Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Electricity
295
quite involved, and transmission line electrical characteristics are often represented
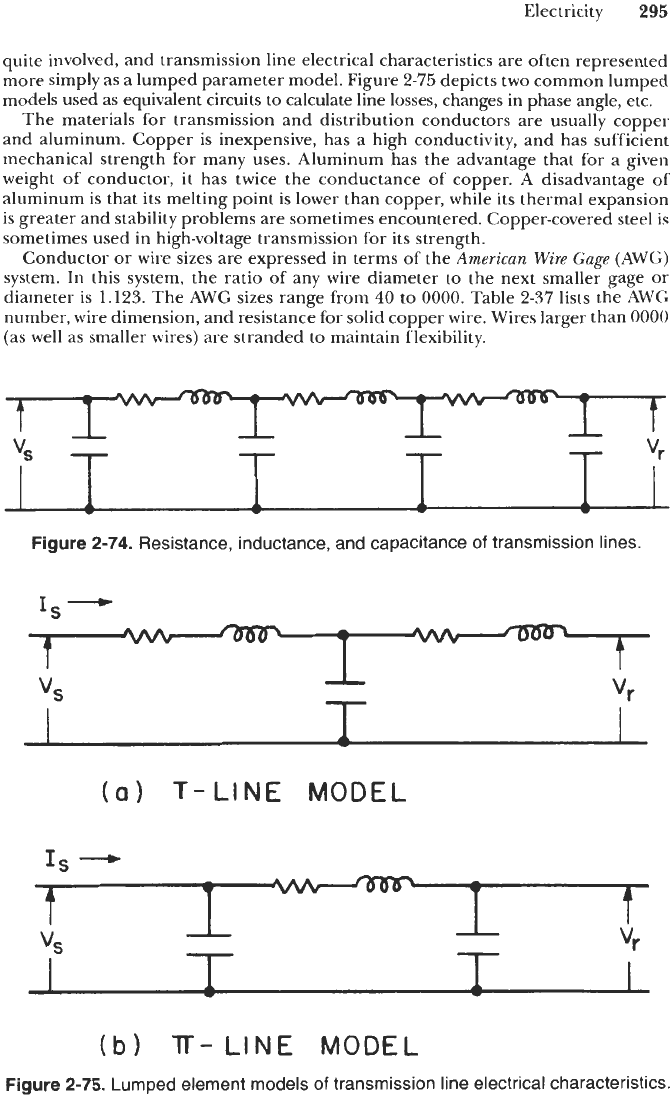
more simply as a lumped parameter model. Figure
2-75
depicts two common lumped
models used as equivalent circuits to calculate line losses, changes in phase angle, etc.
The materials for transmission and distribution conductors are usually copper
and aluminum. Copper is inexpensive, has a high conductivity, and has sufficient
mechanical strength for many uses. Aluminum has the advantage that for a given
weight of conductor, it has twice the conductance of copper.
A
disadvantage of
aluminum is that its melting point is lower than copper, while its thermal expansion
is greater and stability problems are sometimes encountered. Copper-covered steel is
sometimes used in high-voltage transmission for its strength.
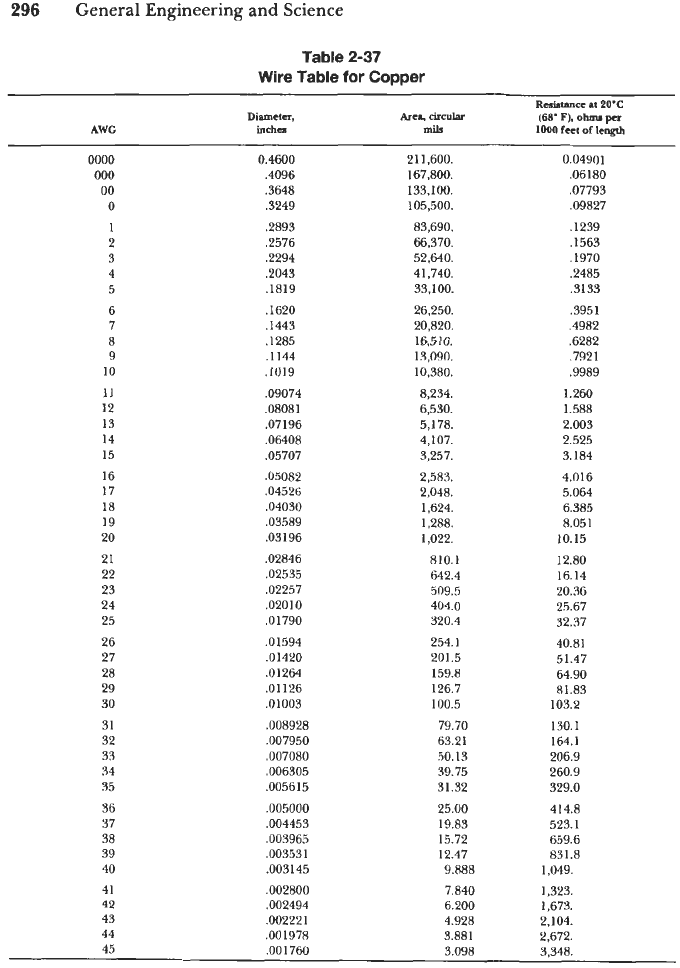
Conductor or wire sizes are expressed in terms of the
American
Wire
Gage
(AWG)
system. In this system, the ratio of any wire diameter to the next smaller gage or
diameter is
1.123.
The AWG sizes range from
40
to 0000. Table
2-37
lists the AWG
number, wire dimension, and resistance for solid copper wire. Wires larger than 0000
(as well as smaller wires) are stranded to maintain flexibility.
Figure
2-74. Resistance, inductance, and capacitance
of
transmission lines.
IS
-
t
t
(01
T-LINE MODEL
(b)
T-LINE
MODEL
Figure
2-75. Lumped element models
of
transmission line electrical characteristics.
296
General Engineering and Science
Table
2-37
Wire
Table
for
Copper
~
AWG
Dinmeter,
inches
Resistance
at
20.C
(68'
F),
oh
per
Area, circular
mils
Iwo
feet
of
lmgrh
0000
0.4600
211.600. 0.04901
000
.4G96
167,800.
.06180
00
.3648 133,100.
,07793
0
.3249 105,500. .09827
1 ,2893 83,690. .1239
2 ,2576 66,370. ,1563
3 ,2294 52,640. .1970
4 .2043 41,740. .2485
5
,1819 33,100. .3133
6
,1620 26,250. ,3951
7 ,1443 20,820. .4982
8
,1285 16,510.
,6282
9 ,1144 13,090. ,7921
10
,1019 10,380. ,9989
11
.09074 8,234. 1.260
12 ,08081 6,530. 1.588
13 .07196 5,178. 2.003
14
.ow08
4,107. 2.525
15 ,05707 3,257. 3.184
16
,05082 2,583. 4.016
17 ,04526 2,048. 5.064
18 ,04090 1,624. 6.385
19
,03589
1,288.
8.051
20 ,03196 1,022. 10.15
21 ,02846 810.1 12.80
22
.a2535 642.4 16.14
23 ,02257 509.5 20.36
24 .02010 404.0 25.67
25 ,01790 320.4 32.37
26
,01594 254.1 40.81
27 ,01420 201.5 51.47
28
.01264 159.8 64.90
29
,01126
126.7 81.83
30
.01003
100.5 103.2
31 ,008928 79.70 130.1
32 ,007950 63.21 164.1
33 .007080 50.13
206.9
34 ,006305 39.75 260.9
35 ,005615 31.32 329.0
36 .005000 25.00 414.8
37 ,004453 19.83 523.1
38 ,003965 15.72 659.6
39 ,003531 12.47 831.8
40 .003145 9.888 1,049.
41
.002800
7.840 1.323.
42 ,002494 6.200 1.673.
43 ,002221 4.928 2.104.
44 .001978
3.881
2.672.
45 ,001
760
3.098 3.348.
Chemistry
297
Interior wiring design and installation for most commercial and industrial uses
should follow the
National
Electrical
Code
(NEC) which has been a national standard
since
1970
with the passage of the Occupational Safety and Health Act
(OSHA).
Some localities, however, may not accept the NEC and require that their own (more
stringent) standards be followed.
For
further information on this subject, refer to References
1
and 41 through
45.
CHEMISTRY
Introduction
The material in this section is divided into three parts. The first subsection deals
with the general characteristics of chemical substances. The second subsection is
concerned with the chemistry of petroleum; it contains a brief review of the nature,
composition, and chemical constituents of crude oil and natural gases. The final
subsection touches upon selected topics in physical chemistry, including ideal gas
behavior, the phase rule and its applications, physical properties of pure substances,
ideal solution behavior in binary and multicomponent systems, standard heats of
reaction, and combustion of fuels. Examples are provided to illustrate fundamental
ideas and principles. Nevertheless, the reader is urged to refer to the recommended
bibliography [47-521 or other standard textbooks to obtain a clearer understanding
of the subject material. Topics not covered here owing to limitations of space may be
readily found in appropriate technical literature.
Characteristics
of
Chemical Compounds
The general nature of chemical compounds and their physical behavior are briefly
reviewed in the following subsection.
Chemical Bonds
Two types
of
chemical bonds, ionic and covalent, are found in chemical compounds.
An ionic bond results from the transfer of valence electrons from the atom of an
electropositive element
(M)
to the atom(s)
of
an electronegative element
(X).
It is due
to coulombic (electrostatic) attraction between the oppositely charged ions, M(cation)
and X-(anion). Such ionic bonds are typical of the stable salts formed by combination
of the metallic elements (Na,
K,
Li,
Mg,
etc.) with the nonmetallic elements
(F,
C1, Br,
etc.).
As
an example, the formation of the magnesium chloride molecule from its
elemental atoms is shown by the following sequence:
Mg
+
Mg2+
+
2e
2C1+ 2e
+
2C1-
Mg2+
+
2C1-
tj
Cl-Mg2'Cl- or MgCl,
where e represents
an
electron. The ionic charges resulting from the gain or
loss
of
electrons are called electrovalences. Thus, the electrovalence of magnesium is +2 and
of chlorine
-1.
Ionic compounds are mostly inorganic substances.
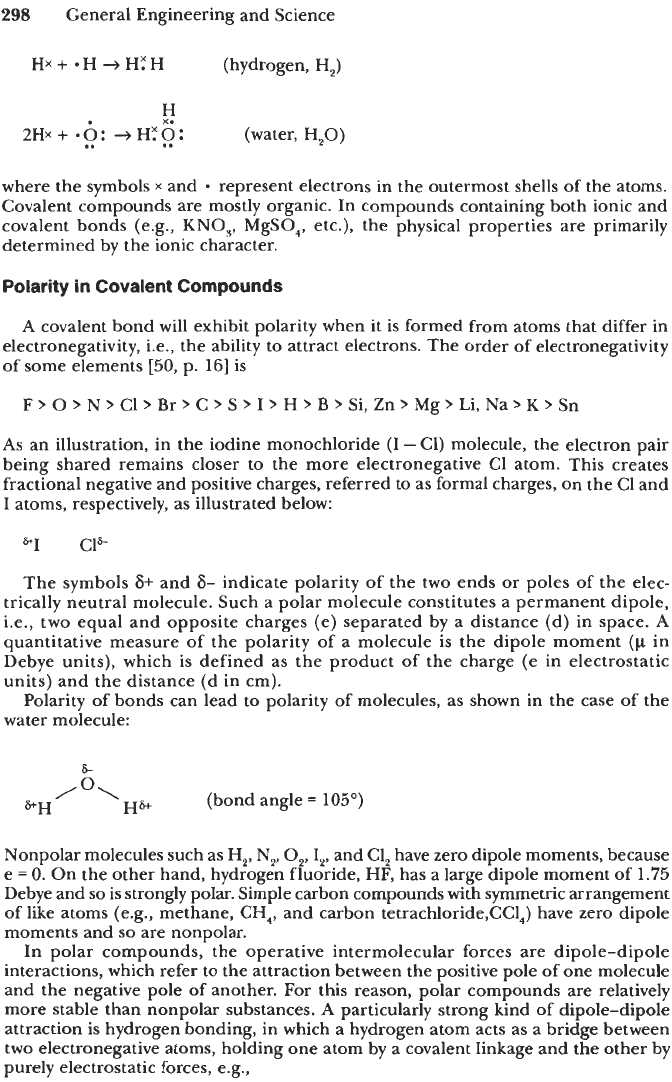
A
nonionic compound is made up of covalent bonds only.
A
covalent bond results
from shared electron pairs between two atoms. It consists of electrostatic attraction
between each electron and both nuclei, e.g.,
298
General Engineering and Science
H
X.
2Hx
+
-6:
+
H":
(water,
H,O)
..
where the symbols
x
and
-
represent electrons in the outermost shells of the atoms.
Covalent compounds are mostly organic. In compounds containing both ionic and
covalent bonds (e.g.,
KNO,,
MgSO,,
etc.), the physical properties are primarily
determined by the ionic character.
Polarity in Covalent Compounds
A
covalent bond
will
exhibit polarity when it is formed from atoms that differ in
electronegativity, i.e., the ability to attract electrons. The order of electronegativity
of some elements
[50,
p.
161
is
F>
0
>
N
>
C1>
Br
>
C
>
S
>
I
>
H
>
B
>
Si, Zn
>
Mg> Li, Na,
Ky
Sn
As
an illustration, in the iodine monochloride (I
-
Cl) molecule, the electron pair
being shared remains closer
to
the more electronegative C1 atom. This creates
fractional negative and positive charges, referred to as formal charges, on the C1 and
I atoms, respectively, as illustrated below:
*I
CIS-
The symbols
6+
and
6-
indicate polarity of the two ends or poles of the elec-
trically neutral molecule. Such a polar molecule constitutes a permanent dipole,
i.e., two equal and opposite charges (e) separated by a distance
(d)
in space.
A
quantitative measure of the polarity of a molecule is the dipole moment
(p
in
Debye units), which is defined as the product of the charge (e in electrostatic
units) and the distance (d in cm).
Polarity
of
bonds can lead to polarity of molecules, as shown in the case
of
the
water molecule:
(bond angle
=
105")
H"
/O\
*H
Nonpolar molecules such as
H,,
N,,
O,,
I,,
and C1, have zero dipole moments, because
e
=
0.
On the other hand, hydrogen fluoride,
HF,
has a large dipole moment of
1.75
Debye and
so
is strongly polar. Simple carbon compounds
with
symmetric arrangement
of like atoms (e-g., methane, CH,, and carbon tetrachloride,CCl,) have zero dipole
moments and
so
are nonpolar.
In polar compounds, the operative intermolecular forces are dipole-dipole
interactions, which refer to the attraction between the positive pole of one molecule
and the negative pole of another. For this reason, polar compounds are relatively
more stable than nonpolar substances.
A
particularly strong kind of dipole-dipole
attraction is hydrogen bonding, in which
a
hydrogen atom acts as a bridge between
two electronegative atoms, holding one atom by
a
covalent linkage and the other by
purely electrostatic forces, e.g.,

Chemistry
299
Hydrogen fluoride H
-
F.
.
.
F-H
Water
H-O...O-H
I
H
I
H
where the sequence of dots indicates the hydrogen bridge. This bond has a strength
of
5
kcal/g-mole versus
50-100
kcal/g-mole for the covalent bond, but it
is
much
stronger than other dipole-dipole interaction
[49,
p.
271.
For hydrogen bonding to
be important, both electronegative atoms must belong to the group:
F,
0,
N.
The intermolecular forces operative in nonpolar compounds are also electrostatic
in nature. These weak van der Waals forces involve attraction between nonbonded
atoms and are effective over short ranges only.
Physical Properties
The degree of polarity has considerable influence on the physical properties of
covalent compounds and it can also affect chemical reactivity. The melting point
(mp) and boiling point (bp) are higher in ionic substances due to the strong nature of
the interionic forces, whereas the covalent compounds have lower values due to the
weak nature of intermolecular forces.
The mp and bp increase in the order of nonpolar to polar to ionic compounds.
Associated liquids, in which the molecules are held together by hydrogen bonds,
show higher bp than nonassociated polar compounds of similar molecular weights.
Ionic compounds in solution and in molten state are good conductors of electricity.
Melts and solutions of covalent substances are nonconducting. Inorganic substances
rarely undergo combustion, whereas (organic) covalent compounds do
so
readily.
Solubility
Ionic compounds can dissolve appreciably in highly polar solvents (e.g., water, liquid
ammonia, sulfuric acid). In solution, each ion is surrounded by a cluster of solvent
molecules and is said to be solvated; if the solvent is water, the ion is said to be
hydrated. To reduce the attraction between solvated ions of opposite charges, the
solvent must also have a high dielectric constant. Besides high polarizability and
dielectric constant, water can also form hydrogen bonds and, hence, is an excellent
solvent. Water can solvate cations at its negative pole and anions via hydrogen bonding.
The solubility of nonionic compounds is largely dictated by their polarity, in
accordance with the axiom, “like dissolves like.” That is, nonpolar compounds dissolve
in nonpolar solvents and polar substances dissolve in polar solvents.
Petroleum Chemistry
Petroleum chemistry is concerned with the origin, composition, and properties of
naturally occurring petroleum deposits, whether in liquid (crude oil or petroleum),
gaseous (natural gas), or solid (tars and asphalts) form. All of them are essentially
mixtures of hydrocarbons. Whereas natural gas contains a few lighter hydrocarbons,
both crude oil and tar deposits may consist of a large number of different hydrocarbons
that cannot be easily identified for molecular structure or analyzed for composition.
For general literature on the subject of petroleum chemistry consult References
54
through
58.

300
General Engineering and Science
Crude
Oils
Elemental compositional analysis indicates that the ratio of hydrogen to carbon
atoms is approximately
1.85:l
in typical crude oils
[51,
p.
281;
the other elements,
chiefly sulfur, nitrogen, and oxygen account for less than
3%
by weight in most light
crudes. Table
2-38
illustrates the elemental compositions of typical crude oils and
asphalts. Sour crudes contain significantly larger amounts of sulfur-containing
compounds. Traces of phosphorous and heavy metals such as vanadium, nickel, and
iron are also present.
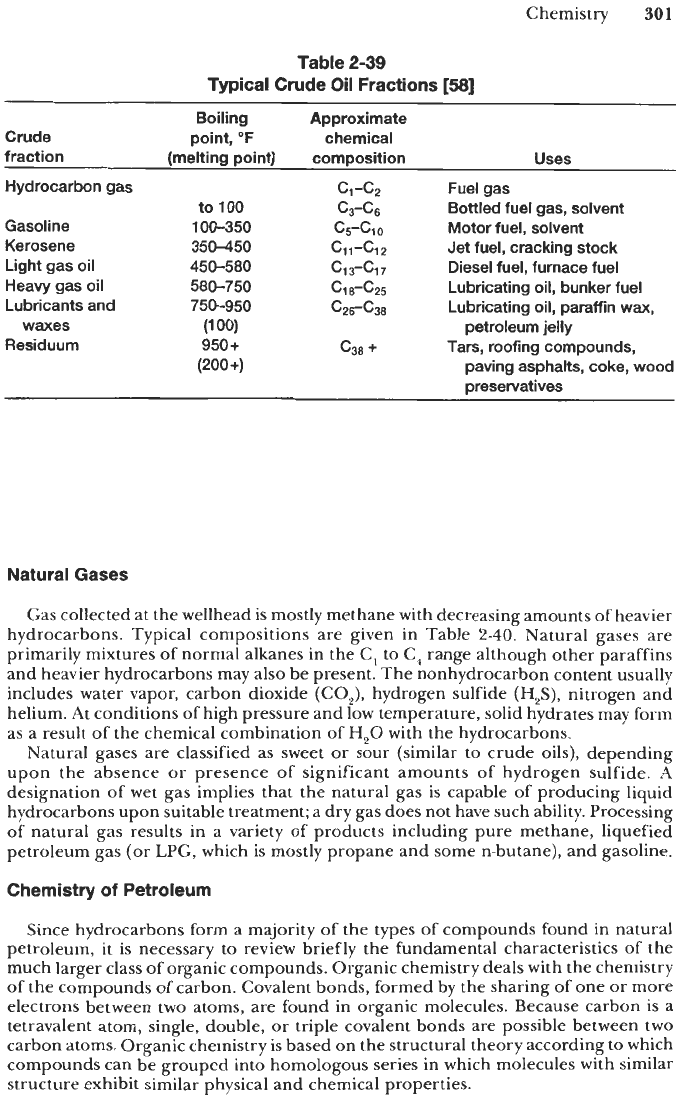
In refining operations, crude oils are subjected to fractional distillation by which
they are separated into different fractions according to the boiling point range of the
compounds and their end use or application (see Table
2-39).
A
“base” designation
is
given in the refining industry
to
classify crude oils:
(i)
A
paraffin-base
crude contains predominantly paraffins and small amounts
of
naphthenes
or asphalt. Upon distillation, it yields fine lubricating oils from the gas-oil fraction
and paraffin wax from the solid residue.
(ii)
An
asphalt-base
crude contains mostly
cyclic compounds (primarily naphthenes), which, upon distillation, produce high
yields of black, pitchlike, solid residue, asphalt, and heavy fuel oil. (iii)
A
mixed-base
crude has characteristics intermediate between the above two categories.
(iv)
An
aromatic-base
crude contains large amounts
of
low-molecular weight aromatics together
with naphthenes, and small amounts of asphalt and paraffins.
Tars and Asphalts
These are semisolid or solid substances formed in nature from crude oils after the
volatile components have evaporated and the remainder has undergone oxidation
and polymerization. They are also referred to as bitumens, waxes, and pitch. These
materials are believed to consist of mixtures of complex organic molecules of high
molecular weight.
As
with crude oils, which contain thousands of different chemical
compounds, an exact chemical analysis for identification and composition
is
impractical to perform on the solid deposits of petroleum.
Table
2-38
Elemental Composition
of
Natural Petroleum
(Percentage by Weight)
[55,56,57]
Element
Most
Crude
Oils
Typical’
Crude
Asphalt
Carbon
83-87“ 84.5 84
Hydrogen
ll-15a
13.0
10
Sulfur
trace
-
8b
1.5
3
Nitrogen
trace
-
1
.6b
0.5
1
Oxygen
trace
-
1
.8b
0.5
2
Metals trace
-
1,000
ppmb
trace
-
1,000
ppm
Source
a:
Reference
56;
Source
b:
Reference
55;
Source c: Reference
57
(Ni,
V,
etc.)
Chemistry
301
Table
239
Typical
Crude
Oil Fractions
[SS]
Boiling Approximate
Crude point, "F chemical
fraction (melting point) composition Uses
Hydrocarbon gas
Gasoline
Kerosene
Light gas
oil
Heavy gas oil
Lubricants and
waxes
Residuum
to
100
100-350
350-450
450-580
580-750
750-950
950+
('1
00)
(200+)
C38
+
Fuel gas
Bottled
fuel gas, solvent
Motor fuel, solvent
Jet fuel, cracking stock
Diesel fuel, furnace fuel
Lubricating
oil,
bunker fuel
Lubricating oil, paraffin wax,
Tars, roofing compounds,
petroleum
jelly
paving asphalts, coke,
wood
Dreservatives
Natural Gases
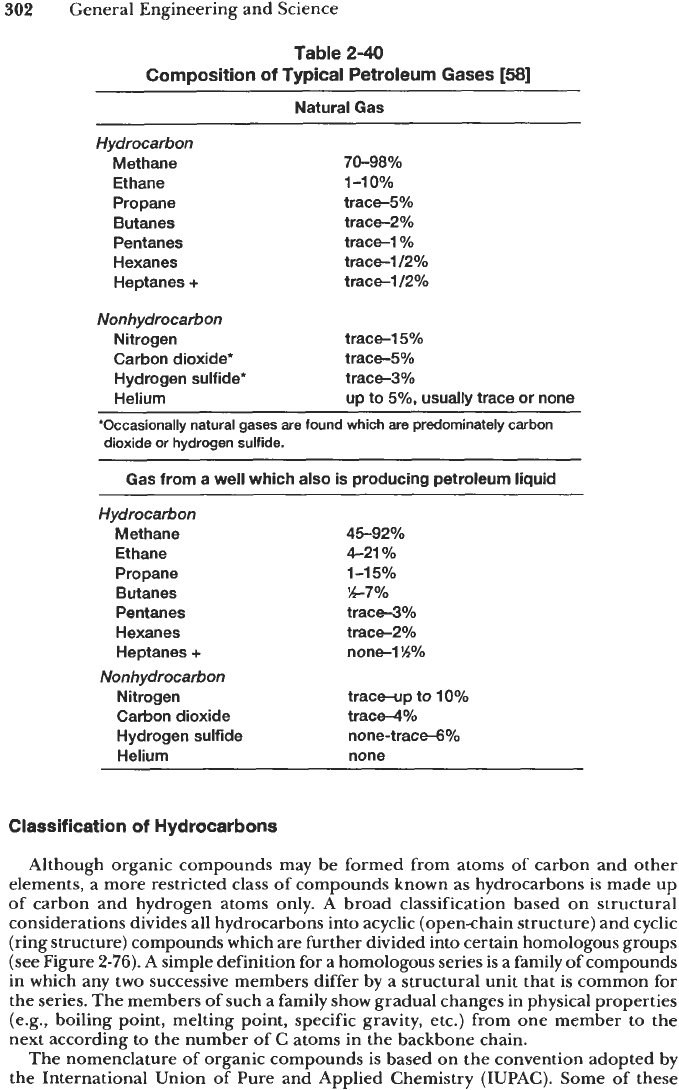
Gas collected at the wellhead is mostly methane with decreasing amounts of heavier
hydrocarbons. Typical compositions are given in Table
2-40.
Natural gases are
primarily mixtures of normal alkanes in the
C,
to C, range although other paraffins
and heavier hydrocarbons may also be present. The nonhydrocarbon content usually
includes water vapor, carbon dioxide (CO,), hydrogen sulfide
(H,S),
nitrogen and
helium. At conditions
of
high pressure and low temperature, solid hydrates may form
as a result of the chemical combination of
H,O
with the hydrocarbons.
Natural gases are classified as sweet
or
sour (similar to crude oils), depending
upon the absence or presence of significant amounts
of
hydrogen sulfide.
A
designation
of
wet gas implies that the natural gas
is
capable of producing liquid
hydrocarbons upon suitable treatment; a dry gas does not have such ability. Processing
of
natural gas results in a variety
of
products including pure methane, liquefied
petroleum gas
(or
LPG, which is mostly propane and some n-butane), and gasoline.
Chemistry
of
Petroleum
Since hydrocarbons form a majority
of
the types of compounds found in natural
petroleum,
it
is necessary to review briefly the fundamental characteristics of the
much larger class
of
organic compounds. Organic chemistry deals with the chemistry
of the compounds
of
carbon. Covalent bonds, formed by the sharing of one
or
more
electrons between two atoms, are found in organic molecules. Because carbon is a
tetravalent atom, single, double, or triple covalent bonds are possible between two
carbon atoms. Organic chemistry is based on the structural theory according
to
which
compounds can be grouped into homologous series in which molecules with similar
st.ructure exhibit similar physical and chemical properties.
302
General Engineering and Science
Table
2-40
Composition of Typical Petroleum Gases
[SI
Natural Gas
Hydrocarbon
Methane
Ethane
Propane
Butanes
Pentanes
Hexanes
Heptanes
+
70-98%
1-10%
trace-5%
trace-2%
trace-1
YO
trace-112%
trace-112%
Nonhydrocarbon
Nitrogen trace-1 5%
Carbon dioxide' trace-5%
Hydrogen sulfide' trace-3%
Helium
up to 5%, usually trace or none
*Occasionally natural gases are found which
are
predominately carbon
dioxide
or
hydrogen sulfide.
Gas from a well which also is producing petroleum liquid
Hydrocarbon
Methane
Ethane
Propane
Butanes
Pentanes
Hexanes
Heptanes
+
Nonhydrocarbon
Nitrogen
Carbon dioxide
Hydrogen sulfide
Helium
4592%
4-21
Yo
1-1
5%
'%7%
trace-3%
trace-2%
none-1
%YO
trace-up to
10%
trac&%
none-trac&%
none
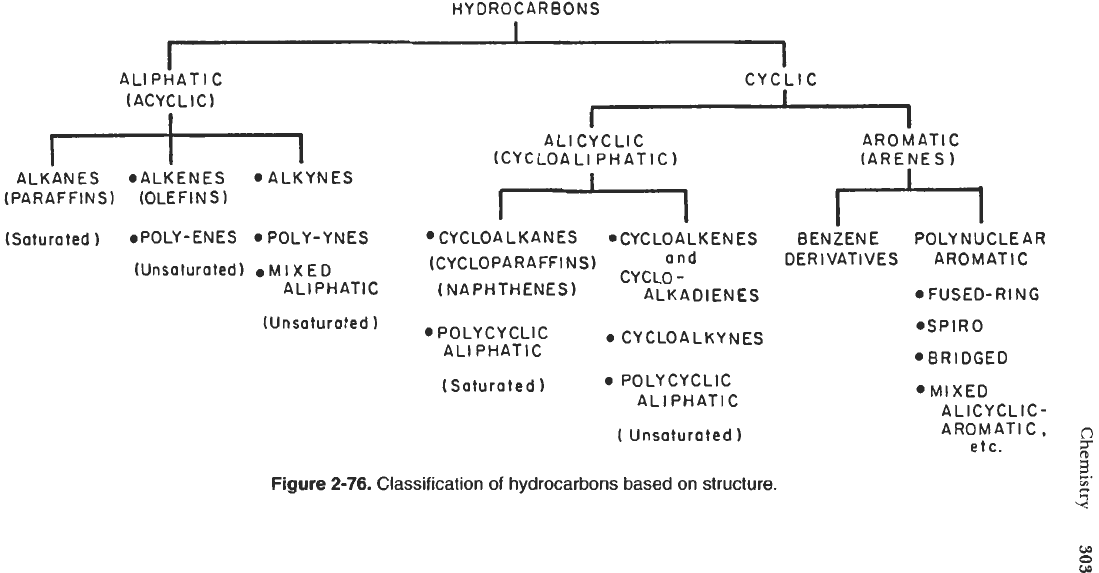
Classification
of
Hydrocarbons
Although organic compounds may be formed from atoms
of
carbon and other
elements, a more restricted class of compounds known as hydrocarbons is made up
of
carbon and hydrogen atoms only. A broad classification based on structural
considerations divides all hydrocarbons into acyclic (openchain structure) and cyclic
(ring structure) compounds which are further divided into certain homologous groups
(see Figure
2-76).
A simple definition for a homologous series is a family of compounds
in which any two successive members differ by a structural unit that is common for
the series. The members of such a family show gradual changes in physical properties
(e.g., boiling point, melting point, specific gravity, etc.) from one member to the
next according to the number of C atoms in the backbone chain.
The nomenclature of organic compounds is based on the convention adopted by
the International Union of Pure and Applied Chemistry (IUPAC). Some of these
HY DROCARBONS
ALIP~ATIC
CYCLIC
(ACYCLIC
1
ALKANES *ALKENES *ALKYNES
(PARAFFINS
1
(OLEFINS
ALI CYCLIC
(CY C LOA
LI
P
H
AT
1
C
1
ARO MAT
1
C
(ARENES)
(Saturated
1
oPOLY-ENES POLY-YNES
CY CLO A
L
K
A N
E
S
CYC LO A LK EN ES
B
EN
i
EN E
POLY
N
U C LE A
R
and DER
1
VATIVES
A
RO
M
AT
I
C
(CYC LOPA
R
A
FFI
N
S
1
(Unsaturated) .MIXED CY CLO
-
FUSED-RI N
G
(NAPHTHENES) ALKADl EN
ES
ALIPHATIC
(Unsaturated
1
.
poLycycLlc
0
CYCLOALKYNES
ALI PHATIC
.
POLYCYCLIC
(Saturated
1
AL
I
PH
AT
I
C
(
Unsaturated
1
Figure
2-76.
Classification of hydrocarbons based on structure.
@SPIRO
@BRIDGED
MIXED
ALICYCLIC-
AROMATIC,
n
etc.
R
v)
2.
CI
1
u:
w
0
w
304
General Engineering and Science
guidelines are presented in Section C of the
CRC
Handbook
of
Chemistry
and
Physics
[62]
and should be referred to for
a
better understanding of the rules.
A
brief discussion
of the various classes of hydrocarbons follows.
Aliphatic
Hydrocarbons.
These are “acyclic” hydrocarbons with an open-chain
structure, which can be either straight (Le., linear) or branched. The former type are
called normal (or n-) aliphatic compounds. Unsaturation is manifested in the form
of double or triple bonds.
Alkanes.
These have the general formula CnHPn+!, where n is the number of carbon
atoms in the alkane molecule, and n
2
1.
These are also known as “paraffins”
or
“saturated aliphatic hydrocarbons,” since all of the carbon atoms in the chain
are
connected by single covalent bonds. Continuous
or
straight-chain alkanes are called
normal paraffins
or
n-alkanes (e.g., methane, CH,; ethane, C,H,; propane, C,H,;
n-butane, n-C,H,,; etc.). The corresponding
alkyl
groups, methyl, -CH,; ethyl, -C,H,;
n-propyl, -C,H,;. n-butyl, -C,H,; etc., are generally represented by the symbol
R-.
Branched-cham alkanes, also known as “isoparaffins” or “isoalkanes,” are possible
when n
2
4.
The prefix “iso” is used when two methyl groups are attached to a terminal
carbon atom
of
an otherwise straight chain and the prefix “neo” when three methyl
groups are attached in that manner. Branched-chain alkanes are sometimes regarded
as normal alkanes with attached substituent alkyl groups. An example is
2,2,4trimethlypentane (isooctane)
Isomers
are
substances having the same molecular formula and molecular weight,
but differing in physical and chemical properties. Since branched and straight-chain
alkanes with the same molecular formula can exist as distinct structures having
different geometrical arrangement
of
the atoms, they are termed
structural
isomers.
One example is C,H,, (butane) which has two isomers:
HH CHS
I1
I
II
I
H3C-C-C-CH3 and H-C-CHS
HH CH3
[CH,CH,CH,CH, (n-butane)] [(CH,),CH (isobutane)]
As
the carbon number increases
in
the
chain, the number of possible structural isomers
grows very rapidly
as
a
result of increased branching possibilities. For example, C,H,,
has only three isomers,
