Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Evaporation 211
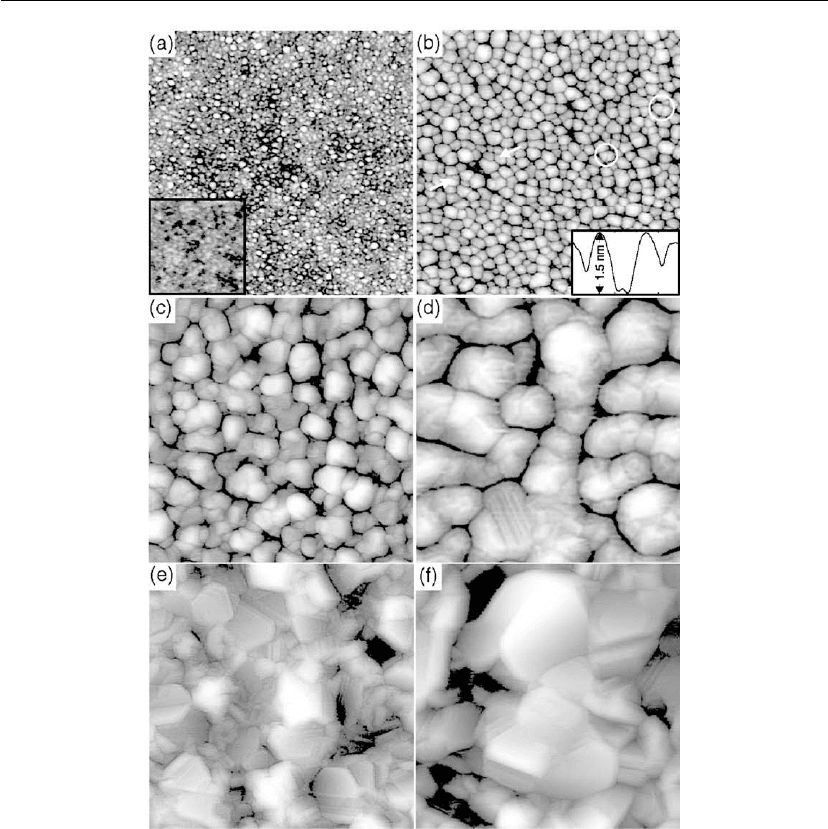
Figure 4.47: STM topographs of Ag films deposited at 300 K with thicknesses of (a) 0.3 nm,
(b) 1.0 nm, (c) 2.7 nm, (d) 8.5 nm, (e) 15.9 nm, and (f) 30.0 nm. Image size = 160 × 160 nm. The
inset in (a) shows an STM image (60 × 60 nm) of the amorphous substrate, and in (b), a line scan
between the arrows in the topograph, which reaches down to the substrate. The circles in
(b) enclose islands with GBs.
thicknesses of 1{1/2} atomic layers. He further observed that chemisorption of even a half
monolayer of oxygen severely inhibited epitaxial growth.
Sherman et al. [185] studied the deposition of thick Mo films onto a rolled Mo sheet substrate
as a function of deposition temperature. They observed polycrystalline deposits at all
212 Chapter 4
Figure 4.48: Cross-sectional SEM images of the CuInS
2
thin films deposited at different flux
angles: (a) =40
; (b) =80
without substrate rotation; and (c) =80
o
with substrate
rotation ω = 0.033 rev/s.
temperatures except in the range of 973–1188 K, where the surface oxide MoO
3
is unstable
and evaporates rapidly, thereby leaving behind a ‘clean’ Mo surface on which epitaxial growth
can readily occur aided by the high surface mobility at the elevated deposition temperature.
Once a continuous film has formed, the subsequent evolution to the final structure of the thin
film is poorly understood at present. It undoubtedly depends on the factors mentioned above,
which in turn influence the primary variables of nucleation rate, growth rate, and surface
mobility of the adatom. The problem has been tackled by Van der Drift [186] and is also the
subject of a paper by Thornton [187].
The microstructure and morphology of thick single-phase films have been extensively studied
for a wide variety of metals, alloys, and refractory compounds. The structural model was first
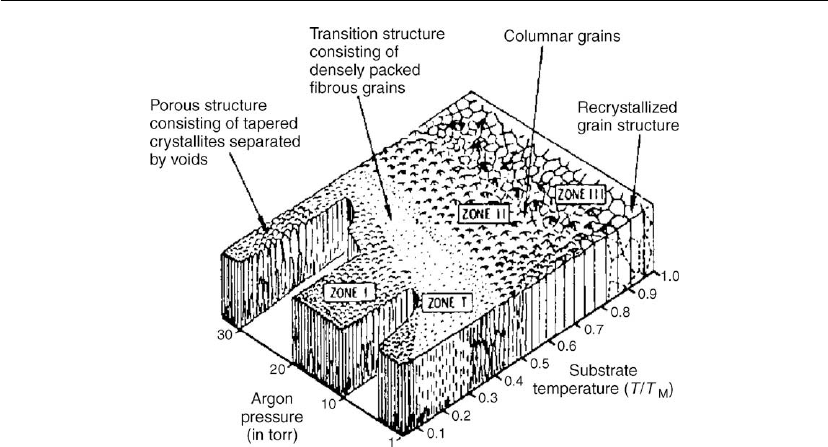
proposed by Movchan and Demchishin [123] (Figure 4.49), and was subsequently modified by
Figure 4.49: Structural zones in condensates [123].
Evaporation 213
Figure 4.50: Structural zones in condensates [187].
Thornton as shown in Figure 4.50. Movchan and Demchishin’s diagram was arrived at from
their studies on deposits of pure metals and did not include the transition zone of Thornton’s
model, zone T, which is not prominent in pure metals or single-phase alloy deposits, but
becomes quite pronounced in deposits of refractory compounds or complex alloys produced
by evaporation, and in all types of deposits produced in the presence of a partial pressure of
inert or reactive gas, as in sputtering or ion-plating processes.
The evolution of the structural morphology is as follows.
At low temperatures, the surface mobility of the adatoms is reduced and the structure grows as
tapered crystallites from a limited number of nuclei. It is not a full-density structure but
contains longitudinal porosity of the order of a few hundred angstroms’ width between the
tapered crystallites. It also contains a high dislocation density and has a high level of residual
stress. Such a structure has also been called ‘Botryoidal’ and corresponds to zone 1 in Figures
4.49 and 4.50.
As the substrate temperature increases, the surface mobility increases and the structural
morphology first transforms to that of zone T, i.e. tightly packed fibrous grains with weak
grain boundaries, and then to a full-density columnar morphology corresponding to zone 2
(Figure 4.50).
The size of the columnar grains increases as the condensation temperature increases. Finally,
at still higher temperatures, the structure shows an equiaxed grain morphology (zone 3). For

214 Chapter 4
pure metals and single phase alloys, T1 is the transition temperature between zone 1 and zone
2 and T2 is the transition temperature between zones 2 and 3. According to Movchan and
Demchishin’s original model [123], T
1
is 0.3 T
m
for metals, and 0.22–0.26 T
m
for oxides,
whereas T
2
is 0.45–0.4 (T
m
is the melting point in K).
Thornton’s modification shows that the transition temperatures may vary significantly from
those stated above and, in general, shift to higher temperatures as the gas pressure in the
synthesis process increases. It should be emphasized that:
The transition from one zone to the next is not abrupt but smooth. Hence the transition
temperatures should not be considered as absolute, but as guidelines.
All zones are not found in all deposits. For example, zone T is not prominent in pure
metals, but becomes more pronounced in complex alloys, compounds, or in deposits
produced at higher gas pressures. Zone 3 is not seen very often in materials with high
melting points.
The reader is referred to a more extensive description given by Greene in Chapter 12, which
includes a discussion of the effects of substrate surface roughness and pressures.
Most thick deposits exhibit a strong preferred orientation (fiber texture) at low deposition
temperatures and tend toward a more random orientation with increasing deposition
temperature. Figure 4.51 shows the evolution of a large-grained columnar morphology in a Be
deposit from a much larger number of fine grains which were originally nucleated on the
substrate. As growth proceeds, only those grains with a preferred growth direction survive,
presumably owing to considerations of the minimization of surface energy.
Figure 4.51: Photomicrograph of a Be deposit showing the evolution of large columnar grains.
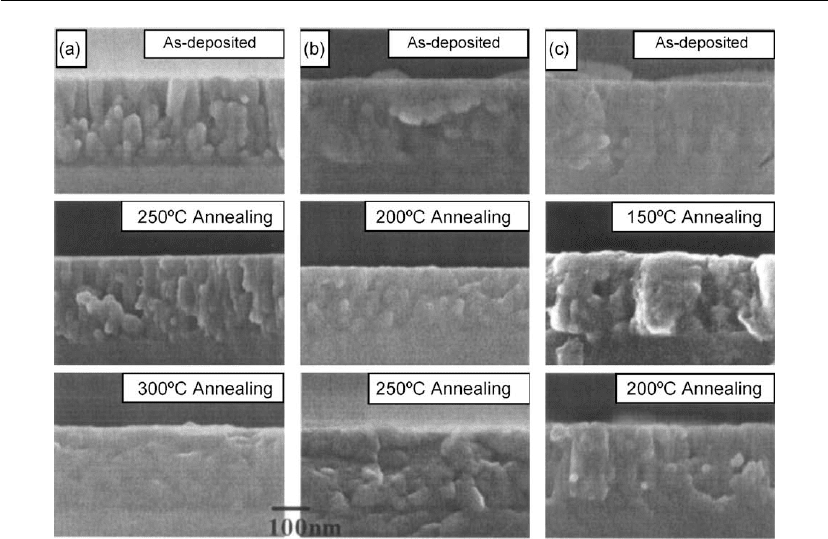
Evaporation 215
Figure 4.52: SEM micrographs of TiO
2
films annealed at various temperatures. Substrate
temperatures: (a) 150
C, (b) 200
C, (c) 250
C.
Chen et al. studied the effects of deposition temperature on the microstructure of TiO
2
films.
Films were deposited by ion assisted deposition in which e-beam evaporation was used to
evaporate Ti
3
O
5
and a Kaufman-type ion-beam source was used to modify the morphology of
the films [188]. Figure 4.52 shows the microstructure of the as-deposited 150
◦
C film which
has a columnar structure. The microstructure persisted up to the annealing temperature of
300
◦
C. The increase in deposition temperature affected the columnar growth of the film. As
the deposition temperature increased columns became denser. The as-deposited samples show
different recrystallization temperatures. The crystallization temperature was determined from
SEM images, X-ray diffraction (XRD) data and refractive indices of the films. For films
deposited at 150, 200 and 250
◦
C, the crystallization temperature was found to be 300, 250 and
200
◦
C, respectively.
Elegant proof of the importance of surface mobility was also provided by Movchan and
Demchishin [123]. Plots of the log of the grain diameter versus the inverse of deposition
temperature in zones 2 and 3 yield straight lines from which activation energies can be
computed. It was found that the activation energy for zone 2 growth corresponded to that for
surface self-diffusion and for zone 3 growth to volume self-diffusion. The morphological
results reported by Movchan and Demchishin for nickel, titanium, tungsten, Al
2
O
3
, and ZrO
2
216 Chapter 4
Table 4.9: Transition temperatures between various structural zones
Material Melting
temp., T
m
(K)
T
1
(K) T
1
(K)/T
m
(K) T
2
(K) T
2
(K)/T
m
(K) Ref.
Ti 1945 653 ± 10 0.3 923 0.5 [98]
Ti 1945 673 ± 20 0.31 Phase
transformation
overlaps
– [113]
Ni 1726 543 ± 10 0.3 723 ± 10 0.45–0.5 [98]
Ni 1726 – – 777 ± 20 0.45 [114]
W 3683 1133 ± 50 0.3 1723 ± 50 0.45–0.5 [98]
Mo 2883 923 ± 20 0.3–0.34 1200 0.44 [189]
Fe 1810 – – Phase
transformation
overlaps
– [190]
Be 1573 473 ± 20 0.29 1023 ± 50 0.63 [191]
Ni–2OCr 1673 500 ± 50 0.3 870 0.52 [192]
ZrO
2
2973 648 ± 10 0.22 1273 0.45–0.5 [98]
Al
2
O
3
2323 623 ± 10 0.26 1173 0.5 [98]
TiC 3340 1070 ± 30 0.31 Not observed
up to 1723 K or
0.51 T
m
– [113]
NbC Obeys the Movchan–Demchishin model [193]
ZrO
2
Obeys the Movchan–Demchishin model [193]
From structures observed at a specific deposition temperature, Au–Cr
58
and V
37
appear to obey the Movchan–Demchishin
model.
have been confirmed for several metals and compounds. The data are given in Table 4.9
[29, 30, 194, 195].
Bunshah and Juntz [196] studied the influence of condensation temperature on the deposition
of titanium. Their microstructures, shown in Figure 4.53, agree substantially with those of
Movchan and Demchishin for zones 1 and 2 and T
1
, the transition temperature between zones
1 and 2. However, they failed to observe zone 3 at the temperatures above 700
◦
C found by
Movchan and Demchishin [123]. The structure was columnar up to 833
◦
C, which is the α:β
phase transformation temperature for titanium. At deposition temperatures above 833
◦
C, the
deposit crystallizes as the β phase and on cooling to room temperature, should transform to the
α phase, resulting in the typical ‘transformed-beta’ microstructure shown in Figure 4.53
(900
◦
C deposit), which could be mistaken for an equiaxed microstructure. Hence, the claim of
such a transition in structure from zone 2 to 3 by Movchan and Demchishin for titanium
deposits is confusing.
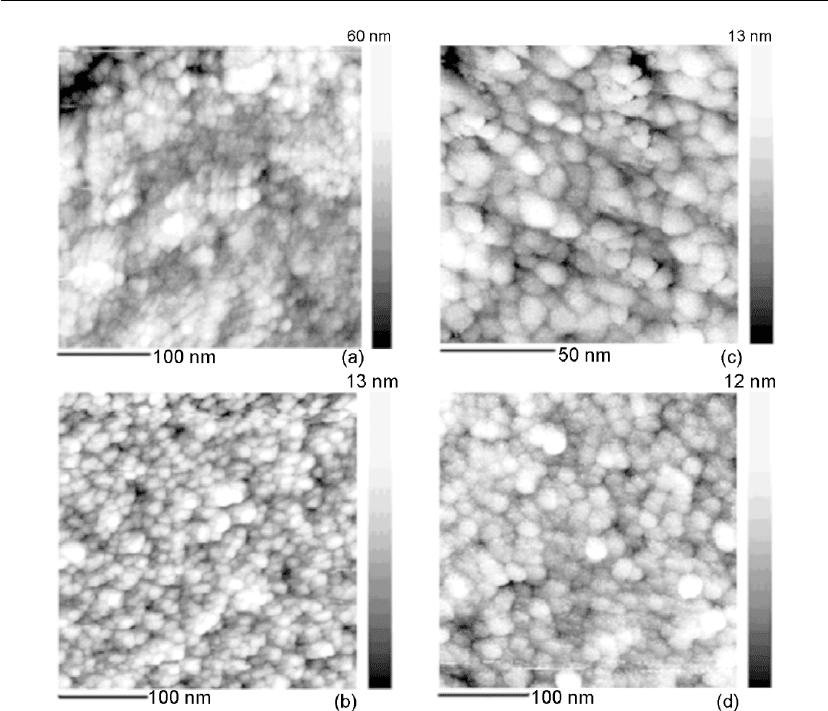
Evaporation 217
Figure 4.53: Structure of titanium deposits at various substrate temperatures [200].
Semaltianos and Wilson [197] investigated the surface morphology of thermally evaporated
gold thin films on mica, glass, silicon, and calcium fluoride substrates, and at different
temperatures. According to Figure 4.54, the average particle size of gold films is 15, 20, and
25 nm for mica, CaF
2
, and Si substrates. However, for glass substrates large accumulations of
gold are formed consisting of many smaller particles. The increase in particle size for Si and
CaF
2
can be due to ionic interaction between the substrate and the film.
Kane and Bunshah [198] observed the change in morphology in deposited nickel sheet. At
425
◦
C deposition temperature, the deposit showed a zone 2 morphology, whereas at 554
◦
C,
the deposit showed a zone 3 morphology.
Chambers and Bower [199] studied the deposition of magnesium, copper, gold, iridium,
tungsten, and stainless steel. Of the photomicrographs presented, gold and magnesium showed
zone 2 columnar morphology at the appropriate substrate temperatures.
218 Chapter 4
Figure 4.54: STM images of gold films grown on (a) glass, (b) mica, (c) CaF
2
, and (d) Si with the
substrates held at room temperature.
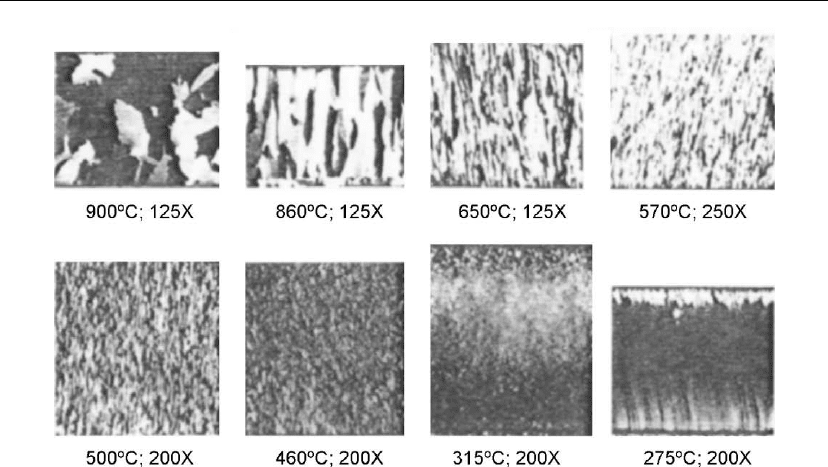
Figure 4.55 shows surface and cross-section photomicrographs of a Ni–20Cr sheet deposited
by Agarwal et al. [194].At950
◦
C, 760
◦
C, 650
◦
C, and 427
◦
C deposition temperatures, the
surface and cross-section showed an equiaxed zone 3 morphology.
Mah and Nordin [201] found that the Movchan–Demchishin model was obeyed by beryllium.
They observed structures corresponding to all three zones with transition temperatures as
predicted by the model.
Neirynck et al. [202] studied the influence of deposition rate and substrate temperature on the
microstructure, adhesion, texture, and condensation mechanism of aluminum and zirconium
coatings on steel substrates and wires in batch and continuous-coating methods.
Kennedy [203] showed a change in morphology from columnar to equiaxed in Fe and
Fe–10Ni alloy with higher deposition temperature. Deposits of Fe–1%Y, which is a two-phase
alloy, showed columnar morphology only, the structure becoming coarser at higher deposition
temperature. The second phase appears to nucleate new grains so that the grain size in
Fe–1%Y alloys is much finer than that of iron.
The microstructure of copper–nickel alloys [31] produced by co-deposition from two sources
showed a single phase, as might be expected for this system, which shows a complete solid
solubility. On the other hand, sequential deposition of Cu and Ni from two sources shielded
from each other onto a rotating substrate produced a microlaminate structure in the deposit
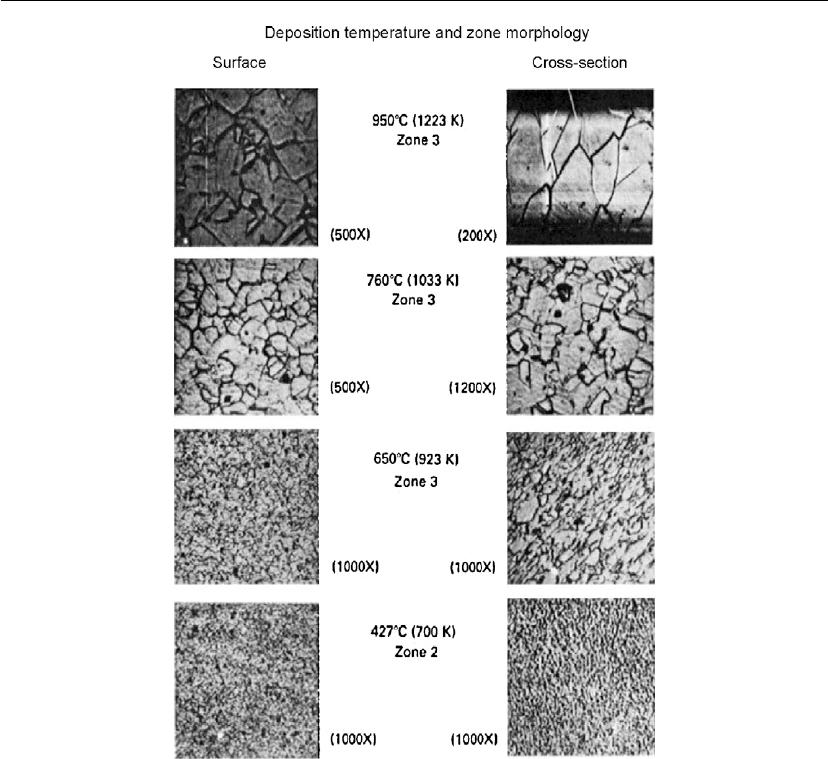
Evaporation 219
Figure 4.55: Photomicrographs of typical Ni–20Cr deposits at various substrate temperatures
[194].
where the laminate size could be varied from 0.01 to 40 m by adjusting the deposition
parameters [204]. Similar structures were also developed in the Fe–Cu [204] and Ti–B
4
C
systems [204].
ln alloy systems showing the presence of several phases, e.g. Ni–B and Cr–Si, the deposits
showed that the phases present corresponded to those expected from the diagram [31].
Smith et al. [27] studied the deposition of the two-phase (α + β) type Ti–6Al–4V alloy
deposited from a single rod-fed source. The microstructure was very similar to wrought
material with the same characteristic α + β morphology present on a finer scale in the
deposited material.

220 Chapter 4
Dispersion-strengthened alloys produced by co-deposition from multiple sources have also
been produced. Paton et al. [31] produced Ni–TiC, Ni–NbC and Ni–ZrO
2
alloys. The particle
size increased from 100 to 1000
˚
A by changing the deposition temperature from 350 to
1000
◦
C. The size of the dispersed carbide phase particles increased on annealing at
1000–1100
◦
C owing to their slight solubility in nickel. On the other hand, the size and
distribution of ZrO
2
dispersion remained constant even after exposure at 1300
◦
C for 5 h, as
shown in Figure 4.56.
Movchan et al. [205] produced Fe–NbC and Fe–Ni–NbC dispersion strengthened alloys by
co-evaporation. The microstructure exhibited columnar morphology, with the inclusion of a
fine dispersion of NbC particles.
Raghuram and Bunshah [206] studied the microstructure of TiC deposits from 500 to 1450
◦
C
(Figure 4.57). They observed the transition from the tapered crystallite (zone 1) to columnar
structure at 973 K, or 700
◦
C (0.3 T
m
). The highest deposition temperature (1450
◦
C) used by
these investigators was not sufficient to produce an equiaxed structure, although this
temperature corresponds to 0.51 T
m
.
Xu et al. investigated the growth texture of MgO films deposited by e-beam deposition at
different inclination angles [207] using SEM. The results are shown in Figure 4.58. Their
study showed that the deposition rate increases with the increasing inclination angle. However,
the surface loses its smoothness and shows more voids at higher angles, especially after 50
◦
.
The energy of the depositing beam of atoms can be increased if some of them are ionized. It
has been shown [23] that a small fraction of the vaporized species from an e-beam-heated
source is ionized owing to collisions with electrons in the plasma sheath above the molten
pool. Bunshah and Juntz [200] biased the substrate to −5000 V during the deposition of
beryllium at 570
◦
C and found that the columnar grain size was markedly refined by the ion
bombardment as compared to the grain size produced without biasing the substrate at the same
deposition temperature. It may be postulated that the ion bombardment causes a localized
increase in temperature at the surface where deposition is occurring, thus causing a higher
nucleation rate and a finer grain size. Similar results have been reported for tantalum [208].
The use of a hollow cathode gun intensifies the degree of ionization of the vapor species,
resulting in a marked increase in kinetic energy of the vaporized atoms [209]. The effects of
substrate bias are, therefore, easier to observe. Increasing the substrate bias results in a change
in morphology from columnar to fine, equiaxed grains for silver deposited on beryllium and
stainless steel [210], and silver and copper deposited on stainless steel [211].
The presence of a gas at high pressures (5–20 m) results in a net decrease in kinetic energy of
the vaporized atoms due to multiple collisions during the transverse from source to substrate.
This degrades the microstructure to lose columnar grains [211] and eventually to an
agglomerate of particles. (This is a way to produce fine powders by evaporation and
