Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.


Evaporation 201
produces a thin plasma sheath on top of the melt. The low-energy secondary electrons from the
plasma sheath are pulled upward into the reaction zone by an electrode placed above the pool
biased to a low positive DC or AC potential (20–100 V), thus creating a plasma-filled region
between the electrode and the electron beam gun. The low-energy electrons have a high
ionization cross-section, thus ionizing or activating the metal and gas atoms and increasing the
reaction probability on collision. Charge-exchange processes between positive ions and neutral
atoms take place in the plasma. In addition, as suggested by Yee [139], transient highly excited
compound species are formed. The formation of the compound is completed most probably on
the substrate from these energetic and excited transient species. The synthesis of TiC by
reaction of Ti metal vapor and C
2
H
2
gas atoms with a carbon to metal ratio approaching unity
was achieved with this process [131, 133]. Moreover, by varying the partial pressure of either
reactant, the carbon to metal ratio of carbides could be varied [133, 140] at will. The ARE
process has also been applied to the synthesis of all five different Ti–O oxides [141]. These
authors noted that in the ARE process (i.e. with a plasma) as compared to the RE process (i.e.
without a plasma), a higher oxide is formed for the same partial pressure of O
2
, thus
demonstrating a better utilization of the gas in the presence of a plasma. The same observation
was noted by Bunshah and Raghuram [137] as well as by Granier and Besson [142] for the
deposition of nitrides.
A variation of the ARE process uses a resistance-heated evaporation source. The basic ARE
process uses e-beam-heated sources, which are expensive and inconvenient for the evaporation
of low melting point, high vapor pressure materials. Nath and Bunshah [138] modified the
ARE process for resistance-heated sources, as shown in Figure 4.43. The metal vapors are
generated from the chamber; the reaction is enhanced by a plasma generated by injecting
low-energy electrons from a heated thoriated tungsten emitter towards a low-voltage anode
assembly. A transverse magnetic field is applied to cause the electrons to go into a spiral path,
thus increasing the probability of electron/atom collision and subsequent ionization.
Modifications of the basic ARE process
The ARE process has substantial versatility since the substrate can be grounded, positively or
negatively biased, or it can be allowed to float electrically. There are several modifications of
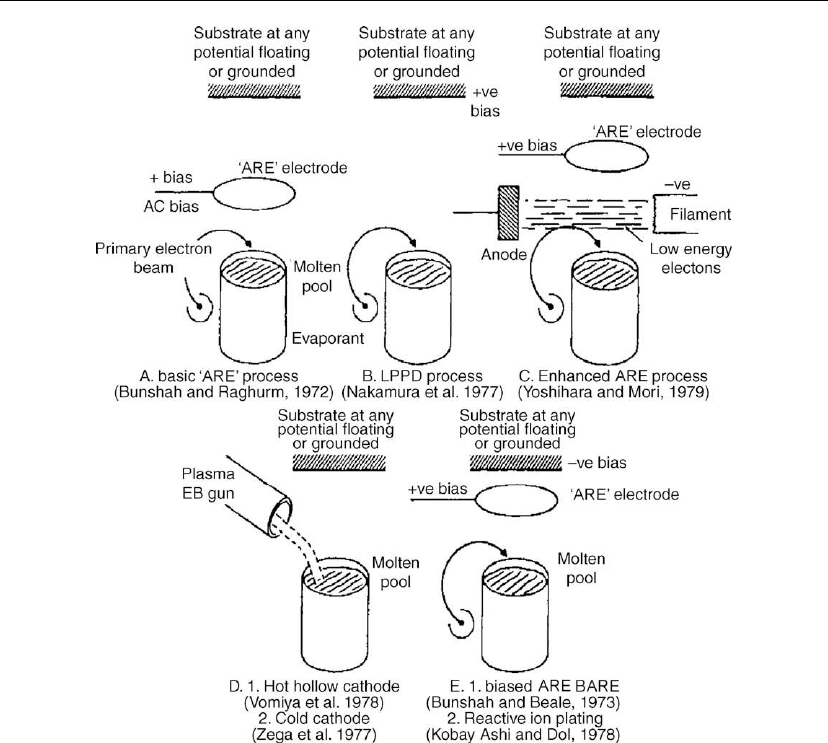
the basic ARE process, as illustrated in Figure 4.44.
1. Enhanced ARE process [143, 144]. This is the conventional ARE process using e-beam
heating with the addition of a thermionic electron emitter (e.g. a tungsten filament) for the
deposition of refractory compounds at lower deposition rates as compared to the basic
ARE process. The low-energy electrons from the emitter sustain the discharge, which
would otherwise be extinguished since the primary e-beam (used to melt the metal) is so
weak that it does not generate an adequate plasma sheath above the molten pool from
which low-energy electrons can be extracted by positively biased interspace electrode. The
substrate may be biased, grounded, or floating.
202 Chapter 4
Figure 4.44: Basic ‘ARE’ process and later variations.
2. Low-pressure plasma deposition process. Using e-beam evaporation sources, the electric
field may be generated by biasing the substrate positively instead of using a positively
biased interspace electrode. In this case, it is called low-pressure plasma deposition
(LPPD) [145, 146]. However, this version has a disadvantage over the basic ARE process
since one does not have the freedom of choice to ground the substrate, let it float, or bias it
negatively (the BARE process; see no. 4 below).
3. ARE using plasma electron-beam guns. The plasma e-beam gun, instead of the
thermionic e-beam gun, can be used to carry out the ARE process. The hot hollow cathode
gun has been used by Komiya et al. [147] to deposit TiC films, whereas Zega et al. [148]
used a cold cathode discharge e-beam gun to deposit titanium nitride films. The plasma
e-beam sources produce an abundant supply of low-energy electrons for the ARE-type
process.

Evaporation 203
4. Reactive ion-plating processes. If the substrate is biased in the ARE process, it is called
biased activated reactive evaporation (BARE). This bias is usually negative to attract the
positive ions in the plasma. The BARE process has been reinvented and called reactive ion
plating by Kobayashi and Doi [149]. Ishida et al. deposited Ni–TiC films by ion plating
with cation ratios varying between Ti:Ni 0.35 and 3 [150]. Reactive ion plating (RIP) is
very similar to the reactive evaporation process in that metal atoms and reactive gases react
to form a compound aided by the presence of a plasma. Since the partial pressures of the
gases in reactive ion plating are much higher (> 10
−2
torr) than in the ARE process
(> 10
−4
torr), the deposits can become porous or sooty. The plasma cannot be supported by
lower pressure in the simple diode ion plating process; therefore, Kobayashi and Doi [149]
introduced an auxiliary electrode biased to a positive low voltage (as originally conceived
for the ARE process) to initiate and sustain the plasma at low pressure (∼ 10
−3
torr). This
is no different than the ARE process with a negative bias on the substrate reported much
earlier by Bunshah [135], which was designated by him as the biased ARE (or BARE)
process.
5. Another variation of reactive ion plating using a triode configuration [151] involves
injection of electrons into the reaction zone between the e-beam-heated evaporation source
and the negatively biased substrate from a heated tungsten filament transversely to the
metal vapor path. These low-energy electrons are pulled across the reaction zone by a
positively biased anode located opposite to the cathode. The arrangement is very similar to
that shown in Figure 4.33 except for the use of an e-beam-heated evaporation source, and
is also very similar to triode sputtering. This adds versatility as well as complexity to the
process through the addition of another process variable.
6. Murayama [152] uses an e-beam-heated source with a negatively biased substrate and RF
activation of the reactants by means of a coil electrode of aluminum wire in the reaction
zone to deposit oxide and nitride films.
7. ARE process using an arc evaporation source. Evaporation of metals using a low-voltage
arc in the presence of a plasma and a negatively biased substrate is used by Snaper [103,
104] and Dorodnov [153] to deposit nitride and carbide films, with N
2
and hydrocarbon
reactive gases, respectively.
8. Reactive arc filtered evaporation. The system consists of conventional bell jar vacuum
system and uses a magnetic field steering arc and a plasma duct formed from a
free-standing and isolated high-current solenoid [154]. The advantage of this system is that
it preserves the target material and achieves higher ionization rates with respect to the
ion-plating process.
Recent developments in the ARE process
New techniques based on ARE are being developed for synthesis of novel and unique
materials. The emphasis of such developments is generally on two aspects: (1) new approaches

204 Chapter 4
to produce the vapor species; and (2) new plasma excitation and confinement techniques and
development of modified plasma excitation geometries.
Westerwaal et al. studied the nucleation of MgH
2
films by the ARE system [155]. Their results
showed that they had 10% unreacted Mg remaining in the structure, but by heat treating the
films and subsequent rehydrogenation procedures they were able to obtain 100% MgH
2
films.
New approaches to produce the various species The basic process involves evaporation of
the constituent metal alloy or compound using e-beam or resistance/induction-heated sources.
However, it is difficult to use this approach with certain materials such as boron and carbon.
Two possible solutions can be used to overcome these difficulties: (1) use a low melting point
compound of the respective element, and (2) use a pulsed laser beam where the pulse rate and
pulse width can be appropriately adjusted to control the rate of material generation and
fragmentation. Moreover, in many cases, the energy of the laser beam can also be used as a
source for plasma excitation.
Both of these approaches have been explored. A process developed by Bunshah et al. [156] for
the synthesis of cubic boron nitride involves boric acid as an reactant, which can be easily
evaporated from a resistance-heated tungsten boat. In addition to the ease of evaporation, this
process excludes the toxicity problems associated with fine boron particles which can be
produced during e-beam evaporation of boron. A similar approach can be extended to
evaporation of carbon using a low melting point carbon compound such as adamantine. It is
likely that many new materials hitherto difficult to synthesize may possibly be deposited using
this routine. Moreover, this novel approach may contribute to further development in reactive
MBE processes and other vapor deposition processes involving organometallic compound
reactants.
The use of pulsed laser beams in an ARE type of process has been demonstrated in recent
literature on high T
c
superconducting films. Films with high T
c
(90 K) and high critical current
density (0.7 × l0
6
A·cm
−2
at 77 K) have been produced [157]. It is claimed that pulsing of the
laser beam avoids fractionation of the compound and hence good control of film stoichiometry
is achieved. It is also suggested that the photon energy is sufficient to activate the reactive
gas/metal species thereby increasing their reactivity, leading to an increased oxygen
concentration in the deposited films.
New plasma excitation modes and geometries As discussed earlier, the attributes of the ARE
processes are due to the possibility of controlling the plasma parameters independently of the
deposition process.
However, improvement in excitation and confinement of the plasma, as well as control and
optimization of plasma parameters in the ARE processes, are likely to enhance the process
Evaporation 205
capabilities. Recent developments include (1) the use of inductively coupled RF with parallel
plate RF geometries, and (2) the use of multiple filaments and anodes with magnetic
confinement. These enhancements have led to substantial improvements in film properties as
well as process control. Examples are high-rate deposition of a-Si–H films [158], transparent
conducting films on polymeric substrates [159], and TiS
x
and MoS
x
[160, 161] films with
variable x values.
Two additional modes of ionization are being explored. Currently, an auxiliary RF excitation
source similar to that reported by Oeschner [162] is being developed for use in ARE. It is
believed that the high electron density and energy selectivity offered by this source is
likely to enhance advantages of the ARE processes for compound synthesis. Also, work is
underway to integrate electron cyclotron resonance (ECR) excitation at microwave frequencies
with the ARE process. ECR plasmas are characterized by a very high level of ionization and
excitation, and may greatly enhance the use of ARE for the deposition and synthesis of
films.
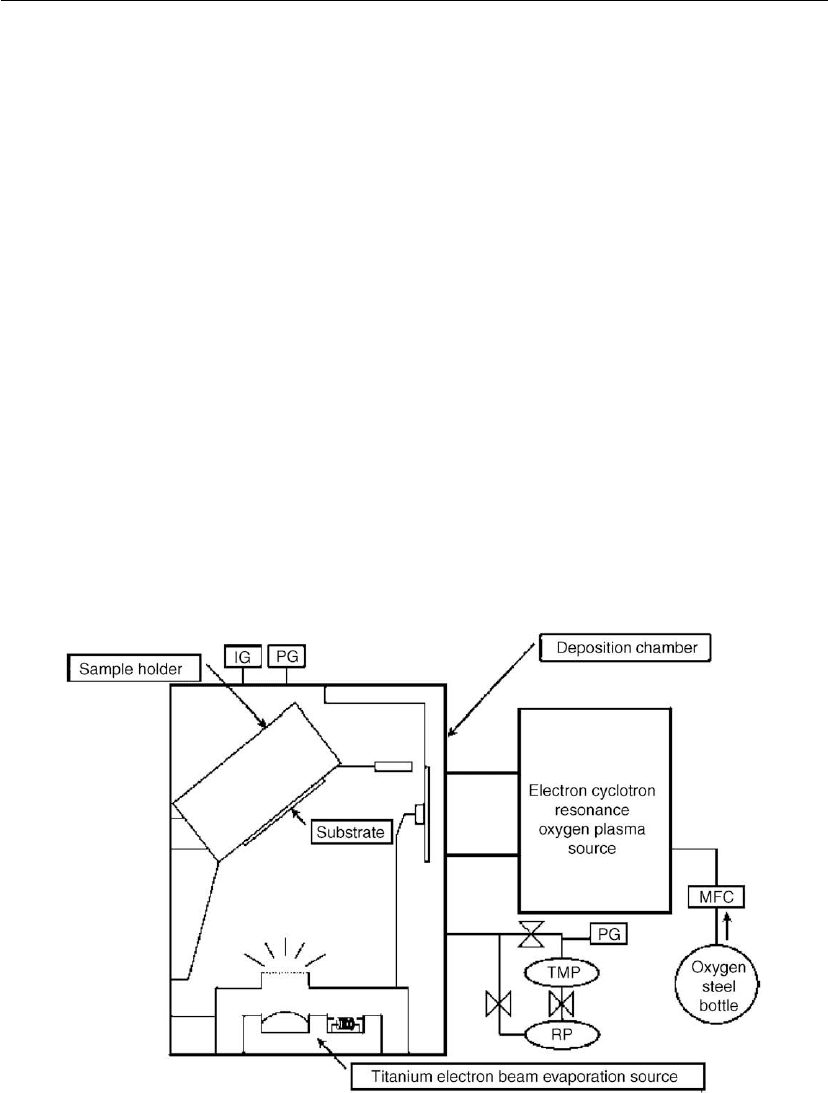
Sakai et al. studied plasma-enhanced reactive evaporation of TiO
2
thin films for photocatalytic
applications [163]. The system consists of a Ti e-beam evaporation source and an ECR oxygen
plasma source, and a sample holder as shown in Figure 4.45. In conventional plasma-enhanced
systems the sputtering rate is low because the target gets oxidized and reduces the deposition
rate. In Sakai’s system the modification is such that the Ti atoms and O
2
gas flow to the
Figure 4.45: Reactive evaporation system [163].

206 Chapter 4
substrate independently so that the target does not get poisoned [163]. According to their
results the crystal structure of the TiO
2
thin film changes as they increase the deposition rate
from 18 nm/min to 145 nm/min. The crystalline phase shifts from anatase to rutile as the
deposition rate increases.
Mechanism of the ARE process
A reactive evaporation process can be simply written as a reaction between the reactants
giving rise to the products. Illustrating this for the deposition of TiC films, one may write:
xTi(vapor) + C
x
H
y
(gas)→xTiC(solid) + yH(gas)
In a plasma-assisted deposition process, the reactants dissociate into fractions/radicals and
ionic species are produced. Therefore a multiplicity of reaction paths are possible and the
overall reaction becomes more complex. Deshpandey et al. [164] studied the synthesis of TiC
and TiN films, evaporating Ti in a plasma of C
x
H
y
gases for the synthesis of TiC films and N
2
or NH
3
with Ti for the synthesis of TiN films. Several spectroscopies were used to carry out
diagnostics on the plasma in the source-substrate volume to determine the species present and
the potential reaction paths leading to film formation. Neutral mass spectrometry (MS),
plasma mass spectrometry (PMS), and optical emission spectroscopy (OES) were used to
examine the nature and relative concentrations of neutral, excited, and ionized species present
in the process.
The main results of these investigations are as follows:
Polymerizing reactions producing higher molecular weight hydrocarbon species are
dominant in the case of methane. Polymerization increases with increasing flow rate of
CH
4
for a given e-beam current. The above reactions lead to the formation of relatively
soft films containing TiC and graphitic phases.
Hard, single-phase TiC films are formed at flow rates of about 50 standard cm
3
min
−1
C
2
H
2
for beam currents in the range of 0.2–0.3 A. Polymerization reactions do not
take place when C
2
H
2
is used as a reactive gas. Species such as carbon, CH, and CH
2
formed in the plasma from the dissociation of C
2
H
2
react with titanium to form TiC.
The PMS and MS data indicate the following possible routes for formation of TiC:
(a) formation of TiC in the plasma volume through reactions such as:
Ti + C→TiC
Ti + CH→TiC + H
Ti + CH
2
→TiC + 2H
Ti + CH
3
→TiC + 3H
followed by condensation of TiC molecules on the substrate; or

Evaporation 207
(b) formation of Ti
x
C
y
or Ti
2
C
y
or Ti
2
C
y
H
z
complexes in the plasma volume followed
by condensation on the substrate to form TiC according to:
Ti
x
C
y
→TiC + C
Ti
x
C
y
H
z
+ CH
2
→TiC + C
y
H
z
Present data are not sufficient to determine which of these two schemes is
dominant in the formation of TiC. PMS and MS sampling of the arriving flux on
the substrate as well as studies with a biased substrate are necessary to resolve
this issue.
Similar studies on the deposition of TiN films revealed the following:
Evaporation of Ti in a N
2
plasma showed that the predominant species leading to hard
stoichiometric TiN films is 2Ti
+
+N
2
+
→ 2TiN. The ratio of Ti
+
/N
2
+
in the plasma was
1.05, i.e. close to unity. When this ratio was increased to 1.5, soft films with excess Ti
in the deposit were produced. Yee [139] also proposed the same reaction path based on
his optical emission spectrographic studies.
Evaporation of Ti in an NH
3
plasma showed similar results. Under conditions where
the Ti
+
/N
2
+
ratio was high, the films were soft and titanium rich. With a higher flow
rate of NH
3
, the N
2
+
concentration in the plasma was higher and the films were hard.
4.8.5 Materials Synthesized by Evaporation-Based Processes
A variety of metals, alloys, and compounds (oxides, nitrides, arbides, sulfides) have been
deposited using evaporation and related processes. In particular, the plasma-assisted variant of
the evaporation process, such as ARE, has been successfully used for deposition of a variety of
compounds for tribological as well as optoelectronic applications. Recently, a modified
process based on the ARE technique has also proved to be successful in synthesizing c-BN
[156, 165]. A representative list of the compounds synthesized by the ARE process is given
below. In a very recent development, the ARE process has been able to deposit Al
2
O
3
films at
very high deposition rates (8 to 12 m/h); these rates are 10–30 times higher than those by
sputter deposition [166].
The compounds synthesized by the ARE process include:
Carbides: TiC, HfC, ZrC, VC, W
2
C, TaC
Carbonitride: Ti (C, N)
Nitrides: TiN, HfN, ZrN, CrAlTiN, TiSiN
Oxides: TiO
2
, ZrO
2
,Al
2
O
3
, SiO
2

208 Chapter 4
Sulfides: TiS
2
, MoS
2
, MoS
3
Superconductors: low T
c
:Nb
3
Ge, CuMo
6
S
8
; high T
c
: YBa
2
CU
3
O
7–8
Photovoltaic materials: a-SiH, CuInS
2
Optoelectric materials: In(Sn)O
2
, ZnO
Novel materials: c-BN, Diamond, i-C, a-C.
4.8.6 Deposition of Nanocomposite Materials
Nanocomposite thin films containing embedding metals in polymer matrices can be prepared
by various techniques. Nanocomposites provide the flexibility of tuning electrical, magnetic,
and other physical properties of the deposited film independently by controlling the chemistry
of the constituents. Biswas et al. deposited Ni nanoparticles with Teflon layers by vapor-phase
tandem evaporation [167]. This technique allows metals and polymers to be evaporated at the
same time. They controlled the Ni nanoparticle size while generating low to relatively high
cluster-volume filling in the polymer. In evaporation of metals Takele et al. tuned the electrical
and structural properties by the simultaneously evaporation of metal (Ag, Au) and polymer
[168]. One of the most crucial factors in this deposition is the sticking coefficient of metal
nanoparticles on polymers. The electrical properties mainly depend on the metal filling factor,
particle size, size distribution, and metal particle/insulator interface properties.
Zaporojtchenko et al. described the effects of metal interaction with various polymer matrices
by evaporating Cu, Ag, and Au on fully cured polymer films [169].
4.9 Microstructure of PVD Condensates
4.9.1 Microstructure Evolution
PVD condensates deposit as single crystal films on certain crystal planes of single crystal
substrates, i.e. by epitaxial growth [170], or in the more general case, the deposits are
polycrystalline. In the case of films deposited by evaporation techniques, the main variables
are: (1) the nature of the substrate; (2) the temperature of the substrate during deposition;
(3) the rate of deposition; (4) the deposit thickness; (5) the angle of incidence of the vapor
stream; and (6) the pressure and nature of the ambient gas phase. Contrary to what might be
intuitively expected, the deposit does not start out as a continuous film one monolayer thick
and grow. Instead, three-dimensional (3D) nuclei are formed on favored sites on the substrates,
e.g. cleavage steps on a single crystal substrate; these nuclei grow laterally and in thickness
(the so-called island growth stage), ultimately impinging on each other to form a continuous
film. The average thickness at which a continuous film forms depends on the deposition
temperature and the deposition rate (both of which influence the surface mobility of the

Evaporation 209
adatom) and varies from 10
˚
A for Ni condensed at 15 K to 1000
˚
A for Au condensed at 600 K.
This familiar model of island growth of a polycrystalline film during the initial stages of
deposition illustrates the case where there is limited interaction between depositing atoms and
the substrate. This is not always the case.
Petrov et al. [171] reviewed film growth processes which include nucleation, coalescence,
competitive grain growth, and recrystallization. They also discussed evolution as a function of
deposition variables including temperature, the presence of reactive species, and the use of
low-energy ion irradiation during growth [171]. Types of substrates, such as amorphous and
polycrystalline, used in PVD processes play an important role in the microstructural
evolution in thin films synthesized by low-temperature PVD. The use of amorphous substrates
allows the isolation of the effects of individual deposition variables on texture development.
However, polycrystalline substrates bias texture through local pseudomorphic epitaxy, with the
same overall microstructure evolution toward the final state driven by the deposition
conditions. On both types of substrates, film growth proceeds via a 3D or Volmer–Weber
model [171].
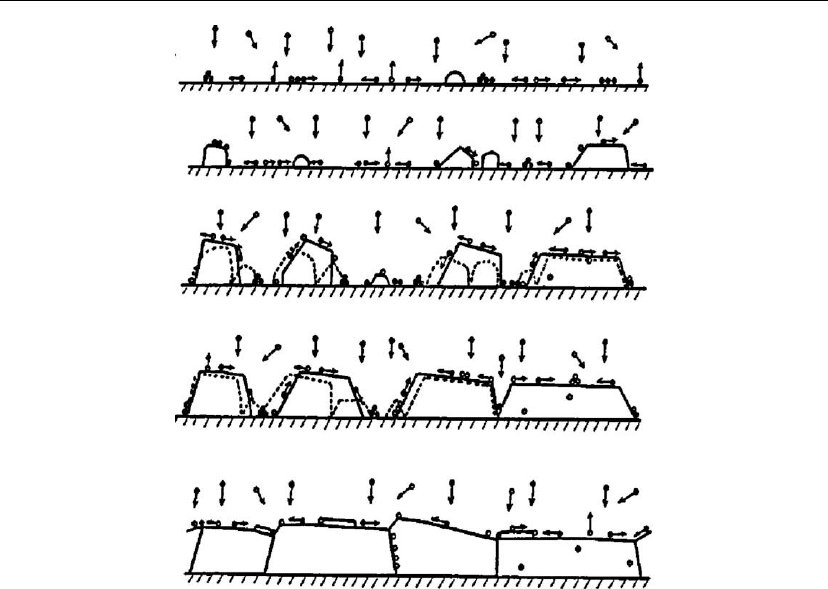
The growth processes controlling microstructure evolution, nucleation, island growth,
impingement and coalescence of islands, grain coarsening, formation of polycrystalline
islands and channels, development of a continuous structure, and film growth, are presented
schematically in Figure 4.46. In the formation of film, grain coarsening, i.e. recrystallization
through grain boundary (GB) migration, can occur both during and after island coalescence.
The nucleation barrier is generally expected to be small, leading to randomly oriented islands,
for low-temperature deposition on amorphous substrates [173, 174]. In situ transmission
electron microscopy (TEM) investigations confirm this for studies of Au/SiO
2
[175, 176] and
In/C [177, 178]. Nucleation kinetics depend on the adatom binding energy, crystal structure of
the substrate material, lattice defects, surface steps, and contamination.
Polop et al. studied the initial stages of polycrystalline Ag film formation deposited by thermal
evaporation on the amorphous Si layer [179]. The deposition temperature was 300 K and the
deposition rate 8 × 10
−3
nm/s. After the deposition the films were analyzed by in situ scanning
tunneling microscopy (STM). Figure 4.47 displays STM topographs of the evolution of Ag
film morphology as a function of the film thickness. Polop’s results show that the film
morphology is distinguished by three regimes: (1) film thicknesses smaller than 0.8 nm – the
nucleation and island formation (Figure 4.47a), (2) film thicknesses between 0.8 and 10 nm –
island growth regime (Figure 4.47b–d) and (3) film thicknesses higher than 10 nm –
continuous film regime (Figure 4.47e–f).
Akkari et al. deposited CuInS
2
films by thermal evaporation with a flux angle with rotation
of the substrate [180].InFigure 4.48, when the flux angle increases, the tilting of the
columns also increases. When the substrate is not rotated the film structure consists of
210 Chapter 4
Figure 4.46: Schematic diagram illustrating fundamental growth processes controlling
microstructural evolution [171, 172].
nanocolumns that are inclined towards the evaporation source. When the substrate is rotated
and the angle is at 80
◦
, the structure takes straight wire forms [180].
Important differences have been observed. Namba and Mori [181] found that by converting a
significant fraction (∼ 10%) of the vapor flux of Ag to positive ions, epitaxial growth of a
single crystal Ag film on a single crystal NaCl substrate biased to −3000 V was observed,
whereas with vacuum evaporation, the Ag film was polycrystalline. No clear explanation is
possible except to note that the mobility of the deposited species is much greater when partially
ionized than for neutral vapor species. The effective surface temperature of the growing film is
much higher owing to ion bombardment, thus permitting greater surface mobility and resulting
in epitaxial growth. Taylor [182] used low-energy electron diffraction (LEED) techniques to
study the epitaxial deposition of Cu onto a single crystal [183] face of tungsten under ultrahigh
vacuum conditions. This represents the case where there is appreciable bonding between
depositing atoms and the substrate. The deposit on a clean tungsten surface was a uniformly
thin [184] Cu film, i.e. no island growth prior to the formation of a continuous film even at
