Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.


Chemistry
325
The aniline
cloud
point
is a measure of the paraffinicity
of
a fuel oil. A high value
denotes a highly paraffinic oil while a low value indicates an aromatic, a naphthenic,
or a highly cracked oil. Theflush
point
represents the temperature
to
which a liquid
fuel can be heated before a flash appears on its surface upon exposure to a test flame
under specified conditions. A knowledge of the flash point is needed to ensure safe
handling and storage without fire hazards.
API
gravity.
The specific gravity of petroleum
or
petroleum products is often
expressed in terms of "degrees API" on a scale defined by
"API
=I-
141e5
131.5
ti
where
G
is the specific gravity of the liquid at
6OoF,
with reference to water at
60°F.
Thus, an API gravity of
10"
corresponds to
G
=
1.
U.O.P.
Characterization Factor
(K).
This is used as a qualitative index of the
paraffinicity of an oil stock. By definition,
K
=
(TB)'/3/G,
where T,
is
the average
boiling point
(OR)
at one standard atmosphere, and
G
is the specific gravity at
60°F.
The U.O.P. characterization factor has been found to vary within a homologous series;
it is therefore not a true measure of the hydrocarbon type. Nevertheless, graphical
correlations have been developed between
K,
API
gravity, molecular weights,
T,,
and critical temperatures for a number of petroleum fractions [Refer to
K.
M.
Watson
and
E.
F.
Nelson, Ind. Eng. Chem.,
25,
p.
880
(1933)l.
Characterization factors have
also been correlated with kinematic viscosities,
API
gravity, and
T,.
Physical Chemistry
Basic Definitions
The
formula
weight
of an element (or a compound
or
a species) is obtained as the
sum of the weight contributions from the constituent atoms making up its chemical
formula. The formula weight
of
an element
is
its atomic weight and that of a compound
is its molecular weight.
The
equivalent weight
of an ion
(or
an element) is the ratio of its formula weight to
its valence. According to an alternative definition that is also suitable for compounds,
an equivalent weight represents the amount of a substance which will react with one
atomic weight of hydrogen
or
its chemical equivalent.
One
gramatom
(or pound-atom)
is
the mass
in
grams
(or
pounds) of a given element
that
is
numerically equal to its atomic weight.
Thus,
the number of gram-atoms of
an
elementary substance is m/A, where m is the mass (in grams) and A, its atomic weight.
One
grum-mole
(or
pound-mole) is the mass in grams (or pounds) of a given
compound that
is
numerically equal to its molecular weight
(M).
Thus, the number
of
pound-moles of a compound contained in a mass
of
m pounds is m/M.
One
grum-equivulent
(or pound-equivalent) represents the mass in grams
(or
pounds)
of a material that is numerically equal to its equivalent weight.
Avogadro's
number
represents the number
of
atoms (or molecules) present in one
gram-atom
(or
gram-mole) of
any
elementary substance
(or
compound). It has
a
value
of
6.02205
x
loz3
[63].
The
density
(p)
of a substance is defined as its mass per unit volume, expressed as
g/cm3, lb/ft3, etc. The
specific volume
(
v
)
of a substance is the reciprocal of its density
and
is
expressed as cms/g, ft3/lb, etc.

326
General Engineering and Science
Thespecdficggmvity
(G)
of
a substance is the ratio of the density
(p)
of the substance
to the density
(pmf)
of a reference substance at specified conditions. That is,
For solids and liquids, the density is
a
weak function of pressure and, therefore, the
temperatures T and Tcef are usually stated.
Also,
the reference substance is commonly
taken as water at
4°C
at which
prer
=
1.000
g/cm’
=
62.43
Ib/ft’. If a single temperature
is stated, it implies that both densities have been measured at that temperature.
In the case of gases and vapors, it is imperative that the temperature as well as
pressures be clearly indicated in referring to specific gravity. Air is normally chosen
as the reference fluid for gaseous substances.
Compositions
of
Multicomponent Systems
The relative amounts of the individual components (or species) making up a mixture
or solution can be expressed in a variety of ways, depending upon the system at
hand.
A
volumetric, mass, or molar basis may be employed to represent the
compositions of multicomponent systems.
Volumetric Basis.
The volume fraction
(vi)
of component
i
in a mixture is the fraction
of the total volume
(V)
of the mixture that is attributable to that component, at the
stated temperature and pressure. Thus, if
Vi
denotes the actual volume of component
i present in a total volume
V
of the mixture, then
and the volume percent of
i
=
lobi.
Instead, if
V
represents the volume of one mole
of the mixture, then
Vi
must be interpreted as the “partial molar volume of com-
ponent
i”
in the mixture.
The above method is commonly used for gases and infrequently for liquid mixtures.
At atmospheric conditions when ideal gas behavior
is
realized, the total volume of the
mixture equals the sum of the pure-component volumes
(
Vp).
That is,
V
=
Vu
and
Vi
=
Vp.
In such a case, Vpcan be obtained directly from the ideal gas law,
without recourse to measurement, and hence, the volumetric composition can be
readily computed. On the other hand, in non-ideal (i.e., real) mixtures and solutions,
V
#
Vp
and the measurement
of
the actual component volumes
Vi
becomes a difficult
undertaking. In these systems, a volume change-either shrinkage or expansion-is
experienced upon mixing of the components. In addition, thermal effects may
accompany the formation of the mixture or solution. The volumetric composition
of
liquid mixtures is expected to vary with temperature owing to the density dependence
on temperature.
When a gas mixture contains water vapor, the volumetric analysis is stated either
on a
wet
basis that includes the water vapor or on
a
dry
basis (moisture-free basis) that
excludes the water vapor.
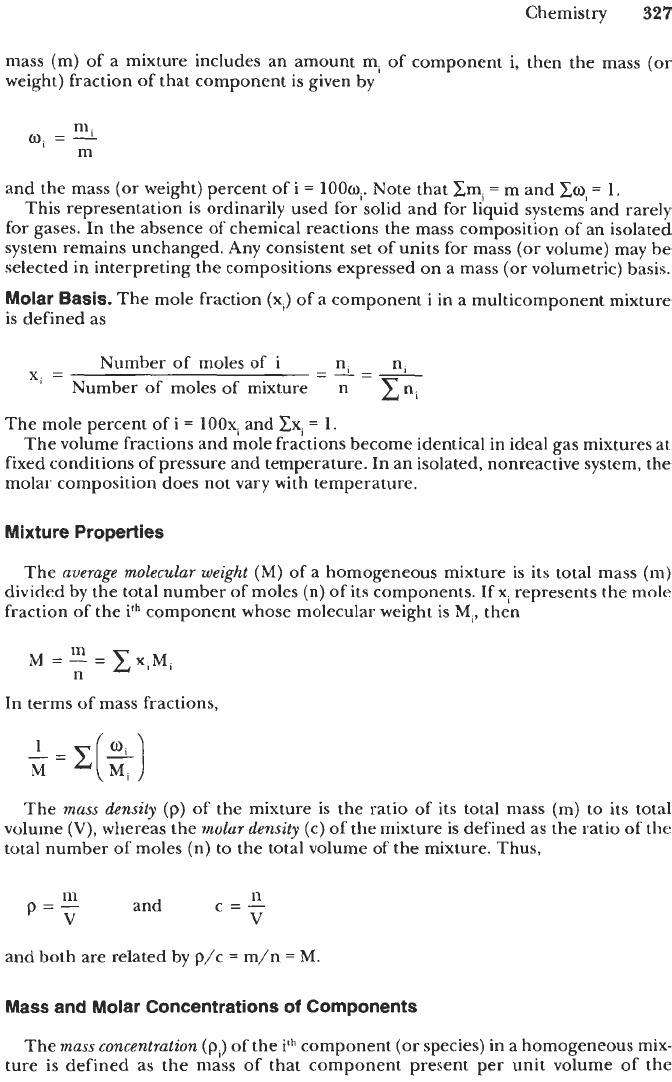
Weight (or Mass) Basis.
The terms “weight fraction” and “weight percent” are often
used as synonymous for “mass fraction” and “mass percent,” respectively. If the total
Chemistry
327
mass (m) of a mixture includes an amount m, of component i, then the mass (or
weight) fraction of that component is given by
and the mass (or weight) percent of
i
=
loow,.
Note that zmi
=
m and
Eoi
=
1.
This representation is ordinarily used for solid and for liquid systems and rarely
for gases. In the absence
of
chemical reactions the mass composition of an isolated
system remains unchanged.
Any
consistent set
of
units for mass (or volume) may be
selected in interpreting the compositions expressed on a mass (or volumetric) basis.
Molar Basis.
The mole fraction (xi)
of
a component i in a multicomponent mixture
is defined as
-3-
-
"i
Number
of
moles of
i
x,
=
Number of moles
of
mixture
n
ni
The mole percent
of
i
=
lOOx, and xxi
=
1.
The volume fractions and mole fractions become identical in ideal gas mixtures at
fived conditions
of
pressure and temperature. In an isolated, nonreactive system, the
molar composition does not
vary
with temperature.
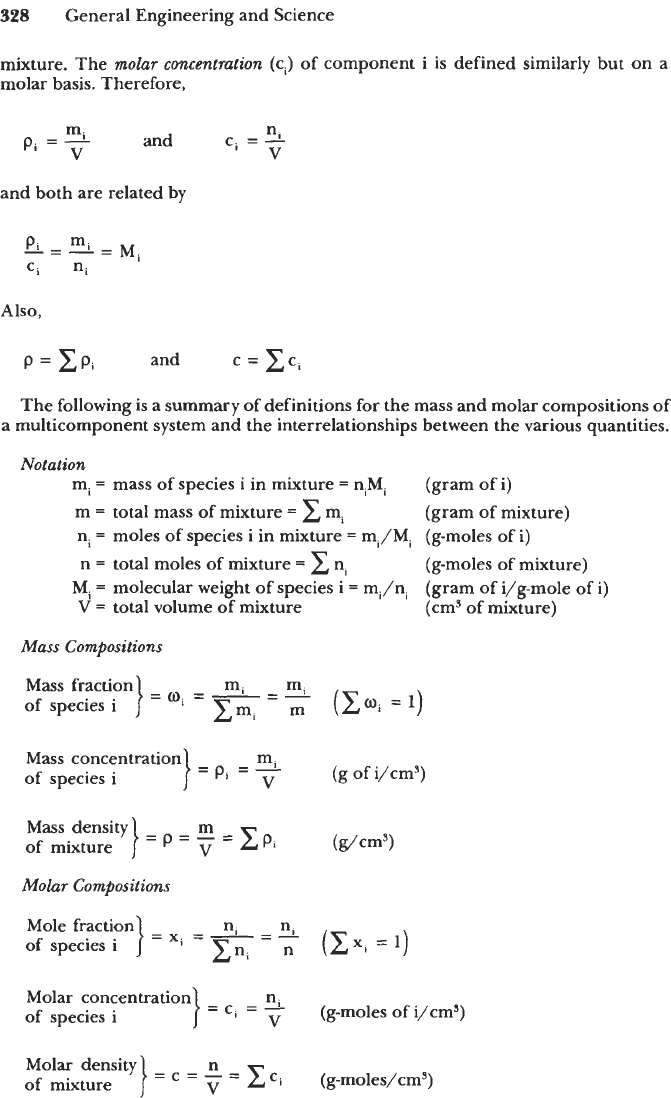
Mixture Properties
The
average
molecular
weight
(M)
of a homogeneous mixture is its total mass
(m)
divided by the total number
of
moles (n) of its components. If xi represents the mole
fraction of the
ith
component whose molecular weight is
Mi,
then
m
n
M
=
-
=
ExiMi
In terms of mass fractions,
The
mass
density
(p)
of the mixture is the ratio of its total mass (m) to its total
volume
(V),
whereas the
molar
density
(c)
of the mixture is defined as the ratio of the
total number of moles (n) to the total volume
of
the mixture. Thus,
n m
V
V
c=-
pz-
and
and both are related by p/c
=
m/n
=
M.
Mass and
Molar
Concentrations of Components
The
mass
concentration
(p,)
of the
ilh
component (or species) in a homogeneous mix-
ture
is
defined as the mass of that component present per unit volume
of
the
328
General Engineering and Science
mixture. The
molar concentration
(ci)
of component i is defined similarly but on a
molar basis. Therefore,
Ci
=
"i
m.
p.
=
I
and
'V
V
and both are related by
Also,
p=cpi
and
C=~C,
The following is a summary
of
definitions for the
mass
and molar compositions
of
a multicomponent system and the interrelationships between the various quantities.
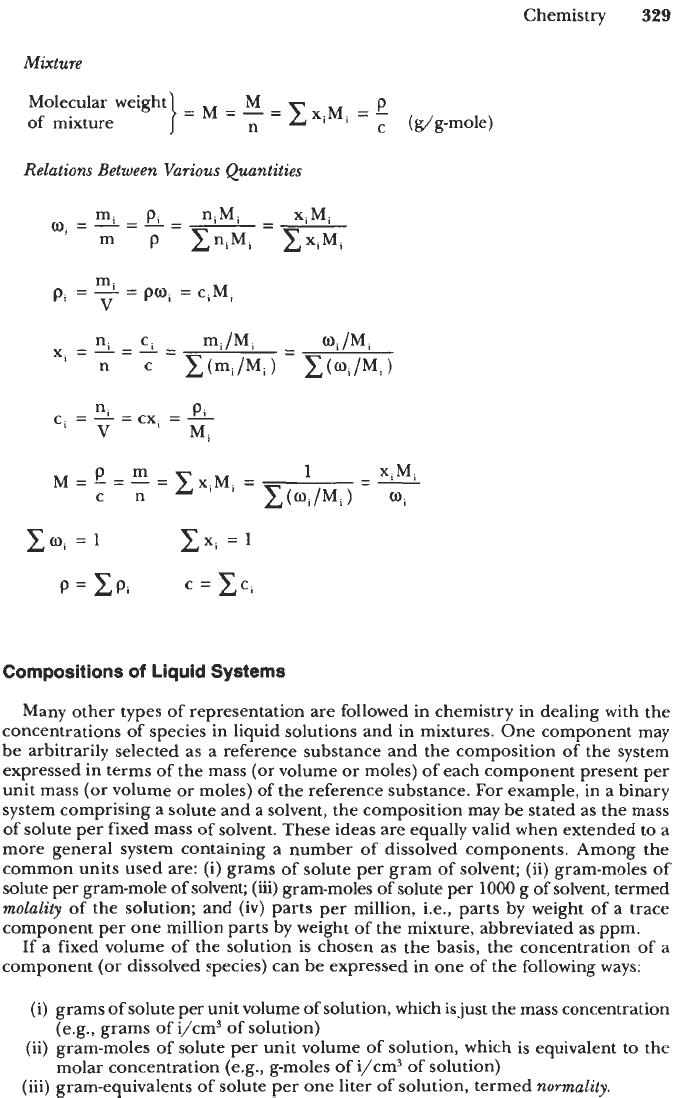
Notation
mi
=
mass of species i in mixture
=
niMi (gram of i)
m
=
total mass of mixture
=
C
mi (gram
of
mixture)
ni
=
moles
of
species
i
in mixture
=
m,/Mi (g-moles of i)
n
=
total moles
of
mixture
=
C
ni (g-moles of mixture)
Mi
=
molecular weight
of
species i
=
mi/ni (gram of i/g-mole
of
i)
V
=
total volume of mixture (cm3
of
mixture)
Mass
Compositions
Mass fraction
-
m.
of species i
}-mi=%-:
--
(Zmi=l)
Mass concentration mi
of species
i
Molar Compositions
Mole fraction
-
of species i
Molar concentration
-
n.
of
species i
=
ZCI
n
of
mixture
(xxi
=
1)
(g-moles
of
i/cms)
(
g-moles/cm3)
Chemistry
329
Compositions
of
Liquid Systems
Many other types of representation are followed in chemistry in dealing with the
concentrations of species in liquid solutions and in mixtures. One component may
be arbitrarily selected as a reference substance and the composition of the system
expressed in terms of the mass (or volume or moles) of each component present per
unit mass (or volume or moles) of the reference substance. For example, in a binary
system comprising a solute and a solvent, the composition may be stated as the mass
of solute per fixed mass of solvent. These ideas are equally valid when extended
to
a
more general system containing a number of dissolved components. Among the
common units used
are:
(i)
grams of solute per gram of solvent; (ii) gram-moles of
solute per gram-mole of solvent; (iii) gram-moles of solute per
1000
g of solvent, termed
moZaZity
of
the solution; and (iv) parts per million, i.e., parts by weight of a trace
component per one million parts by weight of the mixture, abbreviated as ppm.
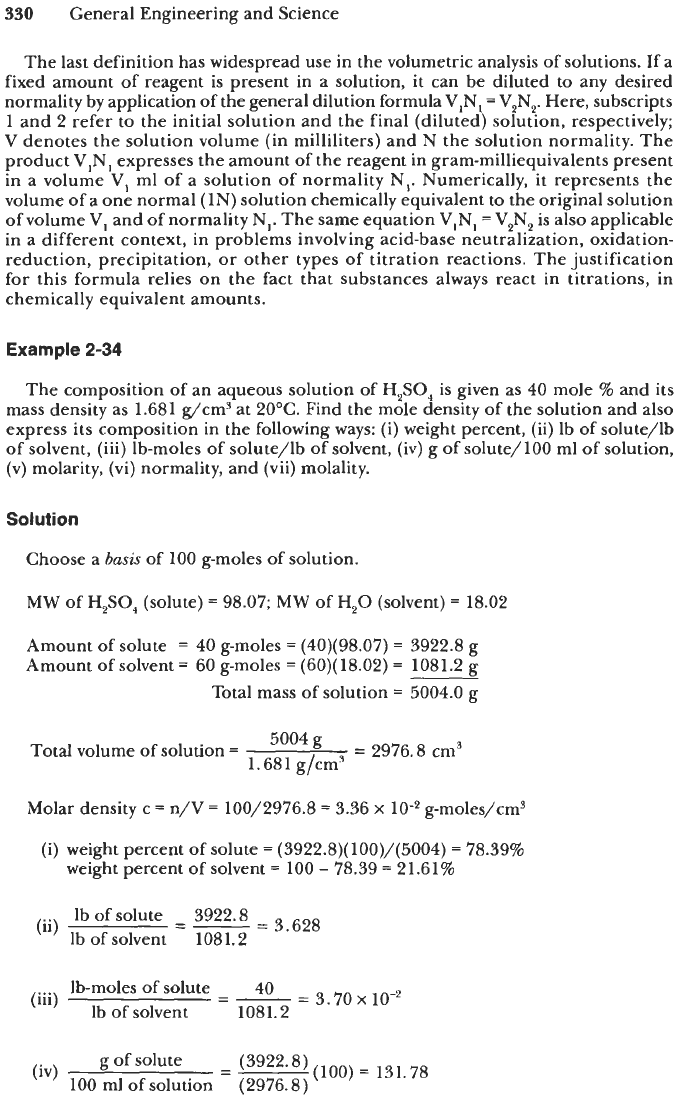
If
a
fixed volume of the solution is chosen as the basis, the concentration of a
component (or dissolved species) can be expressed in one of the following ways:
(i)
grams of solute per unit volume of solution, which isjust the mass concentration
(ii)
gram-moles of solute per unit volume of solution, which is equivalent to the
(iii) gram-equivalents
of
solute per one liter of solution, termed
normality.
(e.g., grams of i/cm3 of solution)
molar concentration (e.g., g-moles of i/cm3 of solution)
330
General Engineering and Science
The last definition has widespread use in the volumetric analysis of solutions. If a
fixed amount of reagent is present in a solution, it can be diluted to any desired
normality by application of the general dilution formulaV,N,
=
V,N, Here, subscripts
1 and 2 refer to the initial solution and the final (diluted) solution, respectively;
V denotes the solution volume (in milliliters) and
N
the solution normality. The
product V,N, expresses the amount of the reagent in gram-milliequivalents present
in a volume VI ml of a solution of normality
N,.
Numerically, it represents the
volume of a one normal (1N) solution chemically equivalent to the original solution
of volume
V,
and of normality
N,.
The same equation V,N,
=
V2N2 is
also
applicable
in a different context, in problems involving acid-base neutralization, oxidation-
reduction, precipitation, or other types of titration reactions. The justification
for this formula relies on the fact that substances always react in titrations, in
chemically equivalent amounts.
Example
2-34
The composition of
an
aqueous solution of H,SO, is given as 40 mole
%
and its
mass density as 1.681 g/cm3 at
20°C.
Find the mole density of the solution and also
express its composition in the following ways: (i) weight percent, (ii) lb of solute/lb
of solvent,
(iii)
lb-moles of solute/lb of solvent,
(iv)
g of solute/100 ml of solution,
(v)
molarity, (vi) normality, and
(vii)
molality.
Solution
Choose a
baris
of 100 g-moles of solution.
MW
of
H,SO,
(solute)
=
98.07;
MW
of
H,O
(solvent)
=
18.02
Amount of solute
=
40 g-moles
=
(40)(98.07)
=
3922.8 g
Amount
of
solvent
=
60
g-moles
=
(60)( 18.02)
=
1081.2 g
Total mass of solution
=
5004.0
g
5004 g
1.681 g/cmJ
Total volume of solution
=
=
2976.8 cm’
Molar density c
=
n/V
=
100/2976.8
=
3.36
X
lo-, g-moles/cm3
(i) weight percent of solute
=
(3922.8)( 100)/(5004)
=
78.39%
weight percent of solvent
=
100
-
78.39
=
21.61%
lb of solute
lb
of
solvent 1081.2
3922.8
-
3.
628
=--
(ii)
_--
40
-
3.70~
lo-*
Ib-moles of solute
-
(iii)
Ib of solvent 1081.2
--
(3922.8)(1~~)
=
131.78
gof solute
-
(iv)
100 ml of solution (2976.8)

Chemistry
331
-- -
(40)
(1000)
=
13.44
M
g-moles of solute
(v)
Molarity
=
1000 ml of solution (2976.8)
(vi)
Equivalent weight of solute=
-
MW
- -
-
".07
=
49.03 g/g-eq.
valence 2
g-eq. of solute
1000 ml
of
solution
Normality
=
=
2(Molarity)
=
26.87N
(vii)
Molality
=
-
-
(40)(1000)
=
37.00 molal
g-moles of solute
1000
g
of
solvent (1081.2)
pH
Scale
and
Buffer
Solutions.
The
pH
of
a
solution
is defined as the logarithm
of
the reciprocal
of
the hydrogen ion concentration (or activity) [H']
of
the
solution, i.e.,
pH
=
-
log [H']
A
pOH scale may be defined analogously for the hydroxyl ion concentration by
pOH
=
-
log [OH-]
Because the ionic product of water
Kw
=
[Hi] [OH-]
=
1.04
x
at 25"C, it follows
that
pH
=
14
-
pOH. Thus, a neutral solution (e.g., pure water at 25°C) in which
[H']
=
[OH-] has a pH
=
pOH
=
7. Acids show a lower
pH
and bases a higher pH than
this neutral value of
7.
The hydrogen ion concentrations can cover a wide range,
from
-1
g-ion/liter or more in acidic solutions to g-ion/liter or less in alkaline
solutions [53, p. 5451.
Buffer
action refers to the property of a solution in resisting
change
of
pH upon addition of an acid
or
a base. Buffer solutions usually consist of
a mixture
of
a weak acid and its salt (conjugate base) or of a weak base and its salt
(conjugate acid).
The
ionicstrength
(p)
of
a solution is a measure of the intensity of the electrical field
due to the ions present in the solution. It
is
defined by
where c,
is
the molal concentration of the ionic species
i
(g-ions
i/lOOO
g
solvent),
and
L,
is
the valence
or
charge on the ion
i.
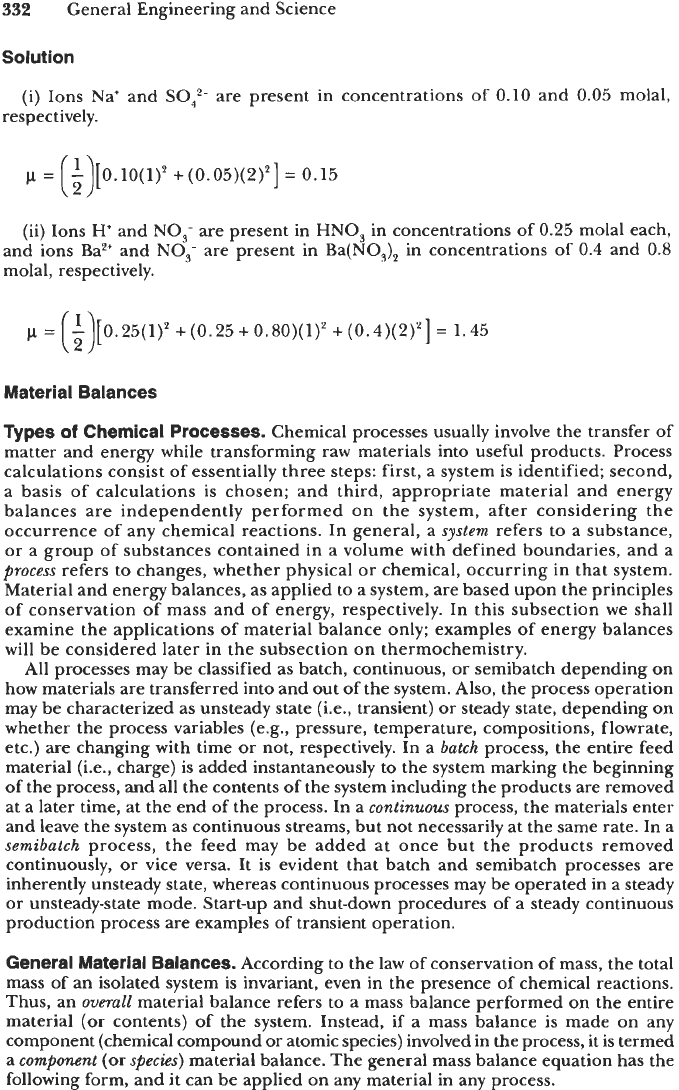
Example
2-35
Find the ionic strength of
(i)
0.05 molal sodium sulfate (Na,SO,) solution, and
(ii)
0.25 molal nitric acid
(HNO,)
and 0.4 molal barium nitrate (Ba(NO,),) together
in one solution.
332
General Engineering and Science
Solution
(i) Ions Na' and
SO:-
are present in concentrations
of
0.10
and 0.05 molal,
respectively.
p
=
-
[0.10(1)2
+(0.05)(2)*]
=
0.15
(3
(ii) Ions
H
and
NO,-
are present in
HNO,
in concentrations of
0.25
molal each,
and ions Ba2+ and
NO;
are present in Ba(NO,), in concentrations
of
0.4 and
0.8
molal, respectively.
p
=
-
[0.25(1)2+(0.25+0.80)(1)2+(0.4)(2)2]
=
1.45
(3
Material Balances
Types
of
Chemical
Processes.
Chemical processes usually involve the transfer of
matter and energy while transforming raw materials into useful products. Process
calculations consist of essentially three steps: first, a system is identified; second,
a basis of calculations is chosen; and third, appropriate material and energy
balances are independently performed on the system, after considering the
occurrence of any chemical reactions. In general, a
system
refers to a substance,
or
a group
of
substances contained in a volume with defined boundaries, and a
process
refers to changes, whether physical or chemical, occurring in that system.
Material and energy balances, as applied to a system, are based upon the principles
of conservation of mass and of energy, respectively. In this subsection we shall
examine the applications of material balance only; examples of energy balances
will be considered later in the subsection on thermochemistry.
All processes may be classified as batch, continuous, or semibatch depending on
how materials are transferred into and out of the system. Also, the process operation
may be characterized as unsteady state (i.e., transient)
or
steady state, depending on
whether the process variables (e.g., pressure, temperature, compositions, flowrate,
etc.)
are
changing
with
time
or
not, respectively. In a
butch
process, the entire feed
material (Le., charge) is added instantaneously to the system marking the beginning
of the process, and all the contents of the system including the products are removed
at a later time, at the end of the process. In a
continuous
process, the materials enter
and leave the system as continuous streams, but not necessarily at the same rate. In a
semibutch
process, the feed may be added at once but the products removed
continuously,
or
vice versa. It is evident that batch and semibatch processes are
inherently unsteady state, whereas continuous processes may be operated in a steady
or
unsteady-state mode. Start-up and shut-down procedures of a steady continuous
production process are examples of transient operation.
General Material Balances.
According to the law of conservation of mass, the total
mass of an isolated system is invariant, even in the presence of chemical reactions.
Thus, an
overall
material balance refers to a mass balance performed on the entire
material
(or
contents) of the system. Instead, if a mass balance is made on any
component (chemical compound or atomic species) involved in the process, it is termed
a
component
(or
species) material balance. The general mass balance equation has the
following form, and it can be applied on any material in any process.

Chemistry
333
inflow
[
the:Ilem entering
Mass
outflow
leaving
the system
Net internal
mass generation
reactions within
Net mass
accumulation
+
due tochemical
=
within
the
1
the system
system
1
or
symbolically,
mi"
-
m,,",
+
CR
=
Am
The third term on the left side of the equation has significance in reactive systems
only. It is used with a positive sign when material is produced as a net result
of
all
chemical reactions; a negative sign must precede this term if material is consumed by
chemical reactions. The former situation corresponds to a source and the latter to a
sink for the material under consideration. Since the total mass
of
reactants always
equals the total mass of products in a chemical reaction,
it
is clear that the reaction
(source/sink) term
CR
should appear explicitly in the equation for component material
balances only. The overall material balance, which is equivalent to the algebraic sum
of all of the component balance equations, will not contain any
CR
term.
Differential and Integral Balances.
Two types
of
material balances, differential
and integral, are applied in analyzing chemical processes. The differential mass
balance is valid at any instant in time, with each term representing a rate (i.e.,
mass per unit time).
A
general differential material balance
may be written on any
material involved in any
transient
process, including semibatch and unsteady-state
continuous flow processes:
Net rate
of
internal
Net rate of
or symbolically
-_
dm
-
mi"(t)-m,,,,,(t)+(R(t)
dt
where each of the rate terms on the right side of the equations can, in general, be
functions of time. The actual solution is obtained upon integration
over
time between
the initial and final states of the transient process.
A
special case of the above equation applies to a
continuous steady-state
flow
process
when all of the rate terms are independent of time and the accumulation term is
zero. Thus, the differential material balance for any component
i
in such a process
is
given by
Rate of mass Rate of mass Net rate of internal
(outflow,
~c,ul)i
-(
inflow,
mi"
)i
=
(mass
generation,
and the overall material balance is
mi"
=
m,,
.
When chemical reactions are absent,
we
have
for a continuous flow process at steady-state.
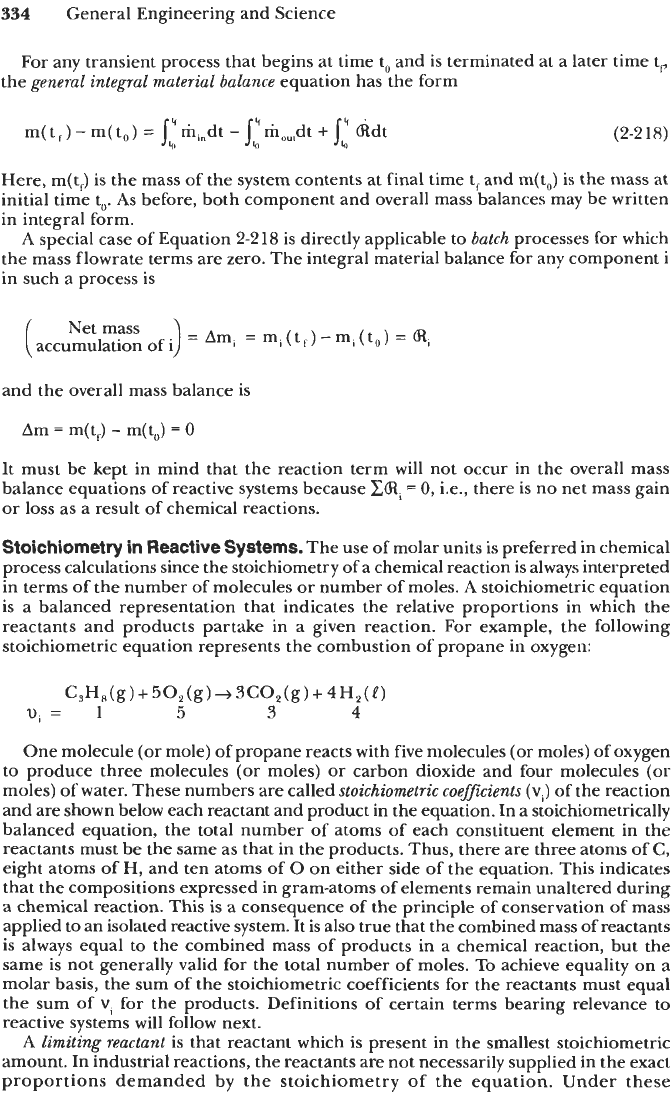
334
General Engineering and Science
For any transient process that begins at time
to
and is terminated at a later time tF
the
general integral material balance
equation has the form
m(
tf)
-
m(to)
=
jq:m,"dt
-
jq:m,,,dt
+
jq:
kdt
(2-2
18)
Here, m(tJ is the mass of the system contents at final time t, and m(t,,) is the mass at
initial time
to.
As
before, both component and overall mass balances may be written
in integral form.
A
special case
of
Equation
2-218
is directly applicable
to
batch
processes for which
the mass flowrate terms are zero. The integral material balance for any component
i
in such a process is
and the overall mass balance is
Am
=
m(t,)
-
m(t,)
=
0
It must be kept in mind that the reaction term
will
not occur in the overall mass
balance equations of reactive systems because
CCRi
=
0,
i.e., there is no net mass gain
or loss as a result of chemical reactions.
Stoichiometry
in
Reactive Systems.
The use
of
molar units is preferred in chemical
process calculations since the stoichiometry of a chemical reaction is always interpreted
in terms of the number of molecules or number of moles.
A
stoichiometric equation
is a balanced representation that indicates the relative proportions in which the
reactants and products partake in a given reaction. For example, the following
stoichiometric equation represents the combustion of propane in oxygen:
One molecule (or mole)
of
propane reacts with five molecules (or moles)
of
oxygen
to produce three molecules (or moles) or carbon dioxide and four molecules (or
moles) of water. These numbers are called
stoichiometric coefficients
(vi)
of the reaction
and are shown below each reactant and product in the equation. In a stoichiometrically
balanced equation, the total number
of
atoms of each constituent element in the
reactants must be the same as that in the products. Thus, there are three atoms of
C,
eight atoms of
H,
and ten atoms of
0
on either side of the equation. This indicates
that the compositions expressed in gram-atoms of elements remain unaltered during
a chemical reaction. This is a consequence of the principle of conservation of mass
applied to
an
isolated reactive system. It is also true that the combined mass of reactants
is always equal to the combined mass of products in a chemical reaction, but the
same is not generally valid for the total number of moles.
To
achieve equality on a
molar basis, the sum of the stoichiometric coefficients for the reactants must equal
the sum of
v,
for the products. Definitions of certain terms bearing relevance to
reactive systems will follow next.
A
limiting reactant
is that reactant which is present in the smallest stoichiometric
amount. In industrial reactions, the reactants are not necessarily supplied in the exact
proportions demanded by the stoichiometry of the equation. Under these
