Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Evaporation 191
Table 4.7: (Continued)
Materials Form of
evaporant
Feeder
mechanism
Filament
temp.
◦
C
Substrate
temp.
◦
C
Comments on films
Cr–SiO (60–100 mol
% Cr)
Sintered pellets,
∼0.7 mm size
Disk and wiper 2050 ± 60 200 Resistor films, SiO content
equals that of source ±1–3%.
Rates: 20–30
˚
As
−1
across 70
cm distance
Cr(15)–Si(85) Powdered alloy Vibrating chute 2000 200–500 Resistor films
Cr
2
Si + TaSi
2
+Al
2
O
3
Mixed powders Worm drive 2500 200–400 Resistor films with ±10%
control
Compounds
AlSb Powder, 100/150 mesh Vibrating trough 1400–1600 700 Imperfect epitaxial films on
Ge
GaP Powder, 100/150 mesh Vibrating trough 1500 540 Epitaxial films on Ge crystals
GaAs Powder, 100/200 mesh Vibrating trough 1450 300–670 Epitaxial films on Ge crystals
above 600
◦
C. Rates:
2–30
˚
As
−1
Powder, 100/200 mesh Worm drive 1400–1800 530 ± 10 Epitaxial films on GaAs
crystals. Rates: 2–5
˚
As
−1
across 21 cm distance
Powder, 100/150 mesh Vibrating trough 1300–1800 475–525 Epitaxial films on Ge crystals
Powder, 40/60 mesh Micrometer
screw and piston
1325 525–575 Highly oriented films on Ge
and GaAs crystals. Rates:
10–25
˚
As
−1
across 10 cm
distance
GaSb Powder, 100/150 mesh Vibrating trough 1650 500 Epitaxial films on Ge crystals
InP Powder, 100/150 mesh Vibrating trough 1400–1650 300 Epitaxial films on Ge crystals
InAs Powder, 100/150 mesh Vibrating trough 1500 500 Epitaxial films on Ge crystals
InSb Granules Vibrating trough 1600 450–460 Epitaxial films on InSb
crystals. n-type, 10
15
–10
17
donors per cm
2
192 Chapter 4
Table 4.7: (Continued)
Materials Form of
evaporant
Feeder
mechanism
Filament
temp.
◦
C
Substrate
temp.
◦
C
Comments on films
Powder, 100/150 mesh Vibrating trough 1650 300–400 Epitaxial films on Ge crystals
Cu
2
S, Cu
2
Se Powders of
250–300 m
Vibrating trough 1400 25 Semitransparent, conductive
films of Cu
1,8
S and Cu
18
Se
BaTiO
2
Sintered powder,
100/200 mesh
Vibrating chute 2300 500–700 Crystalline films, dielectric
constants of 400–700. Rate:
3
˚
As
−1
across 8 cm distance
Various perovskites Sintered powder,
100/200 mesh
Vibrating trough 2050–2300 500–700 Perovskite films, epitaxial on
LiF crystal. Rates: 1–3
˚
As
−1
across 8 cm distance
From Handbook of Thin Film Technology [7]. Copyright © 1970, McGraw-Hill. Used with permission of McGraw-Hill Book Company.

Evaporation 193
was deposited on WC–Co insets and high-speed steel samples. In order to improve the
adhesion of the nanocomposite film, they applied −900 V bias voltages at the beginning of the
deposition. CrN is more malleable than TiN; however, it has better oxidation resistivity. By
adding another improved oxidation-resistant material such as CrN to TiAlN, the wear
resistance of the nanocomposite thin film has been improved [126]. Chang et al. studied the
wear resistance and hardness with TiSiN alloys by cathodic arc plasma evaporation [127].
According to their results the wear mechanism is controlled by counter-materials; however, no
clear relation between bias voltage and wear resistance has been found.
4.8.4.1 Direct Evaporation
Table 4.8 gives the experimental conditions for the direct evaporation of refractory
compounds. Evaporation can occur with or without dissociation of the compound into
fragments. As seen from Table 4.8, the observed vapor species show that very few compounds
evaporate without dissociation. Examples are SiO, MgF
2
,B
2
O
3
, CaF
2
, and other group IV
divalent oxides (SiO homologs like GeO and SnO).
In the more general case, when a compound is evaporated or sputtered, the material is
transformed to the vapor state not as compound molecules but as fragments thereof.
Subsequently, the fragments have to recombine, most probably on the substrate, to reconstitute
the compound. Therefore, the stoichiometry (anion:cation ratio) of the deposit depends on
several factors including the deposition rate and the ratios of the various molecular fragments,
the impingement of other gases present in the environment, the surface mobility of the
fragments (which in turn depends on their kinetic energy and substrate temperature), the mean
residence time of the fragments of the substrate, the reaction rate of the fragments on the
substrate to reconstitute the compound, and the impurities present on the substrate. For
example, it was found that direct evaporation of Al
2
O
3
resulted in a deposit which was
deficient in oxygen, i.e. which had the composition Al
2
O
3–x
[128]. This O
2
deficiency could be
made up by introducing O
2
at a low partial pressure into the environment. In other cases, for
example the direct evaporation of TiB
2
and ZrB
2
, the deposit contains both the monoboride
and diboride phases [129].
4.8.4.2 Reactive Evaporation
The difficulties involved in direct evaporation processes due to fragmentation of the vaporized
compounds are overcome in reactive evaporation where a metal is evaporated in the presence
of the reactive gas; the compound is formed by reaction of the evaporated metal species with
the molecules of the reactive gas. Though this technique has been extensively used to deposit a
variety of oxide films for optical applications, it is generally observed that the films are
deficient in oxygen. It is also observed in some cases, especially in the synthesis of carbide
films, that the deposition rate becomes a limiting factor governing the growth of the films. In
such cases, stoichiometric TiC films could only be deposited at very low rates (∼ 1.5
˚
A/s max.)

194 Chapter 4
Table 4.8: Direct evaporation of inorganic compounds
Compound
Vapor species observed
(in order of decreasing
frequency)
mp,
◦
C T,
◦
C, at which
p*=10
−7
Torr
Comments on actual evaporation
temperatures, support materials used,
and related experience
Oxides
Al
2
O
3
Al, O, AlO, Al
2
O, O
2
,
(AlO)
2
2030 ∼1800 From W and Mo supports at 1850–2250
◦
C.
With telefocus gun at 2200
◦
C, no
decomposition
From W support: Al
2
O
3
films have small
oxygen deficits. O
2
-dissociation pressure at
1780
◦
C: 1.5 × 10
−15
torr
B
2
O
3
B
2
O
3
450 ∼1700 From Pt and Mo supports at 940–1370
◦
C
BaO Ba, BaO, Ba
2
O,
(BaO)
2
,Ba
2
O
3
,O
2
1925 1540 From Al
2
O
3
crucible at 1200–1500
◦
C. From
Pt crucible with only slight decomposition,
pO
2
(1540
◦
C) = 3.5 × 10
−15
torr
BeO Be, O, (BeO)
n
,
n = 1–6, Be
2
O
2530 2230 From W support at 2070–2230
◦
C. With
telefocus gun at 2400–2700
◦
C, no
decomposition
Bi
2
O
3
– 817 1840 From Pt support
CaO Ca, CaO, O, O
2
∼2600 ∼2050 Support materials: ZrO
2
, Mo, W. The latter
two form volatile oxides, molybdates, and
wolframates at 1900–2150
◦
C
CeO
2
CeO, CeO
2
1950 – From W support without decomposition
In
2
O
2
In, In
2
O, O
2
– – From Pt support with only little
decomposition. Vapor species observed at
1100–1450
◦
C. At 1000–1450
◦
CfromAl
2
O
3
crucible, more In
2
O than In
MgO Mg, MgO, O, C
2
2800 ∼1560 Mo or W supports at 1840–2000
◦
form
volatile oxides, molybdates, and wolframates.
With telefocus gun at 1925
◦
C, no
decomposition. From Al
2
O
3
at 1670
◦
C
(Continued)

Evaporation 195
Table 4.8: (Continued)
Compound Vapor species observed
(in order of decreasing
frequency)
mp,
◦
C T,
◦
C, at which
p*=10
−7
Torr
Comments on actual evaporation
temperatures, support materials used,
and related experience
MoO
2
(MoO
3
)
3
, (MoO
3
)
n
,
n = 4.5
795 610 From Mo oven at 500–700
◦
C, the trimer is
the main species. Above 1000
◦
C, there is
some decomposition into MoO
2
(s)+O
2
(g).
At 730
◦
C, the oxygen-decomposition
pressure is 1.1 × 10
−14
torr. From Pt at
530–730
◦
C
NiO Ni, O
2
, NiO, O 2090 1586 From Al
2
O
3
crucible at 1300–1440
◦
C. Heavy
decomposition with po
2
=4× 10
−1
torr at
1586
◦
C
Sb
2
O
3
– 656 ∼450 Lower oxides result if evaporated from W
supports. Pt heaters do not produce
decomposition
SiO SiO – 1025 Usually evaporated from Ta or Mo heaters at
residual gas pressure below 10
−6
torr and at
temperatures between 1150 and 1250
◦
C.
Dissociation into Si and O
2
begins above
1250
◦
C and may lead to oxygen-deficient
films
SiO
2
SiO, O
2
1730 ∼1250 With telefocus gun at 1500–1600
◦
C, no
decomposition. Ta, Mo, W supports are
attacked by SiO
2
and contribute volatile
oxides. From Al
2
O
3
at 1630
◦
C, SiO: vapor
species is present
SnO
2
SnO, O
2
– – From SiO
2
crucible at 975–1250
◦
C. Films
directly evaporated from W support are
slightly oxygen deficient

196 Chapter 4
Table 4.8: (Continued)
Compound Vapor species observed
(in order of decreasing
frequency)
mp,
◦
C T,
◦
C, at which
p*=10
−7
Torr
Comments on actual evaporation
temperatures, support materials used,
and related experience
SrO Sr, O
2
, SrO 2460 ∼1760 From Al
2
O
3
at 1830
◦
C. Evaporation from
Mo or W at 1700–2000
◦
C produces volatile
Mo and W oxides, molybdates, and
wolframates
TiO
2
TiO, Ti, TiO
2
,O
2
1840 – TiO
2
source material decomposes into lower
oxides upon heating. po, at 2000
◦
Cis
10
−10
torr. Nearly stoichiometric films by
pulsed electron-beam heating
WO
2
(WO
2
)
3
,WO
2
1473 1140 From Pt oven at 1040–1300
◦
C. From Pt
support at 1220
◦
C. From W heater with only
slight decomposition; po
3
at 1120
◦
Cis
3 × 10
−10
torr
ZrO
2
ZrO, O
2
2700 – From Ta support at 1730
◦
C, volatile TaO.
From W support, oxygen-deficient films. ZrO
2
source material loses oxygen when heated by
electron beams
Sulfides, Selenides, Tellurides
ZnS – 1830 (p ≈ 150 atm) 1000 From Mo support. Minute deviations from
stoichiometry if allowed to react with residual
gases. From Ta at 1050
◦
C
ZnSe – 1520 (p ≈ 2 atm) 820
CdS S, Cd, S, Si, Su 1750 (p ≈ 100 atm) 670 From Pt oven at 740
◦
C. Films tend to deviate
from stoichiometry. Suitable support
materials: graphite, Ta, Mo, W, SiO
2
,
Al
2
O
3
–coated W: evaporation at 600–700
◦
C
CdSe Se
2
, Cd 1250 660 From Al
2
O
3
crucible

Evaporation 197
Table 4.8: (Continued)
Compound Vapor species observed
(in order of decreasing
frequency)
mp,
◦
C T,
◦
C, at which
p*=10
−7
Torr
Comments on actual evaporation
temperatures, support materials used,
and related experience
CdTe Te
2
, Cd 1100 570 From Ta boat at 750–850
◦
C: film
stoichiometry depends on condensation
temperature
PbS PbS, Pb, S
2
, (PbS)
2
1112 675 From quartz crucible at 625–925
◦
C. From
Mo support. Purest films from quartz furnace
at 700
◦
C; Fe or Mo boats reacts and form
volatile sulfides
Sb
2
S
3
– 546 550 From Mo support
Sb
2
Se
3
Sb
2
, (SbSe)
2
, (Sb
2
),
(SbSe)
611 From graphite at 725
◦
C. From Ta oven at
500–600
◦
C, fractionation and films of
variable stoichiometry
Halides
NaCl NaCl, (NaCl)
2
,
(NaCl)
3
801 670 From Ta, Mo, or Cu ovens at 550–800
◦
C
KCl KCl, (KCl)
2
772 635 From Ni or Cu oven at 500–740
◦
C
AgCl AgCl, (AgCl)
3
455 690 At 710–770
◦
C. From Mo support,
p*=10
−2
torr at 790
◦
C
MgF
2
MgF
2
, (MgF
2
)
2
,
(MgF
2
)
3
1263 1130 From Pt oven at 950–1230
◦
C. From Mo
support. Very little dissociation into the
elements
CaF
2
CaF
2
, (CaF) 1418 ∼1300 From Ta oven at 980–1400
◦
C. From Mo
support
PbCl
2
– 678 ∼430 Direct evaporation possible
From Handbook of Thin Film Technology [7]. Copyright © 1970, McGraw-Hill. Used with permission of McGraw-Hill Book Company.
198 Chapter 4
[130]. This limitation of deposition rate in the case of the reactive evaporation process is due to
the reaction kinetics of the compound formation by this process. The presence of a ‘plasma’ in
the ARE process influences the reaction kinetics by providing activation energy to the reactive
species, thereby making it possible to synthesize compound films at considerably higher rates
[131–133] and lower temperatures.
Hass et al. studied the single and multiple source effect on metal oxide coatings (CeO
2
, ZrO
2
,
Y
2
O
3
) by e-beam directed vapor deposition (DVD) [134]. In this approach, in order to make
the deposition uniform, transonic helium carrier gas was used with the e-beam to transport
metal vapor to a substrate. Metal oxide coatings were then produced by adding oxygen to the
carrier gas. Their model is based on the vapor pressures of each individual evaporation source.
According to their result the phase morphology is similar between a single metal source of
zirconia and reactive deposition of yttria and zirconia source samples.
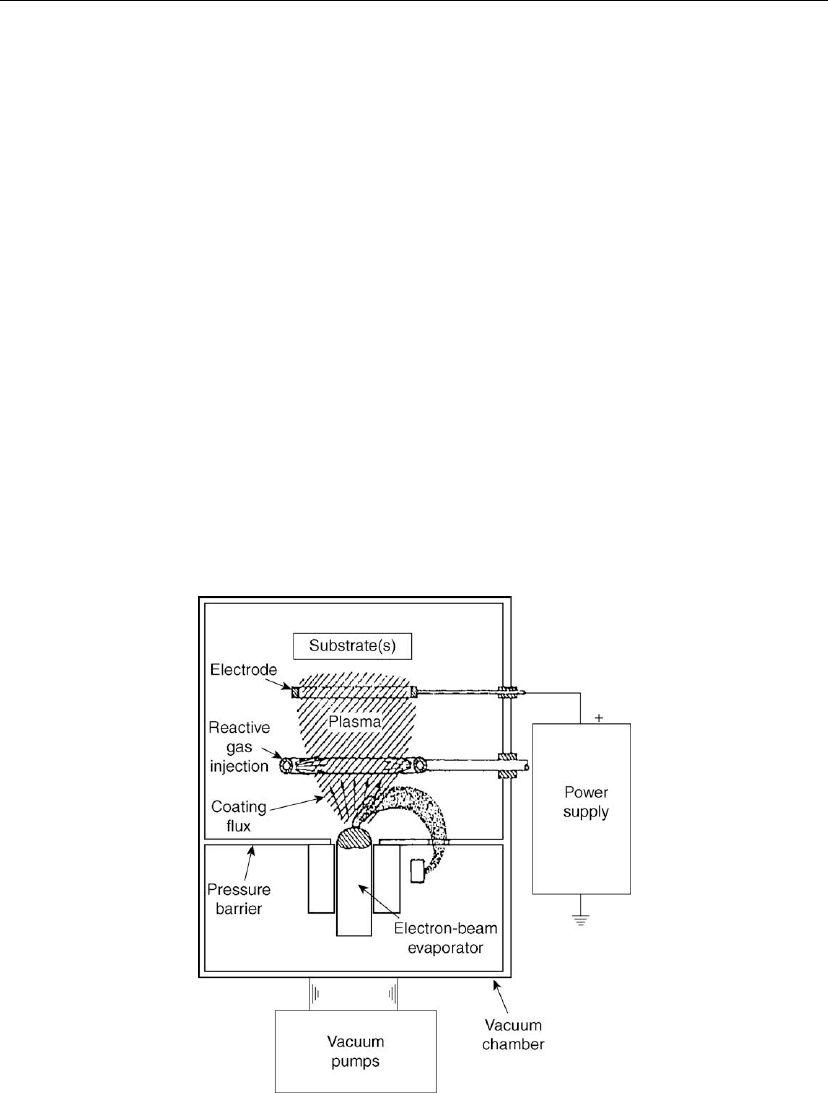
4.8.4.3 Activated Reactive Evaporation
The ARE process generally involves evaporation of a metal or an alloy in the presence of the
plasma of a reactive gas [131, 135]. For example, TiC and TiN coatings are deposited by this
process by evaporating Ti in the presence of C
2
H
2
and N
2
plasma, respectively. The two basic
variants of the ARE process are shown in Figures 4.42 and 4.43. For more information on the
ARE process, refer to a review by Bunshah and Deshpandey [132]. The role of the plasma in
Figure 4.42: Schematic of the activated reactive evaporation (ARE) process.
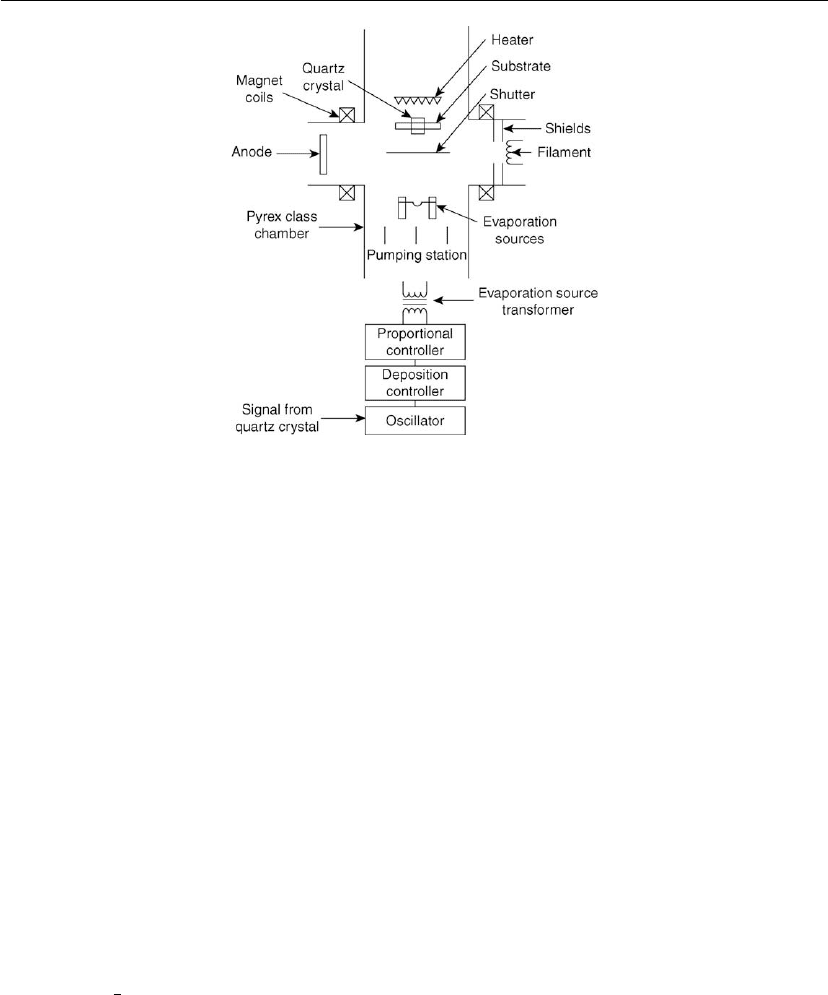
Evaporation 199
Figure 4.43: Activated reactive evaporation (ARE) Process [136] using a resistance-heated
evaporation source.
this process is two-fold:
to enhance the reactions that are necessary for deposition of compound films
to modify the growth kinetics and hence the structure/morphology of the deposits.
In the following section we discuss the above two aspects.
Thermodynamic and kinetic considerations in plasma-assisted deposition processes
For the formation of a compound by any chemical reaction, the corresponding thermodynamic
and kinetic constraints must be satisfied which also apply to the deposition of refractory
compound films by reactive evaporation. In order to understand the role of plasma in
enhancing the chemical reactions essential for the formation of a particular compound, one has
therefore to consider the kinetics of these reactions.
Let us consider the reactions involved in the synthesis of some oxides, carbides, and nitrides
by reactive evaporation. Given below are the reactions for forming Al
2
O
3
, TiC, and TiN.
2Al +
3
2
O
2
→Al
2
O
3
G
◦
=−250 kcal (mol O
2
)
2
at 298 K
2Ti + C
2
H
2
→2TiC + H
2
G
◦
=−7.65 kcal (mol O
2
)
−1
at 298 K
2Ti + N
2
→2TiN G
◦
=−73.5 kcal (mol N
2
)
−1
at 298 K
As can be seen from the above reactions, the thermodynamic criterion of free energy of
formation is satisfied for the respective compounds.

200 Chapter 4
The reaction kinetics in reactive evaporation process can be treated in exactly the same manner
as for reactions occurring in heterogeneous systems of condensed phases. The model for
heterogeneous metallurgical kinetics involves: (1) transport of reactant to the reaction
interface; (2) transport of reaction products away from the reaction interface; (3) the chemical
reaction at the chemical interface; (4) the nucleation of new phase; and (5) heat transfer to or
away from the reaction interface.
For reactive evaporation, this model may be depicted as follows (e.g. for TiC formation):
Reactants
Products
Ti (metal atoms) TiC (deposit)
C
2
H
2
(gas) H
2
(gas)
Reaction interface
On the basis of the above model, the rate-controlling steps in the reactive evaporation process
are: (1) adequate supply of reactants; (2) adequate collision frequency; (3) the rate of chemical
reactions at the interface; and (4) the rate of removal of the reaction products from the interface.
It is easy to satisfy (1), (2), and (4) above for a reactive evaporation process. However,
condition (3), i.e. the rate of reaction, becomes the rate governing step. The ‘plasma’ in the
ARE process influences this step, i.e. the rate of reaction, by providing the necessary activation
energy to the reactive species. The effect of plasma on rate of reaction can be clearly
demonstrated by considering the results of Abe et al. [130] and Bunshah and Raghuram [133]
on deposition of TiC coatings. Abe et al. found that titanium carbide with a carbon to titanium
ratio of 1 could be formed by a reaction between Ti and C
2
H
2
or C
2
H
4
molecules on a
substrate at 300–500
◦
C only if the deposition rate is 1–1.5
˚
A/s. At higher deposition rates, no
TiC was formed. Clearly, the activation barrier could not be overcome at the higher deposition
rates. Bunshah and Raghuram [133, 137] have similarly reported that the deposition of TiC by
reactive evaporation at higher deposition rates (150–200
˚
A/s) required a very high substrate
temperature, exceeding 1000
◦
C. However, in the presence of plasma, these authors reported
that it was possible to deposit TiC at a high rate at a relatively low substrate temperature. The
plasma imparts sufficient energy to the reacting species to overcome the activation energy
barrier, and hence condition (3), i.e. the rate of reaction, no longer remains the rate-governing
step.
Basic variants of the ARE process
The two basic variants of the ARE process are ARE with an e-beam evaporation source [131]
and ARE processes with a resistance-heated source [138].
ARE processes with an electron-beam-heated evaporation source are illustrated in Figure
4.42. In this process, the metal is heated and melted by a high-acceleration-voltage e-beam that
