Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Chemistry
345
n
e
Lu
U
3
u3
w
e
v
a
-
LI
SPEClFlC
VOLUME
(v^)
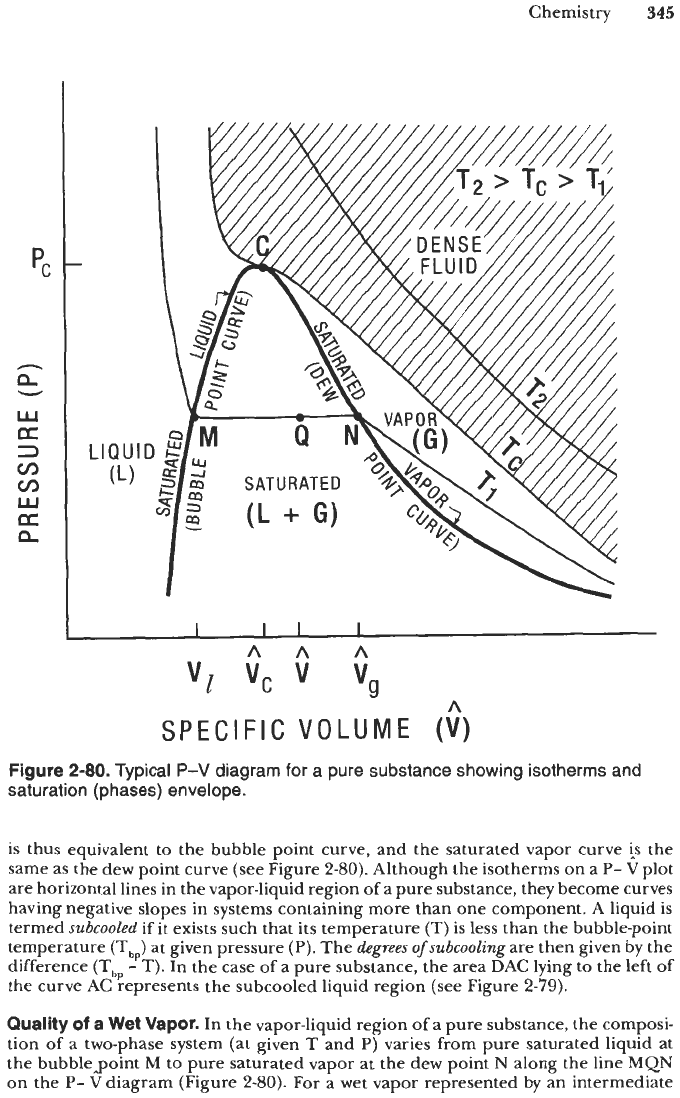
Figure
2-80.
Typical
P-V
diagram for a pure substance showing isotherms and
saturation (phases) envelope.
is thus equivalent to the bubble point curve, and the saturated vapor curve
is
the
same as the dew point curve (see Figure
2-80).
Although the isotherms on a
P-
V
plot
are horizontal lines in the vapor-liquid region
of
a pure substance, they become curves
having negative slopes in systems containing more than one component. A liquid is
termed
subcooled
if
it
exists such that its temperature
(T)
is less than the bubble-point
temperature
(T,J
at given pressure
(P).
The
degrees
ofsubcooling
are then given by the
difference
(Tbp
-
T).
In the case of a pure substance, the area
DAC
lying to the left of
the curve
AC
represents the subcooled liquid region (see Figure
2-79).
Quality
of
a
Wet Vapor.
In the vapor-liquid region
of
a pure substance, the composi-
tion of a two-phase system (at given
T
and
P)
varies from pure saturated liquid at
the bubblepoint
M
to pure saturated vapor at the dew point
N
along the line
MQN
on the
P-
V
diagram (Figure
2-80).
For
a wet vapor represented by an intermediate
546
General Engineering and Science
point
Q,
the
qudity
(4) refers to the mass fraction of the saturated vapor present in
the two-phase mixture. If m, and mg indicate the masses of saturated liquid and
saturated vapor comprising the wet vapor at point
Q,
then its quality
may
be calculated
by the application of the lever rule as
mg
-
?-et
-
lengthMQ
me +mg
cg
-f~,
lengthMN
9'
The origin
o,f
this rule lies in the expression for
the
specific volume
of
the wet
vapor,
V
=
qV
+
(1
-
q)V,. The specific intensive properties of the wet vapor are
obtained readily from the individual properties of its component phases by an
analogous equation:
?
where the symbols
312.
312.,
and
312.
refer to the specific properties of the saturated
vapor, saturated liquis, and the two-phase mixture, respectively.
Clausius-Clapeyron Equation.
This equation
was
originally derived to describe the
vaporization process of a pure liquid, but it
can
be also applied to other two-phase
transitions of a pure substance. The Clausius-Clapeyron equation relates the variation
of
vapor pressure (P*) with absolute temperature
(T)
to
the molar latent heat of vaporization,
kv,
Le., the thermal energy required to vaporize one mole of the
pure
liquid
By neglecting the specific-molar volume of the saturated liquid
?e
in relation to
that of the satura_ted vaporV,, and by assuming the vapor phase to behave as an
ideal gas, i.e., P* V,= RT, the above equation may be arranged into
This suggests that a plot
of
fn
P*
against 1/T should yield
a
line having a local slope
of
(-hv/R).
A straight line is obtained only when is nearly constant, Le., over a
narrow range of temperatures. An integrated version of the Clausius-Clapeyron
equation finds use in correlation of vapor pressure data:
where
P,*
is the known vapor pressure at reference temperature To, and &is the
average value of
hv
between T, and T. The above equation is reasonably valid
when the range IT,
-
TI is small and when the two-phase region of interest is far
away from the critical point.
Example
2-39
point
of
80.1"C.
Estimate the molal latent heat of vaporization for pure benzene at its normal boiling

Chemistry
347
Solution
Two estimates will be made using vapor pressure data from the
CRC
Handbook
[63]
and
the integrated form of Clausius-Clapeyron equation:
Estimate
(2):
From page
D-218
of
the
CRC
Handbook
[63],
P,*
=
1
atm at To
=
80.1"C
=
353.3'K and P*
=
2
atm at T
=
103.8OC
=
377.0'K. With
R
=
1.987 cal/g-mole
OK,
we have
-
h
1
(1.937)
(&
-
377)
=
0.6932=
-
or
-
h,
=
7,740
cal/g-mole
Estimate
(ii):
From page D-208 [63],
P,*
=
400
m-mHg at
To
=
60.6"C
=
3333°K and
The second estimate compares better with the literature value of -7,700 cavg-mole.
P*
=
760 mmHg at T
=
80.1"C
=
353.3'K. Then,
h,
=
7,713 cal/g-mole.
Vapor-Liquid Equilibria in
Binary
and Multicomponent Systems
As
discussed earlier, a N-component system with
N
7
1 does not exhibit a single
boiling point at a given pressure as a pure substance does. Instead, at a constant
pressure, a liquid mixture of fixed composition undergoes a change of phase to
vapor over a range of temperatures lying between the bubble point and the dew
point. Three different approaches-Raoult's law, Henry's law, and the concept of
the equilibrium ratio
or
K
factor-are available
for
computations involving vapor-
liquid equilibria.
Raoult's
Law.
The molar composition of a liquid phase (ideal solution) in equilibrium
with its vapor at any temperature T is given by
xi
=
P,/PF(T)
i
=
1,
2,
. .
.,
N
If
the vapor phase behaves as an ideal gas mixture, then by Dalton's law of partial
pressures,
yi
=
Pi/P
=
xiP:(T)/P
i
=
1,
2,
.
.
.,
N
where xi and
yi
are the respective mole fractions of component i in the liquid and
vapor phases, P:(T) is the equilibrium vapor pressure
of
pure liquid i at system
temperature
T,
Pi is the partial pressure
of
i in the vapor phase, and
P
is the total
pressure
of
the
vapor phase. Each component i is distributed between the two phases
to
an
extent dictated by the relative volatilities
of
the components at the system
temperature. Note that Exi
=
1,
Cyi
=
1,
and
P
=
zxiP,*.
Raoult's law is strictly applicable to ideal liquid solutions at all compositions,
pressures, and temperatures. In an ideal
or
perfect solution, the components are
348
General Engineering and Science
mutually miscible in all proportions and there are no volume or thermal changes
upon mixing. Solutions that approach ideality include mixtures of nonpolar
hydrocarbons belonging to a homologous family (e.g., paraffins). Whereas binary
mixtures of propane-butane, n-hexane-n-heptane, benzene-toluene, etc., show ideal
behavior over a range of compositions, nonideal solutions comprising a solvent
(1)
and a solute
(2)
obey Raoult's law only in the limit of x,
+
1
(i.e., in dilute solutions).
Raoult's law finds use in molecular weight determination of nonvolatile solutes in
dilute solutions, in estimation of equilibrium solubilities of noncondensable gases in
nonpolar liquids, and in vapor-liquid equilibria calculations.
Molecular Weight Determination
by
Application
of
Raoult's
Law.
If a small amount
(m, in grams) of a nonvolatile, nonionized substance (solute,
2)
is dissolved in m,
grams
of
a volatile liquid (solvent,
l),
it experiences a lowering
of
vapor pressure
from the pure solvent value
(P:)
to the solution value (P) at the system temperature,
This is a consequence of Raoult's law because the total vapor pressure of the dilute
solution
(3
<<
1)
is given by
P*
=
xiP:
+
xpP;"
xiP,*.
(a) The
relative
lowering
of
vapor pressure
of
the solvent
is
then
Measurement of P* and P: at one temperature enables determination of
unknown
M,.
(b) Equivalently, the presence of the nonvolatile solute causes an
elevation
of
the
boiling
point
of
the liquid from pure solvent value T, to solution value T. The above
equation
is
coupled with Clausius-Clapeyron equation to yield
where
Av
is the molal latent heat of vaporization of pure solvent at its boiling point
(T,) and R is the universal gas constant. By measuring
ATb
and T, at the
same
total
pressure,
3
can be computed and hence
M,.
(c) An alternative procedure
is
to consider the presence of the solute as responsible
for a
depression
in
thefreezingpoint
of the solvent from the pure liquid value
Tf
to the
solution value
T.
Application
of
Raoult's law together with Clausius-Clapeyron equation
gives a similar result:
where
A,
is the molal latent heat
of
fusion of the solvent at
T,
Techniques
(b)
and
(c) provide more accurate
data
and
therefore better estimates for
hlp
than method
(a).
Example
2-40
A solution is prepared by dissolving
0.91
1
g
of carbon tetrachloride in
50.00
g of
benzene. (i) Calculate the freezing point depression of the solution if pure benzene
has a fp of
5.53"C
and a latent heat of fusion
of
30.45
cal/g. (ii) What will be the
elevation in boiling point of the solution if pure benzene has a nbp of
80.1"C
and a
latent heat
of
vaporization of
7,700
cal/g-mole
[63].

Chemistry
349
Solution
MW
of solute (CCl,)
=
M,
=
153.82
MW
of solvent (C,H,)
=
M,
=
78.12
m,
=
0,911 g
m,
=
50.00 g
(i) Because
3
<<
1, the solution is dilute and Raoult's law may be applied
With
R
=
1.987 cal/g-mole, T,
=
273.2
+
5.53
=
278.73"K, and
h,
=
(30.45)(78.12)
cal/g-mole, we obtain AT,
=
0.595"C, which compares well with an experimental
observation of 0.603"C.
(ii) With
T,
=
273.2
+
80.1
=
353.3"K,
kv
=
7,700 cal/g-mole, we get
Henry's
Law.
This is an empirical formulation that describes equilibrium solubilities
of noncondensable gases in a liquid when Raoult's law fails. It states that the mole
fraction of a gas (solute i) dissolved in a liquid (solvent) is proportional to the partial
pressure of the gas above the liquid surface at given temperature. That is,
xi
=
P,/H,(T)
where the constant of proportionality H,(T) is known as Henry's law constant, with
units of pressure per mole fraction. It
is
a characteristic of the gas-liquid system and
increases with T. Experimentally determined values of Hi are available in the standard
references for various gas-liquid systems [61,62,65,66]. If the gas phase is assumed
to be ideal, then the equilibrium mole fraction of component i in the gas phase is
y,
=
Pi/P
=
xiHi(T)/P
Henry's law is a reasonable approximation in the absence of gas-liquid reactions
when total pressure
is
low to moderate. Deviations are usually manifested in the form
of Hi dependence
on
P
and
phase compositions.
Example
2-41
A
gas mixture has a molal composition of
20%
H,S, 30% CO, and
50%
N,
at
20°C
and a total pressure of
1
atm.
Use Henry's law to calculate the volumes of these gases
that may be dissolved in 1,000 Ib
of
water at equilibrium.
Solution
By
Henry's law, the partial pressure of solute i in
the
gas phase is Pi
=
Hi(T)xi,
where
5
is
the mole fraction of i in solution. Data on Henry's law constant are obtained
350
General Engineering and Science
from Chapter 14 of Perry and Chilton's
Chemical Engineers' Handbook
[62] for gas-
water systems at 20°C.
Gas i: H,S
COZ
NZ
Hi (atm/mole fraction): 4.83
x
10' 1.42
x
lo5
8.04
x
lo4
Assuming ideal-gas law to hold, Pi
=
yip, where P
=
1
atm and
yi
=
mole fraction of i
in the gas phase. The equilibrium mole fraction xi of gas i in solution is then given by
xi
=
Pi/Hi
=
yiP/Hi
"2s
0.20
0.20 4.14
x
lo4
2.30
x
le2
8.86
CO2
0.30
0.30 2.11
x
104
1.17
x
1W
4.52
N2
0.50
0.50
6.22
x
IOd
3.45
x
104 0.13
The above table shows the rest of the required results for the number of moles ni
of each gas i present in the aqueous phase and the corresponding gas volume
Vi
dissolved in it. Since xi
<<
1, the total moles
of
liquid is n (1000/18.02) Ib-moles
in 1000 lb of water and
so
ni
=
xin can be calculated. At T
=
20°C
=
68°F
=
528"R
and P
=
1
atm, the molal volume of the gas mixture
is
The volume
of
each gas dissolved in 1,000 lb of water is then
V,
=
ni
?
Equilibrium Distribution Ratio
or
K
factor.
This is also termed
distribution coefficient
in the literature; it is a widely accepted method of describing vapor-liquid equilibria
in nonideal systems. For any component i distributed between the vapor phase and
liquid phase at equilibrium, the distribution coefficient or
K
factor is defined by
K
=
yi/xi
=
K,(T,P)
The dimensionless
Ki
is regarded as a function of system T and P only and not
of
phase compositions. It must be experimentally determined. Reference 64 provides
charts of
K,
(T,P) for a number of paraffinic hydrocarbons.
Ki
is found to increase
with an increase in system
T
and decrease with an increase
in
P.
Away from the
critical point, it is invariably assumed that the
K
values of component
i
are independent
of the other components present in the system. In the absence of experimental data,
caution must be exercised in the use of K-factor charts for a given application. The
term
distributim
coefficient
is also used in the context
of
a solute (solid or liquid)
distributed between
two
immiscible liquid phases;
yi
and
x,
are then the equilibrium
mole fractions of solute
i
in each liquid phase.
Example
242
The
distribution coefficient for n-heptane (solute i) distributed between ethylene
glycol
(solvent
1)
and
benzene (solvent 2)
at
25°C
is given
as
the
ratio of
mass
fractions

Chemistry
351
(w~,Jw~,~)
=
Ki
=
0.30.
Suppose 60
g
of n-heptane are added to a solvent mixture of 600
g
of
ethylene glycol and 400 g of benzene. Assuming that the solvents are immiscible,
determine the amount of n-heptane dissolved in each liquid phase at equilibrium.
Solution
Consider the equilibrium state of the system assuming that m grams
of
n-heptane
are dissolved in the benzene phase. Then the mass fraction of n-heptane in this phase
is
wiZ
=
m/(400
+
m).
Because (60
-
m) g of i
are
present in the glycol phase, the equilibrium mass fraction
of
i
in this phase
is
=
(60
-
m)/(600
+
60
-
m). Thus,
(60-m) (400+m)
oi,l=
Ki
=
0.3
=
mi.2
(660-m) m
which must be solved for m.
A
quadratic equation in m is obtained by cross-
multiplication and rearrangement of terms:
0.3m (660
-
m)
-
(60
-
m)(400
+
m)
=
0
or
0.7m2
+
538m
-
24,000
=
0
Its roots are
-538
f
d(
538)'
-
4( 0.7)( -24,000)
2(0.7)
m=
Only the positive root is the physically meaningful value and
so
m
=
(-538
+
597.197)/1.4
=
42.28 g
=
42.284/442.28
=
0.0956 in benzene phase
and
ml,,
=
(60
-
42.28)/617.72
=
0.0287 in glycol phase
Check:
Note that
w,,Jw,,,
=
0.0287/0.0956
=
0.3
Thermochemistry
Thermochemistry
is concerned with the study of thermal effects associated with
phase changes, formation of chemical compounds or solutions, and chemical reactions
in general. The amount
of
heat
(Q
liberated (or absorbed) is usually measured either
in a batch-type bomb calorimeter
at
fixed volume or in a steady-flow calorimeter at
constant pressure. Under these operating conditions,
Q
=
Q
=
AU (net change in the
internal energy of the system) for the bomb calorimeter, while
Q
= =
AH
(net
change in the enthalpy of the system) for the flow calorimeter. For a pure substance,

352
General Engineering and Science
the thermodynamic properties
U
and H are functions of the state variables, viz.
temperature (T) and pressure
(P),
and its state of aggregation [e.g., liquid
(e),
gas
or
vapor (g), solid (s), etc.]. Here, we will briefly review certain basic definitions and
terminology employed in the area of thermochemistry and consider some applications
pertaining to combustion of fuels.
Heat
of
Reaction
(AH,).
The heat of a chemical reaction carried out
at
constant
pressure
(P)
is given by the difference between the total enthalpies of the reactants
and products.
where the subscripts i andj refer to reactant
i
and productj, n represents the number
of moles, and symbols
XR
and
&.
imply summations over all reactants (i
=
1,
2,
.
.
.)
and all products
(i
=
1,
2,
. .
.),
respectively. AHr has units of calories or BTUs; its
value depends on the amounts and physical states of the reactants and products as
well as on the reaction conditions. Note that AHr is
negative
for an
exothermic
reaction
in which heat is spontaneously liberated and is
positive
for an
endothermic
reaction in
which heat is absorbed from the surroundings.
Standard-State Enthalpy Changes
(AH').
To expedite calculations, thermochemical
data are ordinarily presented in the form of standard-state enthalpy changes of the
system AH"(T,P), with the requirement that materials start and end at the same
temperature (T) and pressure
(P)
and in their standard states of aggregation, Le.,
It has been traditional to choose the reference state
as
P
=
1
standard atmosphere
and T
=
25°C
(77'F)
in expressing AHo values. Examples include standard heats of
reaction AH:, heats of formation AH:, heats of combustion
AH:,
heats of
vaporization AH: or
a,
heats of solution AH:, etc. To avoid confusion, the standard
state
of
aggregation of each substance taking part in the thermochemical process
must be specified by an appropriate letter symbol adjoining its chemical formula.
The standard state for a gas is the ideal gas at
1
atm and specified T. The standard
state for a solid is its stable crystalline form (e.g., rhombic sulfur) or amorphous form
existing at the specified
P
and
T.
In the absence of such information, the normal
state of aggregation of the material at given
P
and T is assumed. Tabulated values of
standard-state enthalpy changes (AHo) are readily available from a number of sources
including handbooks and textbooks [59-63,65,66].
Standard Heat
of
Reaction.
This is the standard enthalpy change accompanying a
chemical reaction under the assumptions that the reactants and products exist in
their standard states
of
aggregation
at
the same
T
and
P,
and stoichiometric amounts
of reactants take part in the reaction
to
completion at constant
P.
With
P
=
1
atm and
T
=
25°C as the standard state, AH:(T,P) can be written as
AH;(25"C,l am)
=
~:jH~(250C,1 atm)-~viH~(25"C,1
am)
P
R
Because
v
represents the stoichiometric coefficient of a given species, the value of
AH: clearly depends on the way the stoichiometric equation is written for the reaction.
It is conventional to express the above equation in a simplified form as

Chemistry
353
AH:(25"C,1 atm)
=
EujHY -xuiHP
P
R
where it is understood that HP and H,' are to be evaluated at the reaction conditions
of 1 atm and 25°C. Another common practice is to use the number
of
moles ni (or ni)
in place of the stoichiometric coefficient
vi
(or
vj).
Relation Between
Q,
and
Q,.
Let the heats of reaction measured at constant pressure
and at constant volume be
=
AHr(T,P) and
Q
=
AUJT,P). Since H
=
U
+
PV,
it
follows that
(L,
and
Q
are related by
AHr
=
AUr
+
PAV
where AV
=
total volume of products
-
total volume of reactants.
If all of the species are gaseous and obey the ideal gas law, then
AHr
=
AUr
+
RTAn
where An
=
Cpnj
-
zRni
=
net change in the number
of
moles of the system.
The
standard heat
of
formation
(
AH:
)
of a chemical compound is the standard heat
of reaction corresponding to the chemical combination of its constituent elements
to form one mole of the compound, each existing in its standard state at
1
atm and
25°C. It has units of cal/g-mole.
By convention, the standard-state enthalpies of the elements (H:) are taken to be
zero at
1
atm and 25°C
so
that
A#
=
H!o,,,Fnd
.
The
standard heat
of
combustion
(
AH:)
of a chemical substance (usually an organic
compound) is the same as the standard heat of reaction for complete oxidation of
1
mole of the substance in pure oxygen to yield CO,(g) and H,O(P) as products.
A
reference state of 25°C and
1
atm is assumed in quoting standard heats of combustion
in cal/gmole. The value of AH: is always negative because combustion is an
exothermic reaction. Note that the standard heats of combustion
for
carbon and
hydrogen are the same as the heats of formation for CO,(g) and
&O(P),
respectively.
Laws
of
Thermochemistry.
Lavoisier and Laplace (1780) found that the heat
required to decompose a chemical compound into its elements was numerically
equal to the heat generated in its formation under the same conditions of T and
P.
That is, AHd
=
-AH, where the subscript d refers to decomposition reaction
[52,
p.
24;
61,
p.
3031.
An important corollary of this postulate is known as
Hess's
law
of
constant heat
summation
(1840): The overall heat of a chemical reaction is the same whether the
reaction occurs in a single step or multiple steps.
The two basic principles permit the algebraic manipulation of chemical reactions
(represented by their stoichiometric equations and associated enthalpy changes) in
order to achieve desired thermochemical results.
Applications.
(1)
Heats of formation data of reactants and products can be used to
calculate the standard heat of a chemical reaction by applying Hess's law. Thus,
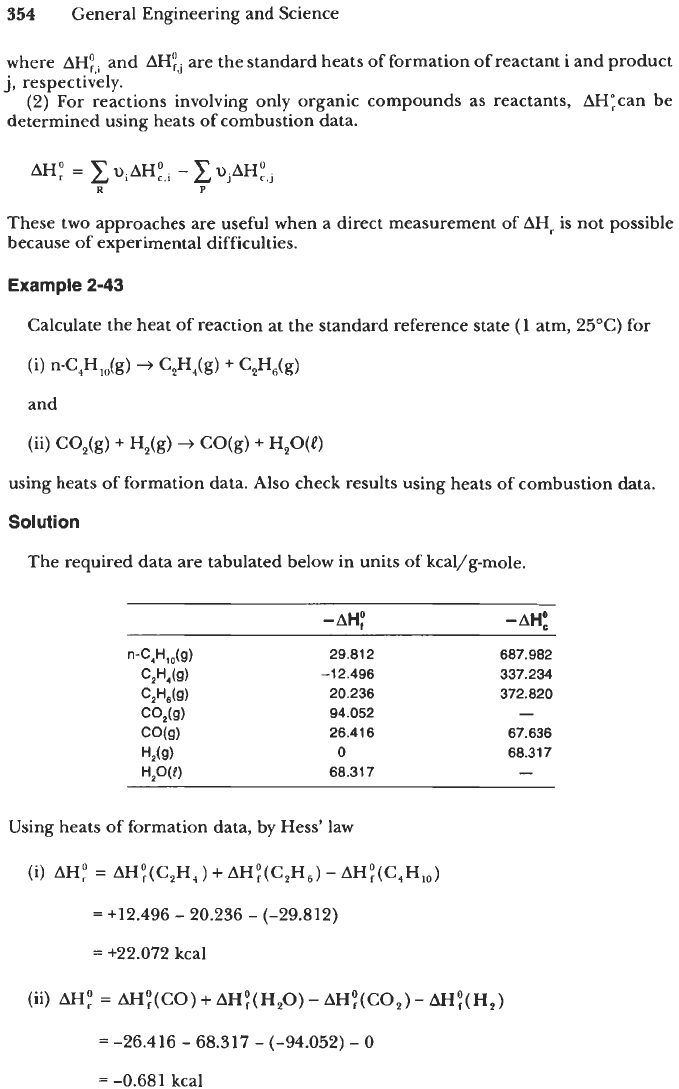
354
General Engineering and Science
where
AH:,i
and
AH:j
are the standard heats of formation of reactant i and product
j,
respectively.
(2)
For reactions involving only organic compounds as reactants, AH",an be
determined using heats of combustion data.
R
P
These two approaches are useful when a direct measurement of
AHr
is not possible
because of experimental difficulties.
Example
2-43
Calculate the heat
of
reaction at the standard reference state
(1
atm,
25°C)
for
(i)
nG,H,"(g)
'zH4($)
+
'ZH,(g)
and
(ii) CO,(g)
+
qg)
+
cow
+
H,W)
using heats of formation data. Also check results using heats
of
combustion data.
Solution
The required data are tabulated below in units of kcal/g-mole.
29.812 687.982
-12.496 337.234
20.236 372.820
94.052
-
26.416 67.636
0
68.317
68.317
-
Using heats of formation data,
by
Hess' law
(i)
AH:
=
AH;(c,H,)
+
AH;(c,H,)
-
AH;(c,H,,)
=
+12.496
-
20.236
-
(-29.812)
=
+22.072
kcal
(ii)
AH:
=
AJ3:(CO)
+
AH:(H,O)
-
AHf(C0,)
-
AH:(H,)
=
-26.416
-
68.317
-
(-94.052)
-
0
=
-0.681
kcal
