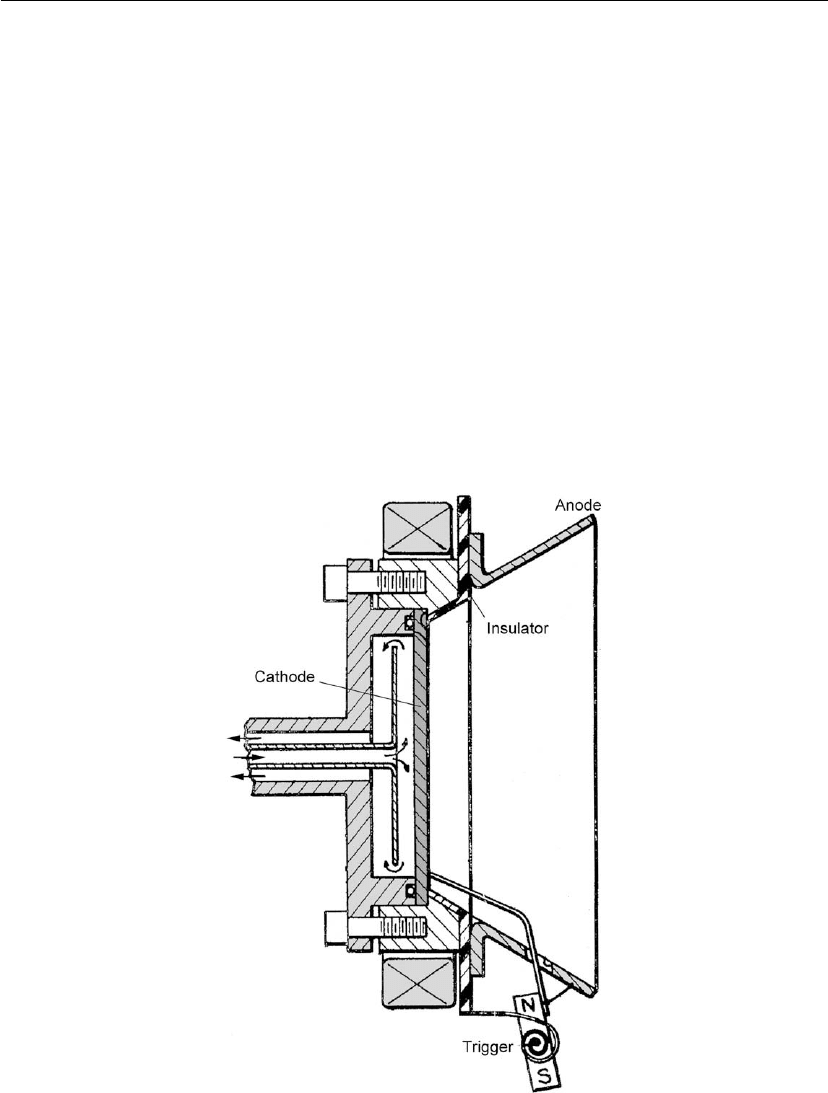
10.7), one can see that the correlation is surprisingly good.
488 Chapter 10
Figure 10.6: Mean ion velocity of vacuum arcs, as calculated by Litvinov [95] and Krinberg [98],
compared to data from Anders and Yushkov [51].
Figure 10.7: Most likely ion velocities for vacuum arc plasmas, converted to ion energies, the
electron temperature near the cathode spot, and the cohesive energy of the cathode material.
(Adapted from [51].)
Unfiltered and Filtered Cathodic Arc Deposition 489
10.3 Arc Plasma Sources
10.3.1 DC Sources
Practically all commercial cathodic arc systems operate with continuous direct current (DC)
arcs to make use of the high deposition rate and reasonably low equipment cost. The typical
arc current is between 40 and 100 A. The lower limit is determined by arc chopping (i.e. the
spontaneous extinguishing of the cathodic arc discharge), whereas the upper limit is
determined by cathode cooling considerations.
Arc sources for industrial coatings were originally designed in the late 1960s and early 1970s,
for example by Sablev [99] and Snaper [100]. Their patents have expired and so the design
features are freely utilized in various modern designs.
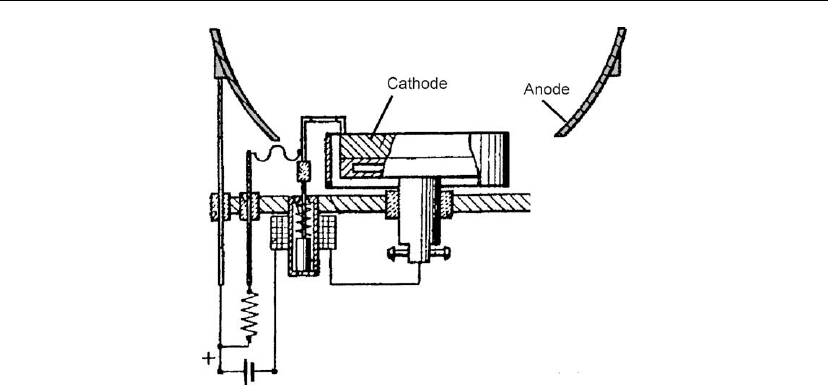
Snaper’s arc patent contains many elements of typical arc sources. Figure 10.8 shows a version
with a magnetically actuated mechanical trigger. The field coil has the dual function of
Figure 10.8: Arc source with magnetically actuated mechanical trigger. (Adapted from [101].)
490 Chapter 10
Figure 10.9: Random arc source by Sablev et al. featuring a mechanical trigger and dedicated
anode. (Adapted from [103].)
removing the trigger from the cathode and increasing the directivity of the ionized beam. The
cathode is held by a ceramic insulator, which limits the area on which arc spots can occur.
Boron nitride has been identified as a preferred insulator material because it does not shatter
when the arc spot burns at the cathode–ceramic interface. Snaper’s source belongs in the
category of random arc sources, because the apparent spot motion is not controlled by a
magnetic field or other means (for spot steering, see Section 10.2.1).
Possible plasma contamination with ceramic material can be avoided when using a gap design,
i.e. when the active part of the cathode is defined by an enclosing floating shield that defines a
narrow gap to the cathode. Should an arc spot ignite inside the gap, the impedance is high and
the arc either extinguishes or another spot on the designated ‘correct’ surface area takes over
electron emission and plasma production. Sablev’s original design [99] became the prototype
for many source versions (Figure 10.9), and other improvements were made, like the labyrinth
gap by Hovsepian [102].
In special cases, namely when the cathode material is of low cohesive energy (like Mg), it can
be clamped with a part made from much higher cohesive energy (like Ta, Mo, W) without
covering (hiding) it because arc spots are unlikely to ignite on those clamp materials.
Yet another way of controlling the location of spot operation is through magnetic steering
(Section 10.2.1), and we speak of ‘steered arc sources’. Steered arc sources can be compact,
with cathodes typically ∼5–10 cm in diameter, or they may have a very large and elongated
cathode, perhaps as long as 1–2 m. Two rules govern the apparent spot motion: (1) the
retrograde motion rule; and (2) the acute angle rule, which can be explained as follows.
