Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Corrosion and Scaling
1305
combinations
of
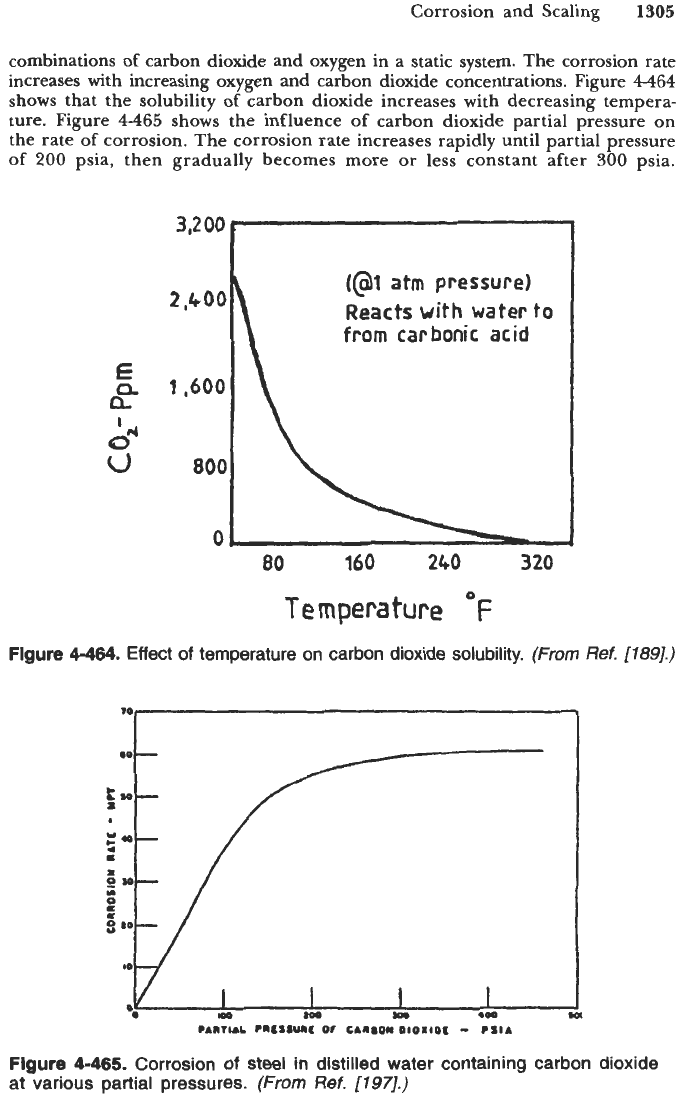
carbon dioxide and oxygen in a static system. The corrosion rate
increases with increasing oxygen and carbon dioxide concentrations. Figure
4464
shows that the solubility
of
carbon dioxide increases with decreasing tempera-
ture. Figure
4-465
shows the influence
of
carbon dioxide partial pressure on
the rate
of
corrosion. The corrosion rate increases rapidly until partial pressure
of
200
psia, then gradually becomes more or less constant after
300
psia.
3,200
t
(@I
atm
pressure)
from
carbonic
acid
2,600
Reacts
with
water
to
1,600
800
0
80
160
260
320
Temperature
OF
Figure
4-464.
Effect
of
temperature on carbon dioxide solubility.
(From
Ref.
[189].)
10
a0
-
PA~TIAL
P~SSSUIC
01
CAl8ON
OIOIIOC
-
PSI.
Figure
4-465.
Corrosion
of
steel in distilled water containing carbon dioxide
at various partial pressures.
(From
Ref.
[797].)
1306
Drilling and Well Completions
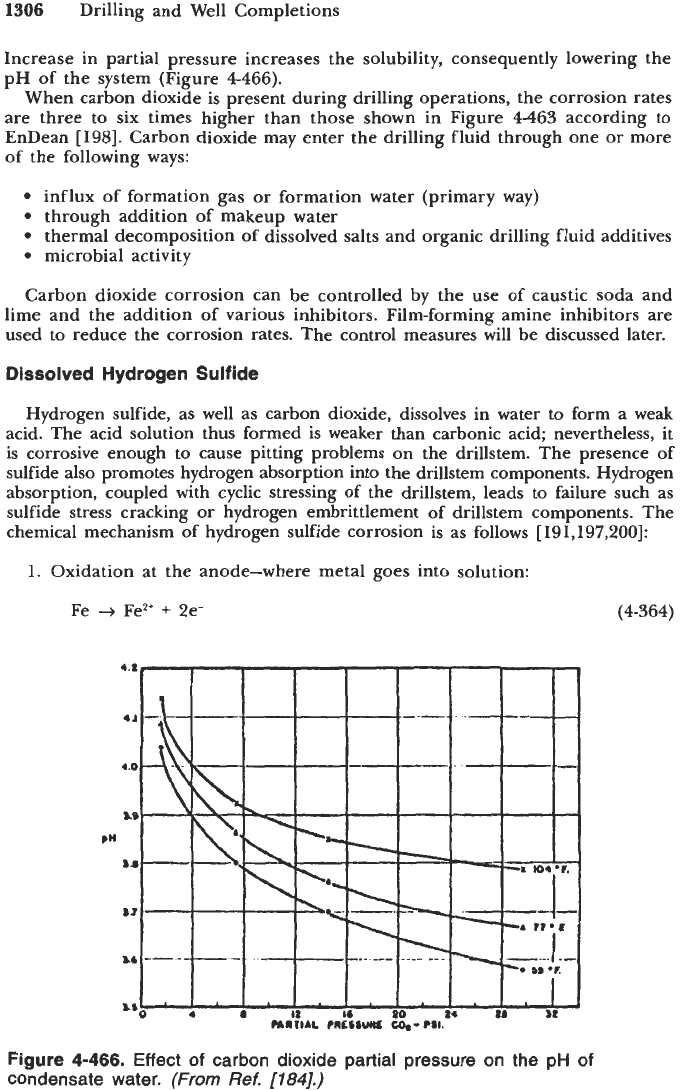
Increase in partial pressure increases the solubility, consequently lowering the
pH of the system (Figure
4-466).
When carbon dioxide is present during drilling operations, the corrosion rates
are three to six times higher than those shown in Figure
4-463
according to
EnDean [198]. Carbon dioxide may enter the drilling fluid through one or more
of the following ways:
influx of formation gas or formation water (primary way)
through addition of makeup water
thermal decomposition of dissolved salts and organic drilling fluid additives
microbial activity
Carbon dioxide corrosion can be controlled by the use of caustic soda and
lime and the addition of various inhibitors. Film-forming amine inhibitors are
used to reduce the corrosion rates. The control measures will be discussed later.
Dissolved Hydrogen Sulfide
Hydrogen sulfide, as well as carbon dioxide, dissolves in water to form a weak
acid. The acid solution thus formed is weaker than carbonic acid; nevertheless, it
is corrosive enough to cause pitting problems on the drillstem. The presence of
sulfide also promotes hydrogen absorption into the drillstem components. Hydrogen
absorption, coupled with cyclic stressing of the drillstem, leads to failure such as
sulfide stress cracking or hydrogen embrittlement of drillstem components. The
chemical mechanism
of
hydrogen sulfide corrosion is as follows
[
191,197,2001:
1.
Oxidation at the anode-where metal goes into solution:
Fe
-+
Fez+
+
2e-
(4-364)
Figure
4-466.
Effect
of
carbon dioxide partial pressure on
the
pH
of
condensate water.
(From
Ref.
[184].)

Corrosion and Scaling
1307
2. Ionization of H,S occurs
H,S
+
H'
+
SH-
(4-365)
The anion, bisulfide
SH-
further dissociates into anionic sulfide
S2-
and
cationic hydrogen ion
H:
SH-
%
Sp-
+
H'
(4-366)
3.
The ion
S2-
reacts with ferrous Fez+ ion
to
form black
iron
sulfide FeS
corrosion product. The hydrogen ions are reduced by electrons produced
by anodic reaction in step
1
and form hydrogen atom HO:
Fez+
+
S2-
+
2H'
+
2e-
+
FeS
+
2H0
(4-367)
In absence of oxygen some hydrogen does manage
to
evolve and polarize the
cathode to some extent. However, if oxygen is present, this polarization does
not occur as discussed earlier, and results in accelerated corrosion attack.
Hydrogen sulfide ionizes in two main stages when dissolved in fluid. The
reactions mechanisms are
H,S
+
NaOH
%
Na'
+
HS-
+
H,O
NaHS
+
NaOH
4
2Na'
+
S2-
+
H,O
These reactions are easily reversed if the solution pH decreases, as can be seen
in Figure
4-467.
*
0
100.0
50.0
10.0
5.0
1.0
0.5
0.1
a!
m
4
c
QI
V
L
Q)
0.
m
3
5
7
9
11
13
PH
Figure
4-467.
Approximate ionization
of
hydrogen sulfide in water at different
pH
values.
(From
Ref.
[191].)

I308
Drilling and Well Completions
If carbon dioxide is present, the solution pH is reduced enough
to
convert
the
sulfide
(S*-)
ion back into the dangerous bisulfide
or
molecular sulfide
(H,S)
state. Figure
4-468
shows the variation of corrosion of a mild steel in distilled
water containing varying concentrations of hydrogen sulfide. The corrosion rate
increases
sharply with increasing concentrations of hydrogen sulfide up to
150
ppm.
It begins to decline rapidly after
400
pprn until it approaches
1,600
ppm. From
1,600
to
2,640
ppm it becomes approximately constant, due to inhibitive
character of deposited iron sulfide at high concentrations.
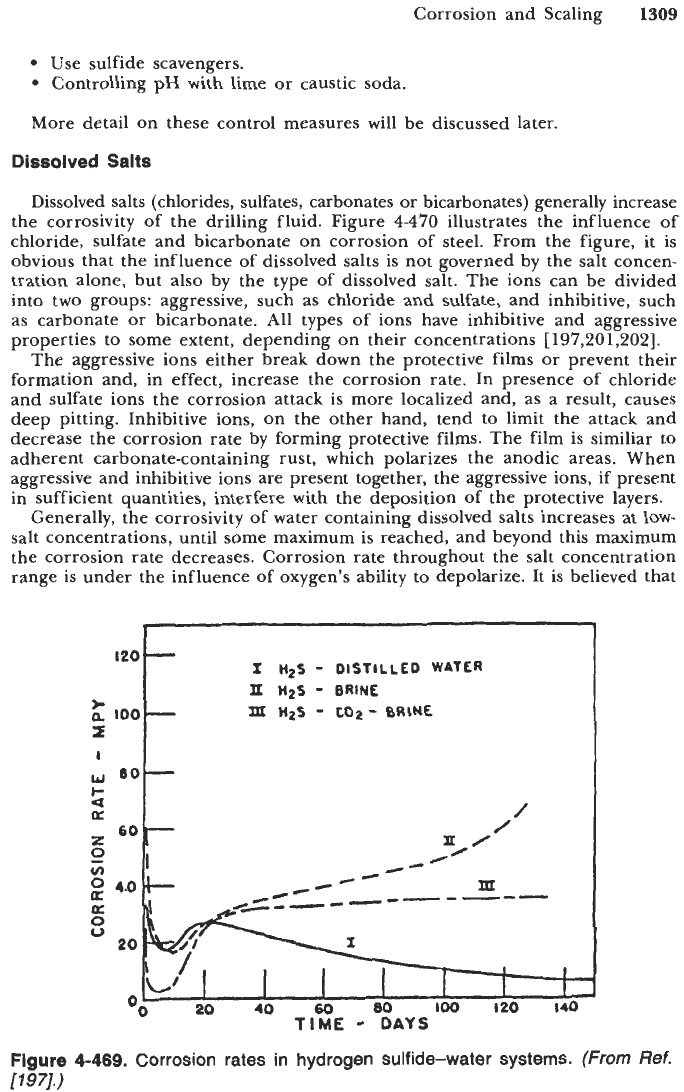
Figure
4-469
shows the effect on corrosion rates
of
1020
steel in different
water systems with dissolved hydrogen sulfide. The difference in corrosion rates
is due to different corrosion products formed in different solutions. In solution I,
kansite forms. Kansite is widely protective as the pyrrhotite coats the surface
giving slightly more protection until a very protective pyrite scale is formed. In
solution 11, only kansite scale forms, resulting in continued increase in the
corrosion rate. Finally, in solution 111, pyrite scale is formed as in solution
I;
however, continued corrosion may be due to the presence of carbon dioxide.
Hydrogen sulfide may enter the drilling fluid in one or more of the follow-
ing ways:
Influx of formation gas
or
formation water is the principal way.
Through addition
of
makeup water.
Thermal degradation of sulfur-containing drilling fluids additives (e.g.,
Chemical reaction of sulfur-containing compounds (e.g., tool joint lubricants).
Microbial activity.
The most effective methods of avoiding or reducing problems associated with
lignosulfates).
hydrogen sulfide corrosion are:
Avoid the use of high-strength steels. Relatively soft steels with low yield
strength (up to Rc
22
and
90,000
psi) are resistant.
DISSOLVED
H2S
-
PPM
Figure
4-468.
Corrosion rate of mild steel in distilled water containing
varying concentrations of hydrogen sulfide.
(From
Ref.
[197].)
Corrosion and Scaling
1309
I20
t
a.
z
1
w
k
a
a
z
60
Use sulfide scavengers.
Controlling pH with lime
or
caustic soda.
More detail on these control measures will be discussed later.
Dissolved
Salts
-
X
M2S
-
DISTILLED
WATER
It
M2S
-
BRINE
m
MzS
-
C02
-
WINE
100-
80-
/
-
/
R0
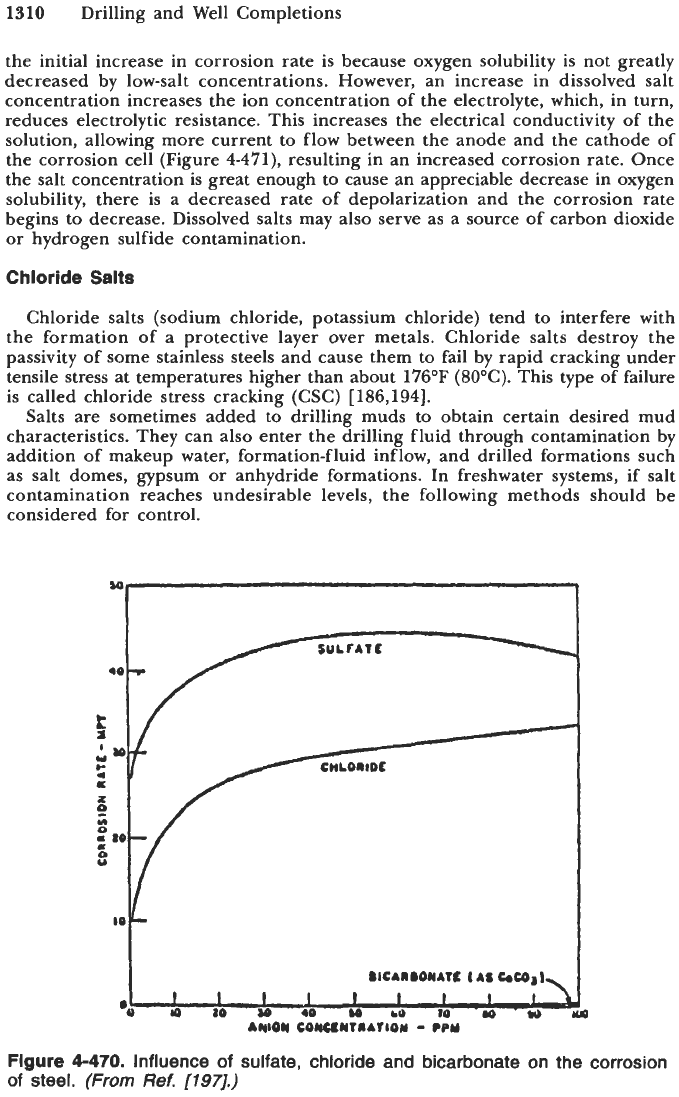
Dissolved salts (chlorides, sulfates, carbonates or bicarbonates) generally increase
the corrosivity of the drilling fluid. Figure
4-470
illustrates the influence of
chloride, sulfate and bicarbonate on corrosion of steel. From the figure, it is
obvious that the influence of dissolved salts is not governed by the salt concen-
tration alone, but also by the type
of
dissolved salt. The ions can be divided
into two groups: aggressive, such as chloride and sulfate, and inhibitive, such
as carbonate
or
bicarbonate. All types of ions have inhibitive and aggressive
properties to some extent, depending on their concentrations
[
197,201,202].
The aggressive ions either break down the protective films
or
prevent their
formation and, in effect, increase the corrosion rate. In presence of chloride
and sulfate ions the corrosion attack is more localized and, as a result, causes
deep pitting. Inhibitive ions, on the other hand, tend to limit the attack and
decrease the corrosion rate by forming protective films. The film is similiar to
adherent carbonate-containing rust, which polarizes the anodic areas. When
aggressive and inhibitive ions are present together, the aggressive ions, if present
in sufficient quantities, interfere with the deposition of the protective layers.
Generally, the corrosivity of water containing dissolved salts increases at low-
salt concentrations, until some maximum is reached, and beyond this maximum
the corrosion rate decreases. Corrosion rate throughout the salt concentration
range is under the influence of oxygen's ability to depolarize. It is believed that
1310 Drilling and Well Completions
the initial increase in corrosion rate is because oxygen solubility is not greatly
decreased by low-salt concentrations. However, an increase in dissolved salt
concentration increases the ion concentration of the electrolyte, which, in turn,
reduces electrolytic resistance. This increases the electrical conductivity
of
the
solution, allowing more current to flow between the anode and the cathode
of
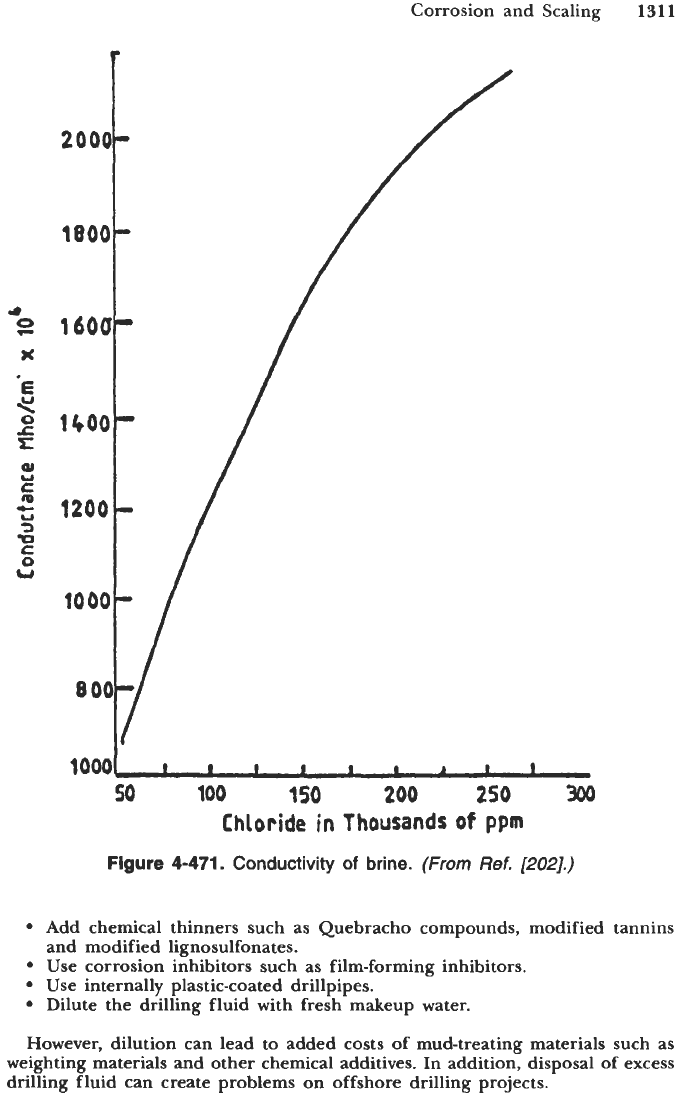
the corrosion cell (Figure 4-471), resulting in an increased corrosion rate. Once
the salt concentration is great enough to cause an appreciable decrease in oxygen
solubility, there is a decreased rate of depolarization and the corrosion rate
begins to decrease. Dissolved salts may also serve as a source of carbon dioxide
or hydrogen sulfide contamination.
Chloride
Salts
Chloride salts (sodium chloride, potassium chloride) tend to interfere with
the formation of a protective layer over metals. Chloride salts destroy the
passivity of some stainless steels and cause them to fail by rapid cracking under
tensile stress at temperatures higher than about 176°F (80%). This type of failure
is called chloride stress cracking
(CSC)
[186,194].
Salts are sometimes added to drilling muds to obtain certain desired mud
characteristics. They can also enter the drilling fluid through contamination by
addition of makeup water, formation-fluid inflow, and drilled formations such
as salt domes, gypsum or anhydride formations. In freshwater systems, if salt
contamination reaches undesirable levels, the following methods should be
considered for control.
I
Figure
4-470.
Influence
of
sulfate, chloride and bicarbonate
on
the corrosion
of
steel. (From
Ref.
[197].)
Corrosion and Scaling
1311
1000(
fi
I
I
I
t
I
A
I
1
so
100
150
200
750
300
Chloride
in
Thousands
of
ppm
Figure
4-471.
Conductivity
of
brine.
(From
Ref.
[202].)
Add chemical thinners such as Quebracho compounds, modified tannins
and modified lignosulfonates.
Use corrosion inhibitors such as film-forming inhibitors.
Use internally plastic-coated drillpipes.
Dilute the drilling fluid with fresh makeup water.
However, dilution can lead to added costs of mud-treating materials such as
weighting materials and other chemical additives. In addition, disposal of excess
drilling fluid can create problems on offshore drilling projects.
1312
Drilling and Well Completions
Organic Acids
Acids are substances that increase the hydrogen ion
(H)
concentration of the
solution they are dissoIved in. This, in turn, reduces the pH of the solution, and
the corrosion rate increases. Acids may
also
attack the metal by dissolving the pro-
tective film on the metal surface. Presence of acid aggravates the oxygen-influenced
attack and also hydrogen sulfide-promoted hydrogen embrittlement [203].
Organic acids can enter the drilling fluid through microbial activity
or
by
thermal degradation of organic, drilling-fluid additives. The acids may also be
formed by chemical reactions between drilling-mud additives
or
a result of other
contamination. Some common acids found in drilling fluids are formic (HCOOH),
acetic (CH,COOH) and carbonic
[H,CO,
(CO, in H,O)].
Corrosion Monitoring and Equipment Inspections
The best way to combat corrosion is to maintain an effective corrosion-
monitoring program to supplement good preventative measures. It is also very
important to keep complete records of monitoring programs, control programs
and failures that occur. The importance of well-qualified responsible personnel
cannot be overemphasized as effective corrosion control depends on their efforts
An effective corrosion control program should be able to detect evidence of corro-
sion and early identify the causes. Therefore, continuous monitoring is essential dur-
ing drilling operations because
the
nature of drilling fluid corrosivity changes as
the hole
is
drilled and different formations
are
penetrated. It is
very
important never
to rely on
any
single method of monitoring corrosion. Several techniques should
be used simultaneously whenever possible, and complete records should be kept.
Linear Polarization Instruments
[201,204,205
1,
Linear polarization instruments provide an instantaneous corrosion-rate data,
by utilizing polarization phenomena. These instruments are commercially avail-
able as two-electrode ”Corrater” and three electrode “Pairmeter” (Figure
4-472).
The instruments are portable, with probes that can be utilized at several locations
in the drilling fluid circulatory systems. In both Corrater and Pairmeter, the tech-
nique involves monitoring electrical potential of one of the electrodes with respect
to one of the other electrodes as a small electrical current is applied. The amount
of applied current necessary to change potential (no more than
10
to 20 mV) is
proportional to corrosion intensity. The electronic meter converts the amount
of current to read out a number that represents the corrosion
rate
in mpy. Before
recording the data, sufficient time should be allowed for the electrodes to reach
equilibrium with the environment. The corrosion-rate reading obtained by these
instruments is due to corrosion of the probe element at that instant
[184].
The limitation of these instruments is that they only indicate overall corrosion
rate. Their sensitivity is affected by deposition of corrosion products, mineral
scales
or
accumulation of hydrocarbons. Corrosivity of a system can be measured
only if the continuous component of the system is an electrolyte.
Galvanic Probe
The galvanic probe continuously monitors the corrosion characteristics of the
drilling fluid. The probe (Figure 4-473) consists of two dissimilar metal elec-
trodes, usually brass and steel. The electrodes are mounted on, but insulated

Corrosion and Scaling
1313
Threaded Connectors
/
Body
2
T
v
I
1
To
Instrument
Threaded Connectors
fletal
Electrodes
3
To
Instrument
Figure
4-472.
Linear polarization instrument probes.
from,
a
threaded high-pressure plug. These electrodes are connected to each
other through a DC ammeter capable of detecting microamperes when the
probes are immersed in an electrolyte. Enough time is allowed for the electrodes
to reach equilibrium and read the current flow through the external loop. The
current is generated by the corrosion process occurring on the electrodes
[184].
The amount of current flow related to the environment is a measure of its
corrosiveness. The probe generally registers low-current flow
(0-10
mA)
in slightly
corrosive environments. However, high-current flows
(40-
100
mA)
have been
recorded in severely corrosive environments. The current intensity generally
depends
on
oxygen concentration of the system, since oxygen depolarizes the
brass cathode, thereby continuing the corrosion process of the cell. Among
various locations
of
surface circulatory system, the instrument can be installed
downstream of deaerator and in the standpipe.
If the instrument indicates current surge in an air-free system, it generally
implies hydrogen sulfide contamination, but the galvanic probe is usually best
suited to detect corrosion influenced by oxygen contamination.

1314
Drilling and Well Completions
1
Other limitations of this instrument are the same as those of linear polariza-
tion instruments discussed earlier.
Threaded
Housing
/
Hollow
Tube
Cavity
/-
&e
sswe
Cuage
Figure
4-474. Hydrogen probe.
