Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.


Chemistry
305
[
nC,H,, (n-pentane)]
[
(
CH,),CHC,H, [(CH,),C
(neopentane)]
(isopentane)]
while
C,,H,,
(decane) has
75,
and
C,,H,
(eicosane) has
366,319
possible isomers.
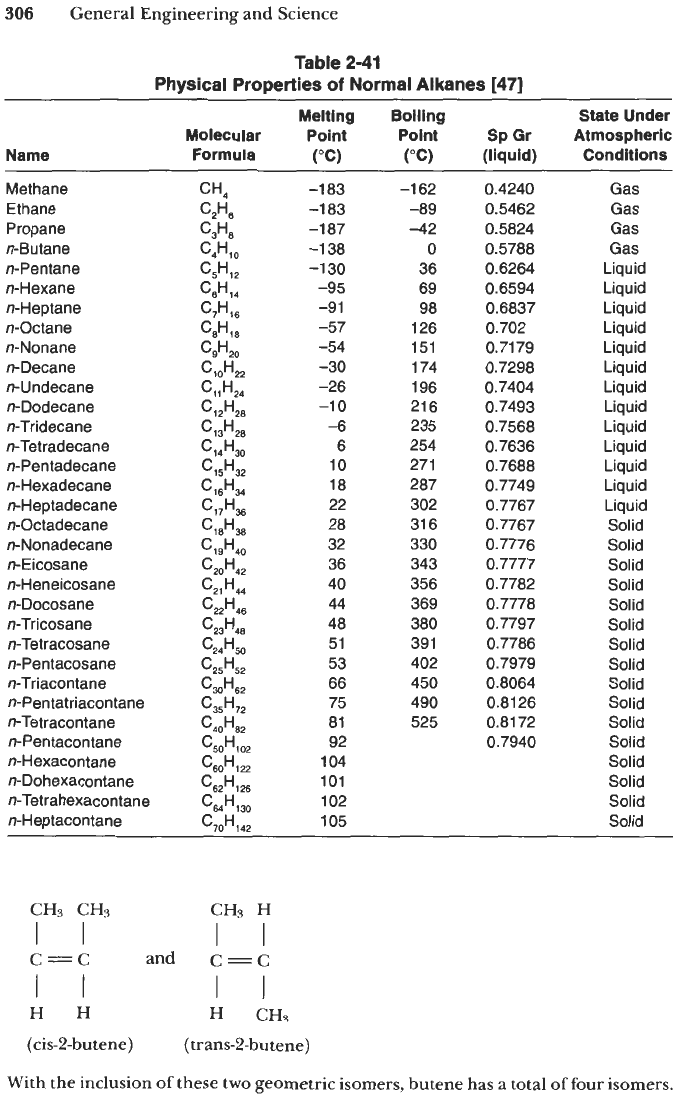
Whereas n-alkanes exhibit smooth and graded variations in physical properties
(see Table
2-41),
the branched members do not
[49,
p.
861.
The structural isomers of
any alkane generally show dissimilar physical and chemical characteristics.
A
branched-
chain isomer has a lower boiling point than a straight-chain isomer, and the more
numerous the branches, the lower its boiling point. Alkanes are either nonpolar or
weakly polar. They are soluble in nonpolar or weakly polar solvents (e.g., benzene,
chloroform, ether), and are insoluble in water and other highly polar solvents. Alkanes
can dissolve compounds
of
low polarity. Chemically, paraffins are stable and quite
unreactive at ordinary conditions. At high temperatures, they can burn completely in
the presence of excess oxygen
or
air to yield
CO,
and
H,O
as products. The combustion
reaction is exothermic.
At ambient temperature and pressure, the first four members of the n-alkane series
(methane to n-butane) are gases, the next thirteen (n-pentane through n-heptadecane)
are liquids, and the higher members from n
=
18 on are solids.
Alkenes.
These are also called "olefins" and have the general formula
CnHPn,
with n
2
2.
They contain a single
C=C
double bond and are named in accordance
with the
IUPAC
convention by specifying the location
of
the double bond from the
terminal carbon atom nearest to it.
The first three members of the olefin series are ethylene, propylene, and butylene
(or
butene). Structural isomers exist when n
2
4,
as a consequence of the positioning
of the double bond in normal alkenes
as
a result of branching in branched alkenes.
In addition,
geometric
isomers
may be possible owing to restricted rotation
of
atoms
about the
C=C
bond. For instance,
C,H,
(butene) has four possible isomers instead
of the expected three:
[CH,CHCH,CH, [CH,CHCHCH, [(CH,),CCH,
(1-butene)] (2-butene)] (isobutene)]
This occurs because 2-butene, itself, can exist in two different structures, the cis-
or the trans- configurations, depending on whether the methyl groups are situated
on the same side or on opposite sides of the main chain.
306
General Engineering and Science
Table
2-41
Physical Properties
of
Normal Alkanes
[47]
Melting Boiling
State Under
Molecular Point
Point
Sp
Gr Atmospheric
Name Formula
(“C)
(“C)
(liquid) Conditions
Methane
Ethane
Propane
n-Butane
n-Pentane
n-Hexane
n-Heptane
n-Octane
n-Nonane
n-Decane
n-Undecane
n-Dodecane
n-Tridecane
n-Tetradecane
n-Pentadecane
n-Hexadecane
n-Heptadecane
n-Octadecane
n-Nonadecane
n-Eicosane
n-Heneicosane
n-Docosane
n-Tricosane
n-Tetracosane
n-Pentacosane
n-Triacontane
n-Pentatriacontane
n-Tetracontane
n-Pentacontane
n-Hexacontane
n-Dohexacontane
n-Tetrahexacontane
n-Heptacontane
-1
83
-1
83
-187
-1
38
-1
30
-95
-9
1
-57
-54
-30
-26
-1
0
-6
6
10
18
22
28
32
36
40
44
48
51
53
66
75
81
92
104
101
102
105
-1
62
-89
42
0
36
69
98
126
151
174
196
21 6
235
254
271
287
302
31 6
330
343
356
369
380
391
402
450
490
525
0.4240
0.5462
0.5824
0.5788
0.6264
0.6594
0.6837
0.702
0.7179
0.7298
0.7404
0.7493
0.7568
0.7636
0.7688
0.7749
0.7767
0.7767
0.7776
0.7777
0.7782
0.7778
0.7797
0.7786
0.7979
0.8064
0.81 26
0.81 72
0.7940
Gas
Gas
Gas
Gas
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Liquid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
Solid
CH3 CHs CHJ H
and
c=c
C=C
II
II
II
I1
H CHs
HH
(cis-2-butene) (trans-2-butene)
With the inclusion
of
these
two
geometric isomers, butene has a total of four isomers.

Chemistry
307
Alkenes also form a homologous series; as the carbon number increases, the number
of
possible isomeric structures for each member increases more rapidly than in the
case of the alkane series.
The physical properties of alkenes [49,
p.
1521
are not very different from the
corresponding members
of
the alkane family. Alkenes are nonpolar or at most weakly
polar. They are insoluble in water but are soluble in concentrated
H,SO,
and liquid
HF.
Analogous to n-alkanes, normal alkenes dissolve in nonpolar or weakly polar
organic liquids such as ethers, CCl,, and hydrocarbons. In general, the cis- isomer
has a slightly higher polarity, a higher boiling point, and a lower melting point than
the trans- isomer, but there are exceptions. Chemically, olefins are more reactive
than paraffins due to the presence of the double bond, which can be split into two
single (stable) bonds.
Alkadienes, alkatrienes, and alkatetraenes (poly-enes).
These are unsaturated
aliphatic hydrocarbons containing two, three, or four
C=C
double bonds, respectively.
Alkadienes are also called “diolefins” or “dienes,” and alkatrienes are also known as
“triolefins” or “trienes.” Alkenes containing multiple double bonds fall under the
general class of “poly-enes.” Double bonds that alternate with single bonds in a straight
chain are said to be
conjugated.
Examples are
CH,= CH
-
CH CH,
(1,3-butadiene)
CH,
=
CH
-
CH
=
CH
-
CH
=
CH,
Double bonds that occur together in a straight chain are said to be
cumulated.
(1,3,5-hexatriene)
Examples are
CH,
=
C
=
CH,
CH,
-
CH
=
C
=
CH
-
CH,
If
the double bonds are separated by more than one single bond, they are said to
[allene (propadiene)]
(2,3-pentadiene)
be
isolated
or
unconjugated.
An example is
CH,
=
CH
-
CH,
-
CH
=
CH,
Although alkadienes have a higher degree
of
unsaturation than alkenes, their
chemical behavior is similar to alkenes, and their physical properties are similar to
alkanes containing the same number of carbon atoms. Common
alkenyl
groups include
(1,4-pentadiene)
CH,,
=
CH
-
CH,
=
CHCH,
-
CH,CH
=
CH
-
CH,CH
=
CHCH,
-
(vinyl) (allyl) (propenyl) (crotyl)
Alkynes.
These contain
a
single triple bond and have the general formula
CnHyn-,,
with n
2
2.
Alkynes are also referred to as “acetylinic compounds.” The simple alkynes
are alternatively named as derivatives of acetylene, e.g.,
HC-
CH
[acetylene (or ethyne)]
CH,C
=
CH
(methylacetylene or propyne)
Rutyne,
C,,H,;,
exists as two isomers:
CH,CH,C
=
CII
CH,C
=
C
-
CH,4
(1-butyne or ethylacetylene)
(2-butyne or dimethylacetylene)
308
General Engineering and Science
Physical properties of alkynes
[49,
p.
2511 are essentially similar to those
of
alkanes and alkenes. These compounds are weakly polar and are insoluble in water,
but they are quite soluble in organic solvents
of
low polarity (e.g., ether, benzene,
CCl,). Chemically, alkynes are more reactive than alkanes but behave like alkenes.
The triple bond appears to be less reactive than the double bond in some reagents
while more reactive in others. In a chemical reaction, the triple bond is usually broken
into a double bond, which may eventually split into single bonds.
Diynes and triynes refer to alkynes containing two
or
three triple bonds;
polyynes
contain multiple triple bonds. A conjugated triyne is a straight-chain hydrocarbon
with triple bonds alternating with single bonds. An examples is
CH,
-
C
When both double and triple bonds occur in the same molecule, the IUPAC system
recommends the use of both endings -ene, and -yne, with the former always preceding
the latter in the name. Common
alkynyl
groups are
C
-
C
=
C
-
C
=
C
-
CH,
(2,4,6-octatriyne)
HCGC- and HC=C-CH,-
(ethynyl) (P‘OPargYU
Cyclic
Hydrocarbons.
These are structures in which the carbon atoms form a ring
instead
of
an open chain. They are also called “carbocyclic” or “homocyclic”
compounds. They are divided into two classes: alicyclic
(or
cycloaliphatic) and aro-
matic compounds.
AIkydk
HydfoCafbOnS.
These refer to cyclic analogues of aliphatic hydrocarbons
and are named accordingly, using the prefix “cyclo-.” Their properties are similar to
their open-chain aliphatic counterparts. Alicyclic hydrocarbons are subdivided into
monocyclic (cycloalkanes, cycloalkenes, cycloalkynes, cycloalkadienes, etc.) and poly-
cyclic aliphatic compounds. Monocyclic aliphatic structures having more than
30
carbon atoms in the ring are known, but those containing
5
or
6
carbon atoms are
more commonly found in nature
[47,
p. 281.
Cycloalkanes
(or
naphthenes).
These are also known as “cycloparaffins
or
“saturated
alicyclic hydrocarbons.” They are quite stable compounds with the general formula
C,Hsn, with n
2
3
for rings without substituent groups. The first two members are
(cyclopropane)
H2C
-
CH2
CHz
-
CH2
(cyclobutane)
I
I
Or
CHz
-
CHz
the next two being cyclopentane
0
and cyclohexane
0.
For
convenience, aliphatic
rings are represented by polygons.
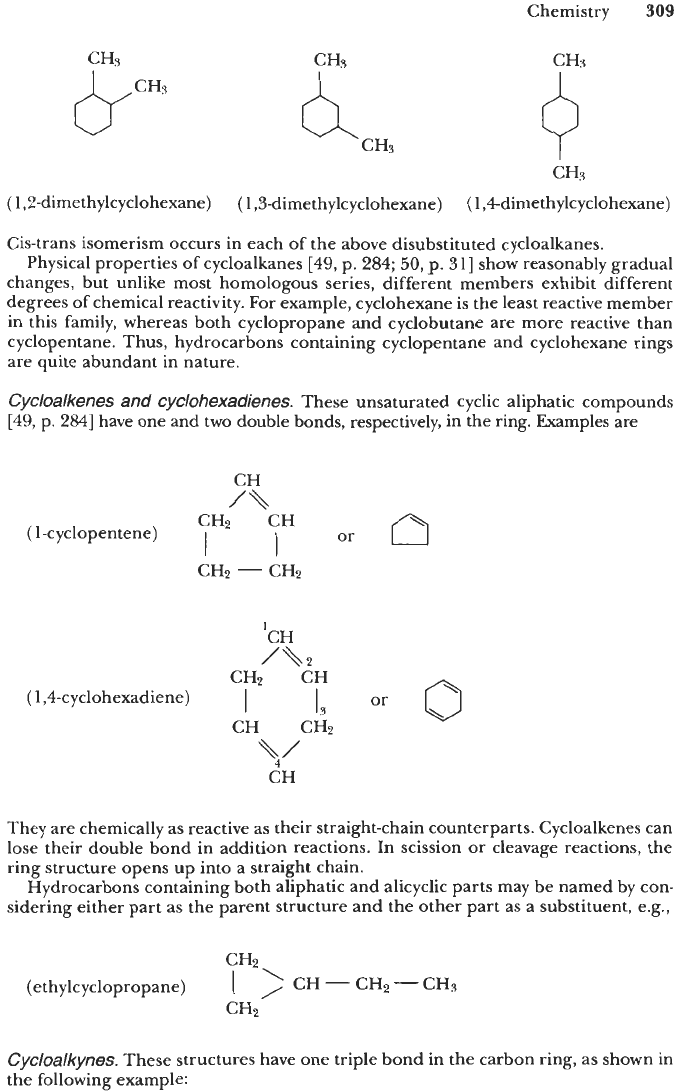
When substituent groups are present, they are identified and their positions
indicated by numbers in naming the compound.
As
an example, dimethylcyclohexane
has three structures:
Chemistry
309
b""
CHs
(
1,2-dirnethylcyclohexane)
(1,3-dimethylcyclohexane)
(
1,4-dimethylcyclohexane)
Cis-trans isomerism occurs in each of the above disubstituted cycloalkanes.
Physical properties of cycloalkanes
[49,
p.
284;
50,
p.
311
show reasonably gradual
changes, but unlike most homologous series, different members exhibit different
degrees of chemical reactivity. For example, cyclohexane is the least reactive member
in this family, whereas both cyclopropane and cyclobutane are more reactive than
cyclopentane. Thus, hydrocarbons containing cyclopentane and cyclohexane rings
are quite abundant in nature.
Cycloalkenes
and
cyclohexadienes.
These unsaturated cyclic aliphatic compounds
[49,
p. 2841 have one and two double bonds, respectively, in the ring. Examples are
or
0
CH2 CH
I
I
(1-cyclopentene)
1
CH
CH2 CH
/\2
(1,4-~yclohexadiene)
I
CH CH2
\/
CH
They are chemically as reactive as their straight-chain counterparts. Cycloalkenes can
lose their double bond in addition reactions. In scission or cleavage reactions, the
ring structure opens up into a straight chain.
Hydrocarbons containing both aliphatic and alicyclic parts may be named by con-
sidering either part as the parent structure and the other part as a substituent, e.g.,
CHz
(ethylcyclopropane)
I
,
CH
-
CH2 -CHJ
CH2
\
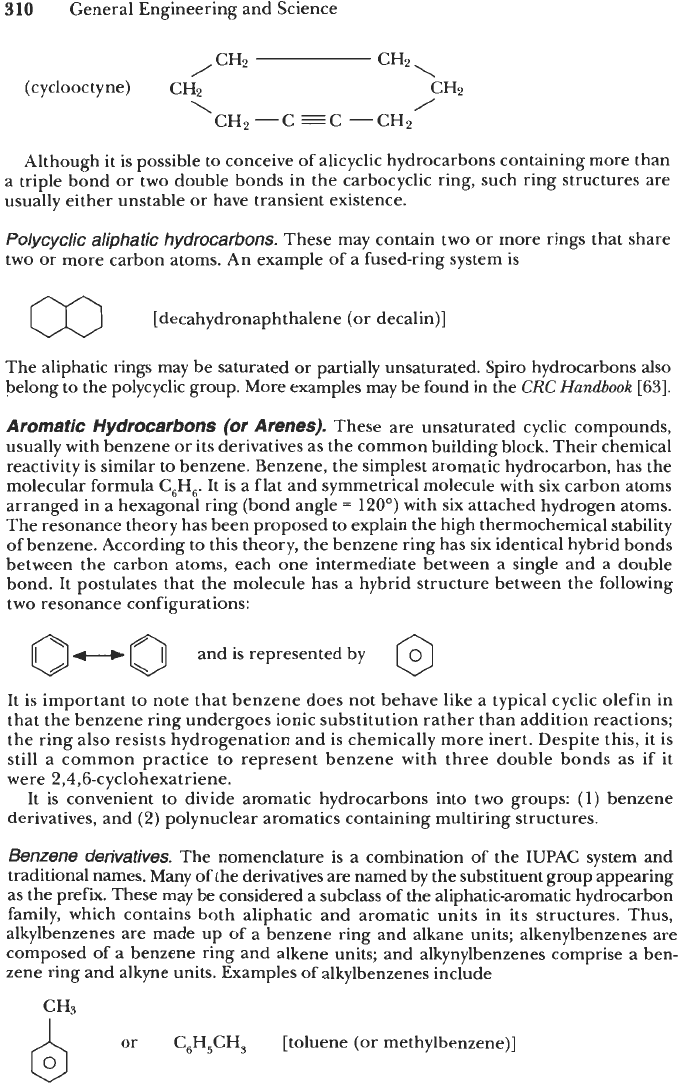
Cycloalkynes.
These structures have one triple bond in the carbon ring, as shown in
the following example:
310
General Engineering and Science
Although it is possible to conceive of alicyclic hydrocarbons containing more than
a triple bond or two double bonds in the carbocyclic ring, such ring structures are
usually either unstable or have transient existence.
Polycyclic aliphatic hydrocarbons.
These may contain two or more rings that share
two or more carbon atoms. An example of a fused-ring system is
[decahydronaphthalene (or decalin)]
03
The aliphatic rings may be saturated or partially unsaturated. Spiro hydrocarbons
also
belong to the polycyclic group. More examples may be found in the
CRC
Handbook
[63].
Aromatic Hydrocafbons
(Of
Arenes).
These are unsaturated cyclic compounds,
usually with benzene or its derivatives as the common building block. Their chemical
reactivity is similar to benzene. Benzene, the simplest aromatic hydrocarbon, has the
molecular formula C,H,. It is a flat and symmetrical molecule with six carbon atoms
arranged in a hexagonal ring (bond angle
=
120") with six attached hydrogen atoms.
The resonance theory has been proposed to explain the high thermochemical stability
of benzene. According to this theory, the benzene ring has six identical hybrid bonds
between the carbon atoms, each one intermediate between a single and a double
bond. It postulates that the molecule has a hybrid structure between the following
two resonance configurations:
and is represented by
It is important to note that benzene does not behave like a typical cyclic olefin in
that the benzene ring undergoes ionic substitution rather than addition reactions;
the ring also resists hydrogenation and is chemically more inert. Despite this, it is
still a common practice to represent benzene with three double bonds as if it
were 2,4,6-~yclohexatriene.
It is convenient to divide aromatic hydrocarbons into two groups:
(1)
benzene
derivatives, and (2) polynuclear aromatics containing multiring structures.
Benzene derivatives.
The nomenclature is a combination of the IUPAC system and
traditional names.
Many
of the derivatives are named by the substituent group appearing
as the prefix. These may be considered a subclass of the aliphatic-aromatic hydrocarbon
family, which contains both aliphatic and aromatic units in its structures. Thus,
alkylbenzenes are made up
of
a benzene ring and alkane units; alkenylbenzenes are
composed of a benzene ring and alkene units; and alkynylbenzenes comprise a ben-
zene ring and alkyne units. Examples of alkylbenzenes include
or C,H,CH, [toluene (or methylbenzene)]
Chemistry
311
If
several groups are attached to the benzene ring, their names as well as their
relative positions should be indicated.
For
example, dimethylbenzene or xylene,
C H
(CH,),,
has three geometric isomers, with prefixes ortho-, meta-, and para-,
iididating the relative positions
of
the
two
methyl groups.
4
CHs
[ortho-xylene (o-xylene or
[meta-xylene (m-xylene or [para-xylene (p-xylene
or
1,2-dimethylbenzene)]
1,3dimethylbenzene)] 1,4-dimethylbenzene)l
Examples
of
an alkenylbenzene and an alkynylbenzene are given below:
CH
=
CH2 CECH
[styrene (phenylacetylene)
(vinylbenzene)]
When the benzene ring is regarded as the substituent group,
it
is called phenyl
group and is represented by
An example
is
3
2
1
CH2
-
CH
=
CH2 or
CH,CHCH,(C,H,)
(3-phenylpropene)
Polynuclear aromatic hydrocarbons.
These consist
of
a variety
of
complex structures
made up of aromatic rings alone, or combinations
of
aliphatic rings, aromatic rings,
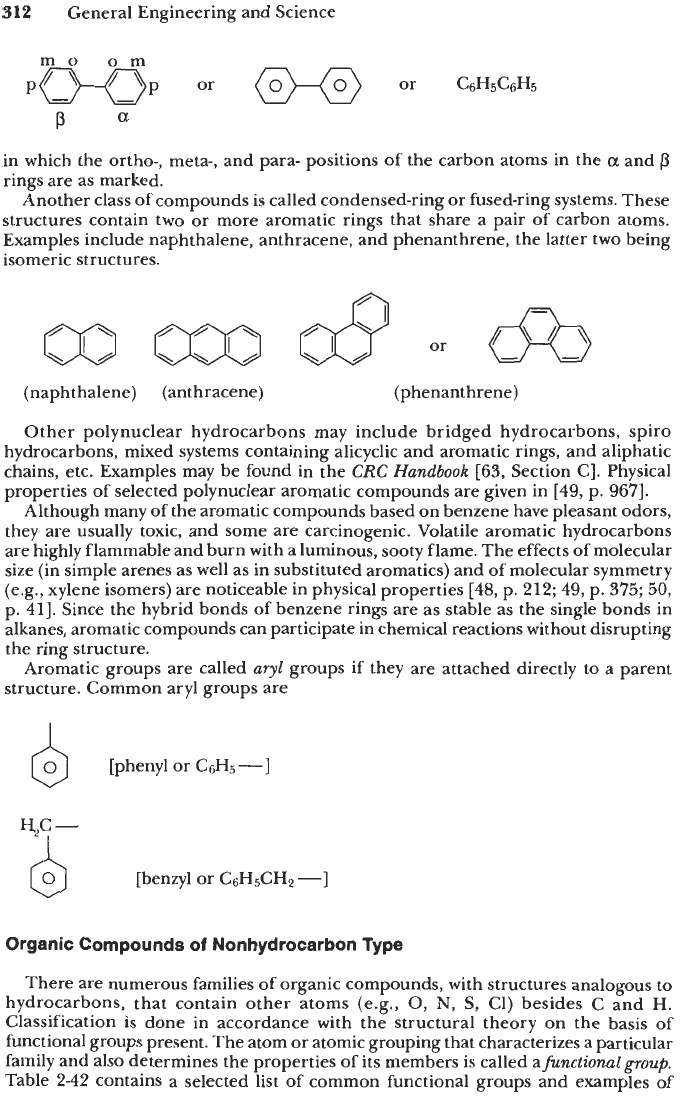
and aliphatic chains, etc. One such class of compounds is biphenyl and its derivatives,
in which
two
benzene rings are connected by a single C
-
C linkage. The structural
formula of biphenyl (or phenylbenzene) is
312
General Engineering and Science
mo om
pmp
/\
/\
or Or C6H5C6H5
Pa
in which the ortho-, meta-, and para- positions of the carbon atoms in the
a
and
p
rings are as marked.
Another class of compounds is called condensed-ring or fused-ring systems. These
structures contain two or more aromatic rings that share a pair of carbon atoms.
Examples include naphthalene, anthracene, and phenanthrene, the latter
two
being
isomeric structures.
mador&
/
\\
(naphthalene) (anthracene) (phenanthrene)
Other polynuclear hydrocarbons may include bridged hydrocarbons, spiro
hydrocarbons, mixed systems containing alicyclic and aromatic rings, and aliphatic
chains, etc. Examples may be found in the
CRC
Handbook
[63,
Section C]. Physical
properties of selected polynuclear aromatic compounds are given in
[49,
p.
9671.
Although many of the aromatic compounds based on benzene have pleasant odors,
they are usually toxic, and some are carcinogenic. Volatile aromatic hydrocarbons
are highly flammable and burn with a luminous, sooty flame. The effects of molecular
size (in simple arenes as well as in substituted aromatics) and of molecular symmetry
(e.g., xylene isomers) are noticeable in physical properties
[48,
p.
212; 49,
p.
375;
50,
p.
411.
Since the hybrid bonds of benzene rings are as stable as the single bonds in
alkanes, aromatic compounds can participate in chemical reactions without disrupting
the ring structure.
Aromatic groups are called aryl groups if they are attached directly
to
a parent
structure. Common aryl groups are
[phenyl or C6H5
-1
6
Organic Compounds
of
Nonhydrocarbon Type
There are numerous families
of
organic compounds, with structures analogous
to
hydrocarbons, that contain other atoms (e.g.,
0,
N,
S,
C1) besides C and
H.
Classification is done in accordance with the structural theory on the basis of
functional groups present. The atom or atomic grouping that characterizes a particular
family and also determines the properties of its members is called afunctional
group.
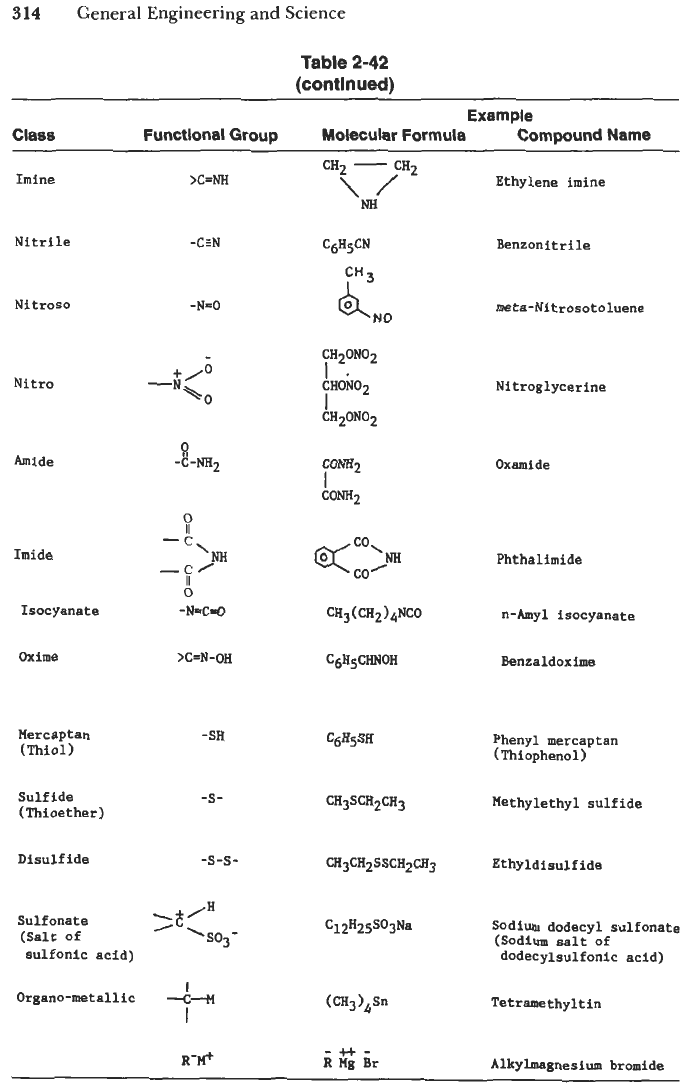
Table
2-42
contains a selected list of common functional groups and examples of
Chemistry
313
Table
2-42
Selected
Functional Groups and Representative Organic Compounds
[58]
Example
Class Functional Group Molecular Formula Compound Name
Alkene
>c=c<
CH,CH
=
CH,
Propylene
Cyclopropylacetylene
CH2\
I
CHC
S
CH
CH2’
Alkyne
-cat-
OH
sec-Butyl alcohol
I
CHjCH2CHCHj
Alcohol
Ether
-OH
-0-
cH30c6H5
Methylphenyl ether
Ethylene oxide
cHvcHz
/O\
>c
-
c<
Epoxide
(Oxirane)
Peroxide
-0-o-
(CH3)3COOC(CH3)3
Di-tert-butyl peroxide
a
-C-H
Aldehyde
C&CHO
Benzaldehyde
8
-C-
Ketone
CH3COCH3
Acetone
(Methyl ketone)
!?
-C-OH
Carboxylic acid
CgHgCOOH
Benzoic acid
a
-c-0-
0
II
-c\
-c
H0
I/
0
Ester
Acid anhydride
CHjCOOCHzCHj
Ethyl acetate
(CH3CO)
20
Acetic anhydride
Halide
Acid halide
-X
8
-c-x
Trichloromethane
(chloroform)
Benzoyl chloride
Amine
Trimethylamine
314
General Engineering and Science
Table
2-42
(continued)
Example
Functlonal Group Motfsular Formula Compound Name
Class
Ethylene imine
Imine
>C=NH
cH<-7
NH
Nitrile -CSN C6HgCN Benzonitrile
Nitroso
-N=O
meta-Nitrosotoluene
Nitro
Amide
Imide
Isocyanate
Oxime
Mercaptan
(Thiol)
+/O
NO
-N
!?
-c-m2
0
‘NH
-7’
CH2ONO2
CHClNO2
I
I
CH2ON02
CONH2
I
corn2
-SH
Sulfide
-s-
(Thioether)
Disulfide
-s-s-
‘‘@
‘~03-
I
Sulfonate
(Salt: of
sulfonic acid)
I
Organo-metallic
-C---fl
I
R-Hf
Nitroglycerine
Oxamide
Phthalimide
n-Amyl isocyanate
Benzaldoxime
CgHgSH Phenyl mercaptan
(Thiophenol)
CH3SCH2CH3 Methylethyl sulfide
CH3CH2SSCH2CH3 Ethyldisulfide
Sodium dodecyl sulfonate
(Sodium salt
of
dodecylsulfonic acid)
1
2H25s03Na
(m3)*Sn Tetramethyltin
-*-
R
Mg Br Alkylmagnesium bromide
