Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Corrosion and Scaling
1295
If calcium or magnesium bicarbonates are present in water, the rise in
temperature decomposes them, and subsequent evolution of carbon dioxide will
result in a higher corrosion rate, while at the same time calcium and magnesium
carbonates may deposit on the metal surface. This scale may be protective, thus
slowing the corrosion rate; however, it can create concentration cells if
it
is
deposited loosely, exposing parts of the surface.
In systems with considerable temperature variations over the metal surface,
the warmer areas will be anodic to the cooler areas. The creation of a cell
usually leads to pitting corrosion of the anodic area.
Velocity
The velocity of fluids over the metal surface has an effect on the corrosion
rate through influencing other factors responsible for corrosion. High velocity
may increase erosion corrosion by either washing away the protective film or
by mechanically agitating the metal surface. On the other hand, stagnant systems
where the fluid velocity is zero may experience deposition of sludge and other
suspended solids. This deposition may create concentration cells, resulting in
pitting corrosion
[196-1981.
Systems with extremely high velocity of turbulent flow give rise
to
pressure
variations that may result in cavitation corrosion. When oxygen is present, low-
velocity areas receive less oxygen and become anodic to the high-velocity areas
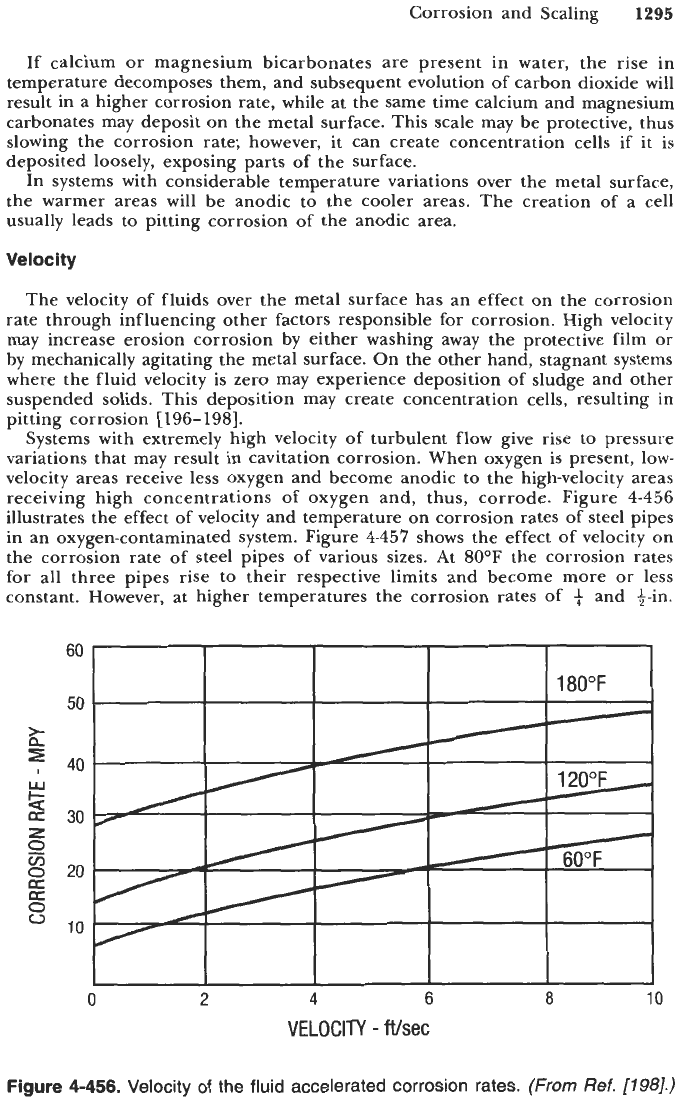
receiving high concentrations of oxygen and, thus, corrode. Figure
4-456
illustrates the effect of velocity and temperature on corrosion rates of steel pipes
in an oxygen-contaminated system. Figure
4-457
shows the effect of velocity on
the corrosion rate of steel pipes of various sizes. At 80°F the corrosion rates
for all three pipes rise to their respective limits and become more
or
less
constant. However, at higher temperatures the corrosion rates of
+
and +-in.
h:
E
W
3
z
v)
0
a
z
8
60
50
40
30
20
10
0
2
4
6
8
10
VELOCITY
-
Wsec
Figure
4-456.
Velocity
of
the fluid accelerated corrosion rates.
(From
Ref.
[198].)

1296
Drilling and Well Completions
1
40
120
20
0
-
-!
1/4"Pipe
j
~
,,,,,
r150°F
-
- - -
1/2" Pipe
--
3/4"
Pipe
*
0
1
2
3
4
FLOW
RATE
-
Wsec
Figure
4-457.
Effect of velocity
of
flow on the initial rate of corrosion of steel
pipe.
(From
Ref.
[197].)
pipes continue to increase as the velocity increases.
A
possible explanation for
this behavior is that the corrosion rate increases with increasing velocity in small-
diameter pipes, probably due to turbulence effects. Thus, the corrosion rate may
be reduced by using either an oversized flow area or by reducing the velocity
and, hence, minimizing the turbulence effect.
The critical velocity, which when exceeded may result in erosion corrosion,
can be calculated by the equation presented in
API RP
14E,
which is
[I991
C
v,
=J;;
(4-35
1)
where
V,
*
maximum allowable velocity in ft/s
C
=
a constant, typically
100-125
y
=
fluid specific weight in lb/ft3
Heterogeneity
Conditions necessary for the onset of corrosion are quite often provided by
heterogeneities. These heterogeneities may very well exist within the metal
or
alloy or may be imposed by external factors. These heterogeneities can give rise
to variations in potential
on
a metal surface immersed in an electrolytic fluid.
The galvanic cell thus formed gives rise to flow of current that accompanies
corrosion
[
1881.

Corrosion and Scaling
1297
High Stresses
Highly stressed areas generally corrode faster than areas of lower stress. This
is due to the fact that the more stressed areas are usually anodic and corrode
more readily. The drillstem just above the drill collars is often susceptible to
abnormal corrosion damage. High stresses and bending moments in this region
may be partially responsible for this failure.
Microbial Activity
Microorganisms are present in most systems in one form
or
another. Their
mere presence does not necessarily mean that they present a problem. Microbial-
influenced corrosion is not
a
very significant problem in drilling operations.
Their activity, however, does introduce corrodents in drilling fluids, reduces the
pH of the environment and can attack the organic additives of the drilling
fluids, thus producing corrosive products. Since the potential for problems does
exist, it becomes necessary to consider the effects on metal corrosion resulting
from microbial activity.
All microbes are classified into two main groups according to their oxygen
requirements. These groups are:
Anaerobic organisms-Flourish in the absence of oxygen in environment with low
Aerobic organisms-Require oxygen for survival.
redox potential.
The most common types of microorganisms found in oil fields that can cause
corrosion related problem are now discussed.
Sulfate Reducers.
Most of the oilfield corrosion problems arise from the
activity of sulfate-reducing bacteria
(SRB)
belonging to genus Desulfovibrio and
one
of
the genus Clostridium. They are anaerobic, but although inactive, they
will survive in systems containing dissolved oxygen. They may grow under scale,
debris
or
other bacterial masses where oxygen cannot penetrate, and in fresh
or
saltwater environments.
SRB
contribution to corrosion of metals is twofold;
by direct corrosion attack, and by attack from products produced as a result of
microbial activity. Figure
4-458
shows schematically the
SRB
direct corrosion
of steel. A simplistic chemical mechanism of this process
is
as follows:
1.
The metal goes into solution at the anode
Fe
-+
Fes+
+
2e-
2. Reaction at the cathode results in molecular hydrogen that polarizes the
cathode. Figure
4-459
shows the cathode polarization:
H,O
*
H+
+
OH-
2H
+
2e-
+
H,
3.
Depolarization of the cathode
by
SRB.
SRB
contain
an
enzyme called hydrog-
enase, which allows the utilization of hydrogen to reduce sulfate to sulfide:

1298
Drilling
and
Well Completions
SOLUTION
r
bs-
I
DESULFOVIBRIO
4
H20
+
S2
-
Fes
1
52
+
4
H20
DESULFOVIBRIO
S04'
+
8H
8H
+
8e+8H
Figure
4-458.
Diagram
of
polarization
of
local cathode by a film
of
hydrogen
gas bubbles (cathodic area to right
of
anode is polarized).
(From Ref.
[208].)
Fe++
H'
H'
Fe"
SOLUTION
Fe++
H'
H'
Figure
4-459.
Diagram
of
the bacterial corrosion of steel or iron by
Desulfovibrio
bacteria (corrosion products are underlined).
(From
Ref.
[208].)

Corrosion and Scaling
1299
R
SO:-
+
8H
+S'-
+
4H,O
4.
Corrosion product reaction
Ferrous ions (Fe2+) combine with sulfide ions
(Sz-)
to form black ferrous
sulfide FeS):
Fe2+
+
S2-
+
FeS
Ferrous ions (Fez+) combine with hydroxyl ions
(OH-)
to form brownish
red ferrous hydroxide [Fe(OH),]:
3Fe2+
+
60H-
+
3Fe(OH),
The overall corrosion reaction equation is
4Fe
+
SO,'-
+
3H20
-+
FeS
+
3Fe(OH),
+
20H-
If the water system contains dissolved carbon dioxide, iron carbonate
may form:
CO,
+
H,O
-+
H,CO,
FeS
+
H2C0,
+
FeCO,
-1
+
HzS
'?
The second way
SRB
influences corrosion of metal is by producing
products such as hydrogen sulfide that can affect corrosion resistance
of metals.
SRB
SO:-
+
2H'
+
4H,
-+
H$
+
4H,O
In the above mechanism, both hydrogen ion and molecule are utilized by
SRB
to convert
SO:-
to H,S. The consumption of hydrogen depolarizes the cathode
and leads to an increased rate
of
corrosion.
Slime-Forming
Bacteria.
Several forms of bacteria produce a slimy capsule
under certain environmental conditions. These organisms are "heterotrophic,"
that is, they obtain their energy from organic sources such as sodium lactate.
The reaction is
2CH,CHOHCOONa
+
MgSO,
+
2CH,COONa
+
CO,
?
+
MgCO,
+
H2S
?
+
H,O
These microorganisms become
a
problem when their numbers are large
enough to produce corrosives such as carbon dioxide and hydrogen sulfide.
Pseudomonas,
Bacillus
and
Flavobacterium
are examples of slime-forming bacteria.
Iron-Oxidizing
Bacteria.
These are aerobic organisms capable of growing in
systems with less than
0.5
ppm oxygen. They oxidize iron from ferrous to the
ferric state by the following mechanism:

1300
Drilling and Well Completions
4FeC0,
+
0,
+
6H,O
+
4Fe(OH),L
+
4CO,?
The carbon dioxide produced can contribute to the corrosion of metal. The
deposits of ferric hydroxide that precipitate on the metal surface may produce
oxygen concentration cells, causing corrosion under the deposits.
Gallionalla
and
Crenothrix
are two examples of iron-oxidizing bacteria.
Sulfur-Oxidizing Bacteria.
These are aerobic bacteria that oxidize sulfur or
sulfur-bearing compounds to sulfuric acid according to the following equation:
2s
+
30,
+
2H,O
+
2H,SO,
The resulting environment is low in pH and extremely corrosive.
Thiobacillus
and
Beggiatoa
are good examples of this form of bacteria.
Other
Microorganisms.
There are several other microorganisms that affect the
corrosion of metal directly or indirectly. Some examples are yeasts and molds,
algae and protozoa. For the present purposes
it
is sufficient to realize that there
micro- are other
organisms capable of presenting corrosion problems.
There are several methods of monitoring microbial-influenced corrosion.
Some methods are as follows:
Sample culturing
Filtration technique
Metal surface examination, Le., use
of
coupons
Methods to prevent or reduce problems associated with microbial corrosion
will be discussed later. Some of them are:
use of effective microbiocides
removal of nutrients
pH adjustment
proper coatings
cathodic protection
monitoring the effectiveness of microbiocides
Corrodents in Drilling Fluids
The principal corrosive agents affecting the drillstem components in drilling
fluids are dissolved gases, dissolved salts and acids. These corrodents may enter
the system from the formations being drilled. They can also be introduced by the
addition of makeup water, or other treating chemicals and processes. Also,
they can be products of thermal degradation of chemicals and microorganism
activity, etc.
Dissolved Oxygen
Oxygen dissolved in aqueous solutions, even in very low concentrations, is a
leading cause of corrosion problems (i.e., pitting) in drilling. Its presence also
accelerates the corrosion rate of other corrodents such as hydrogen sulfide and
carbon dioxide. Oxygen plays a dual role both as a cathodic depolarizer and
an anodic polarizer or passivator. Within a certain range of concentration the

Corrosion and Scaling
1301
attack is dependent on
the
oxygen concentration. Above a certain concentration
it
may act as anodic passivator for certain metals resulting in reduction of the
corrosion rate. It is thought that the concentration of oxygen is sufficient to
cause ferrous to ferric oxidation
at
a faster rate than ferric ion has a chance to
diffuse away from the metal surface. This results in deposition of a protective
film of ferrous hydroxide on the steel surface. The chemical reaction in oxygen
corrosion of steel in an aqueous solution is
2Fe
+
2Hz0
+
0,
+
2Fe2+
+
40H-
4
2Fe(OH),
1
(4-352)
Ferrous hydroxide precipitates from solution. Since this compound is unstable
in oxygenated solution, it is oxidized to the ferric salt:
2Fe(OH),
+
HzO
+
40,
+
2Fe(OH), (4-353)
Step-by-step explanation of the reaction is as follows:
1.
Oxidation occurs at the anode, where the metal goes into solution as it
corrodes:
Fe
-+
Fez+
+
2e-
(4-354)
2.
Reduction occurs at the cathode, where oxygen combines with water and
gains electrons to form the hydroxyl ion:
0,
+
2H,O
+
4e-
+
40H-
(4-355)
3. The hydroxyl ion thus produced combines with ferrous ion produced in
the first step and forms ferrous hydroxide (rust), which coats the cathode:
Fez+
+
2(OH)-
+
Fe(OH),
(4-356)
There are basically
two
ways through which oxygen promotes corrosion. First,
it
is a strong cathodic depolarizer; that is, it retards cathodic polarization. In a
corrosion process with an acid substance (dissolved hydrogen sulfide or carbon
dioxide), hydrogen molecules (H,) formed during reduction reaction accumu-
late at the cathode. The gaseous hydrogen thus forms an insulating blanket
that reduces the current flow and increases the corrosion rate. This process
is
called polarization.
2H'
+
2e-
4
2H0
+
H,
(4-357)
Step
1
Step 2
(atomic hydrogen) (molecular hydrogen)
However, if dissolved oxygen is present, the second step of Equation 4-357
does not occur and instead
2Ho
+
40,
+
H,O
(4-358)
Second, oxygen attacks the metal directly by precipitating iron oxide at the
anode, thus, preventing anodic polarization by Fez+ ions.
1302
Drilling and Well Completions
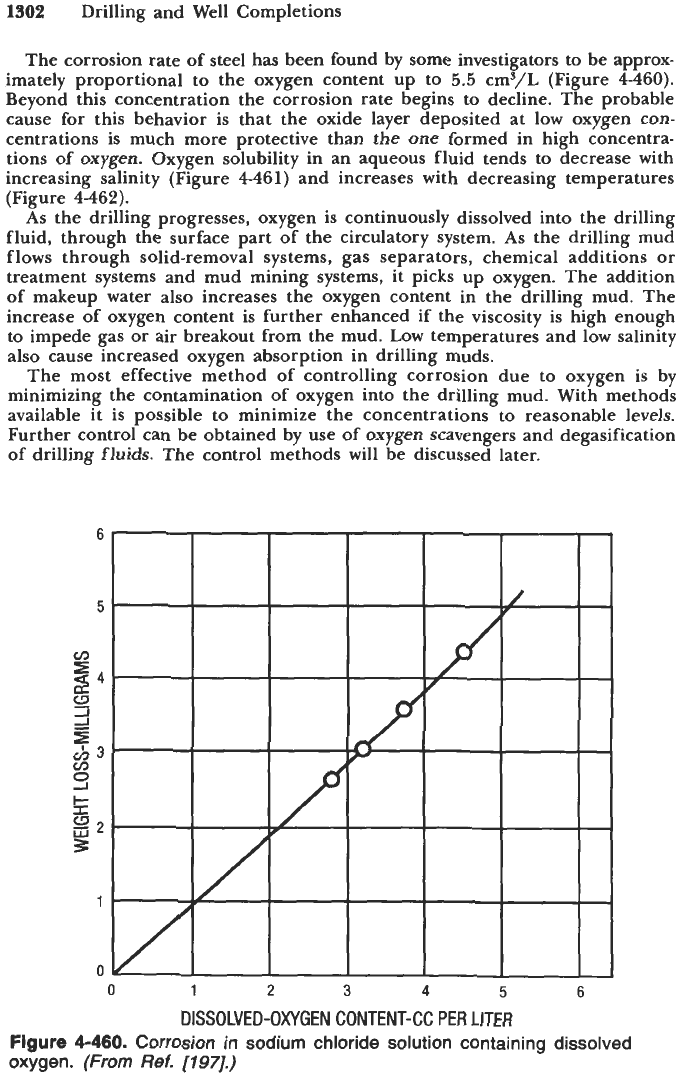
The corrosion rate of steel has been found by some investigators to be approx-
imately proportional to the oxygen content up to
5.5
cmS/L (Figure
4-460).
Beyond this concentration the corrosion rate begins to decline. The probable
cause for this behavior is that the oxide layer deposited at low oxygen con-
centrations is much more protective than the one formed in high concentra-
tions of oxygen. Oxygen solubility in an aqueous fluid tends to decrease with
increasing salinity (Figure
4-461)
and increases with decreasing temperatures
(Figure
4-462).
As
the drilling progresses, oxygen is continuously dissolved into the drilling
fluid, through the surface part of the circulatory system.
As
the drilling mud
flows through solid-removal systems, gas separators, chemical additions or
treatment systems and mud mining systems, it picks up oxygen. The addition
of makeup water also increases the oxygen content in the drilling mud. The
increase of oxygen content is further enhanced if the viscosity is high enough
to impede gas or air breakout from the mud. Low temperatures and low salinity
also cause increased oxygen absorption in drilling muds.
The most effective method
of
controlling corrosion due to oxygen is by
minimizing the contamination of oxygen into the drilling mud. With methods
available it is possible to minimize the concentrations to reasonable levels.
Further control can be obtained by use
of
oxygen scavengers and degasification
of drilling fluids. The control methods will be discussed later.
6
5
CI)
34
2
s2
53
s
s
52
3
c3
1
0
0
1
2
3
4 5
6
DISSOLVED-OXYGEN CONTENT-CC
PER
LITER
Figure
4-460.
Corrosion
in
sodium chloride solution containing dissolved
oxygen.
(From
Ref.
[197].)

Corrosion and Scaling
1303
Dissolved Carbon Dioxide
Carbon dioxide dissolves in water to form a weak acid (carbonic acid), which
reduces the
pH
of
the solution and, consequently, increases its corrosivity.
Corrosion caused by carbon dioxide is generally referred to
as
"sweet" corrosion,
and results in pitting. The mechanism of carbon dioxide corrosion is as follows
[
197,1981:
t
0
I
I
I
I
I
I
0
1
2
3
4
5
6
Ionic
Strength
Figure
4-461.
Oxygen solubility in salt solutions.
(From
Ref.
[797].)
WJICI
io
fumc
acid
ti;O
or
COc
do-.
11
Tempemuie."F.
Figure
4462.
Oxygen solubility in water at varying temperatures.
(From
Ref.
[789].)

1304
Drilling and Well Completions
1.
Carbon dioxide dissolves in water to form carbonic acid
CO,
+
H,O
+
H,CO,
(4-359)
2.
which ionizes first to
H,CO,
H
+
HCO-,
(3-360)
and then to
HCO-,
+
H'
+
CO;'
(4-36
1)
3.
At the anode metal goes into solution as it ionizes to
Fe
+
Fez+
+
2e-
(4-362)
4.
Finally, the carbonate ion combines with ferrous ion to form ferrous
carbonate and hydrogen is evolved:
Fez+
+
2e-
+
2H
+
GO:-
j
FeCO,
+
H,
(4-363)
The corrosion rate of carbon dioxide depends on metallurgy of the material,
oxygen content and solubility of carbon dioxide in the aqueous solution.
Solubility, in turn, depends on the amount of dissolved salt, temperature, partial
pressure of carbon dioxide and oxygen, and velocity of the system. An aqueous
solution that contains both oxygen and carbon dioxide in solution is more
corrosive than the solution that contains only one of these gases equal in
concentration to both gases. Figure
4-463
shows this fact
by
comparing various
I
z
v)
0
a:
0
0
0
a
60
50
40
30
20
0
5
10
15
20
CARBON
DIOXIDE
-
PPM
Flgure
4-463.
Effect
of
carbon dioxide concentration on corrosion rate.
(From
Ref.
121
11.)
