Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Corrosion and Scaling
1265
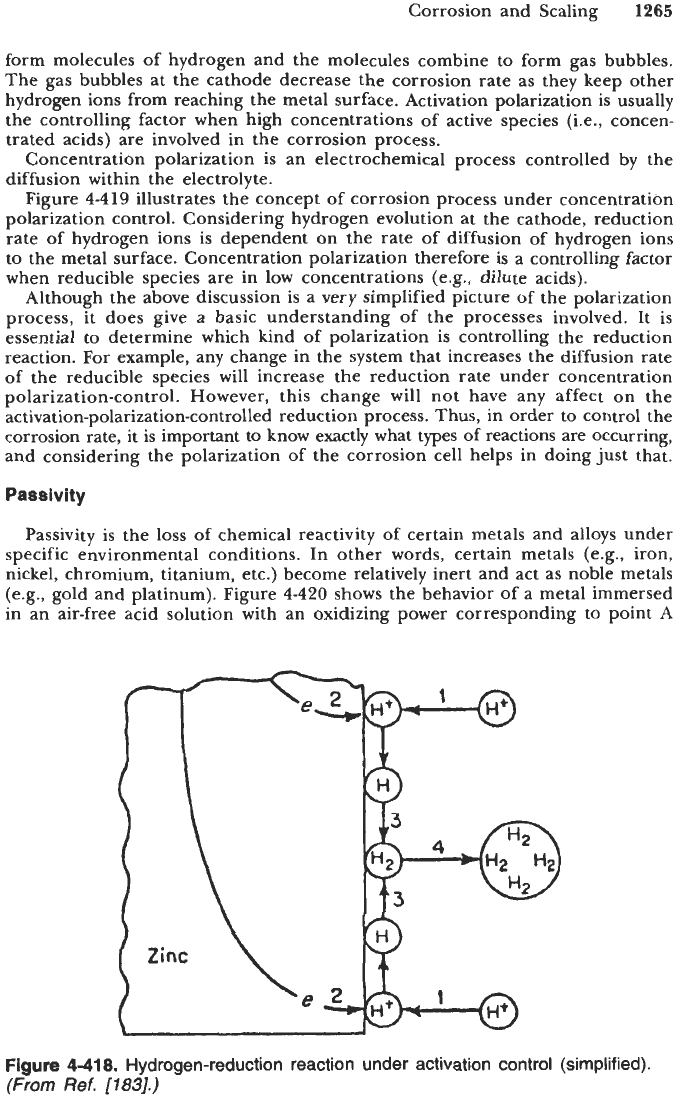
form molecules of hydrogen and the molecules combine to form gas bubbles.
The gas bubbles at the cathode decrease the corrosion rate as they keep other
hydrogen ions from reaching the metal surface. Activation polarization is usually
the controlling factor when high concentrations of active species (i.e., concen-
trated acids) are involved in the corrosion process.
Concentration polarization is an electrochemical process controlled by the
diffusion within the electrolyte.
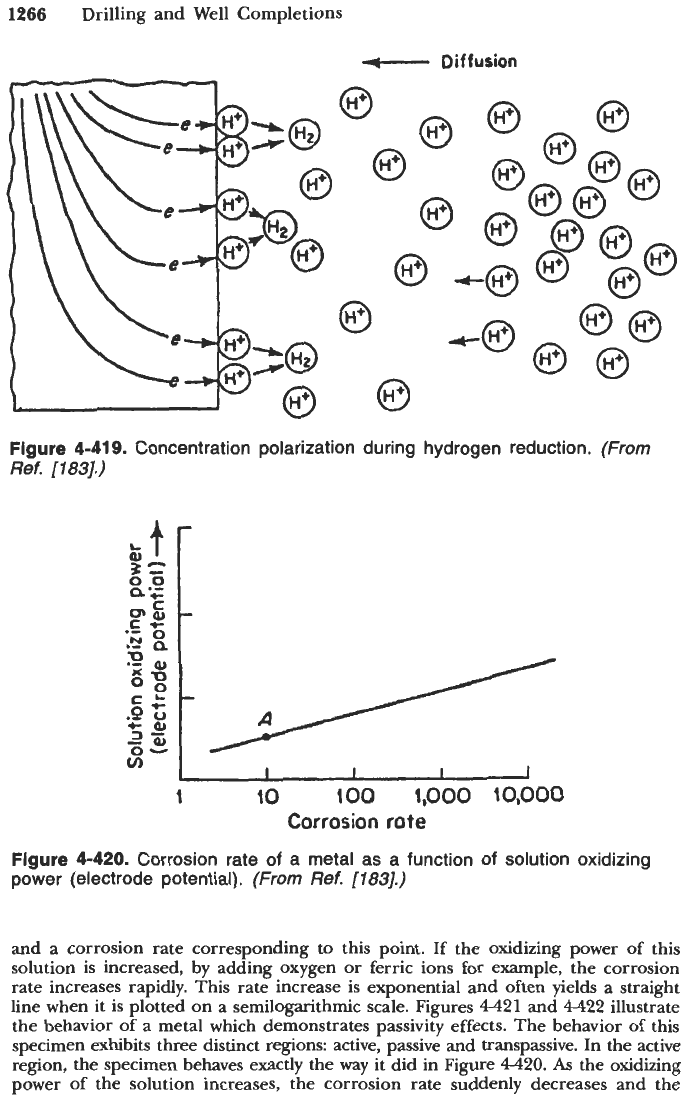
Figure
4-419
illustrates the concept of corrosion process under concentration
polarization control. Considering hydrogen evolution at the cathode, reduction
rate of hydrogen ions is dependent on the rate of diffusion of hydrogen ions
to the metal surface. Concentration polarization therefore is a controlling factor
when reducible species are in low concentrations (e.g., dilute acids).
Although the above discussion is a very simplified picture of the polarization
process, it does give a basic understanding of the processes involved. It is
essential to determine which kind of polarization is controlling the reduction
reaction. For example, any change in the system that increases the diffusion rate
of the reducible species will increase the reduction rate under concentration
polarization-control. However, this change will not have any affect on the
activation-polarization-controlled reduction process. Thus, in order to control the
corrosion rate, it is important to know exactly what types of reactions are occurring,
and considering the polarization of the corrosion cell helps in doing just that.
Passivity
Passivity is the
loss
of chemical reactivity of certain metals and alloys under
specific environmental conditions. In other words, certain metals (e.g., iron,
nickel, chromium, titanium, etc.) become relatively inert and act as noble metals
(e.g., gold and platinum). Figure
4-420
shows the behavior of a metal immersed
in an air-free acid solution with an oxidizing power corresponding
to
point
A
Figure
441
8.
Hydrogen-reduction reaction under activation control (simplified).
(From
Ref.
[183].)
1466
Drilling and Well Completions
4--
Diffusion
Figure
4-41
9.
Concentration polarization during hydrogen reduction.
(from
Ref.
[
1831.)
~
1
10
100
1,000
10,000
Corrosion
rote
Figure
4-420.
Corrosion rate
of
a metal as a function
of
solution oxidizing
power (electrode potential).
(From
Ref.
[183].)
and a corrosion rate corresponding to this point. If the oxidizing power
of
this
solution is increased, by adding oxygen or ferric ions for example, the corrosion
rate increases rapidly. This rate increase is exponential and often yields a straight
line when it is plotted on a semilogarithmic scale. Figures
4421
and
4-422
illustrate
the behavior of a metal which demonstrates passivity effects. The behavior of this
specimen exhibits three distinct regions: active, passive and transpassive. In the active
region, the specimen behaves exactly the
way
it did in Figure
4420.
As
the oxidizing
power
of
the solution increases, the corrosion rate suddenly decreases and the
Corrosion
and
Scaling
1267
t
Solution
oxidizing
power
(electrode
potenti011
F.
Trans passive
--------------------
-
f
A
I
I
I
,I
'-
Pos
s
ive
-----
--
I
Active
-
I
Fe
3-
2-
1-
01
a
J
10-2
1
1
o2
1
o4
1
Ob
CORROSION
RATE
(MPY)
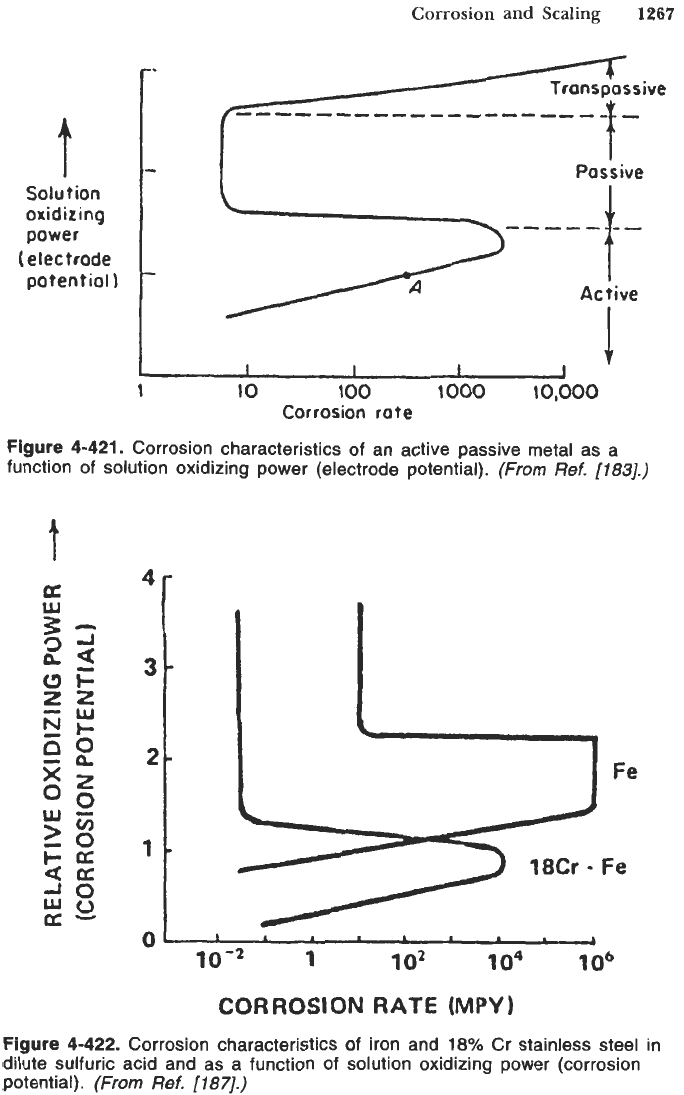
Figure
4-422.
Corrosion characteristics of iron and
18%
Cr stainless steel in
dilute sulfuric acid and as a function of solution oxidizing power (corrosion
potential).
(From
Ref.
[187].)

1268
Drilling and Well Completions
behavior enters the passive zone. Further increase in oxidizing power of the
solution produces hardly any change in corrosion rate. However, at a very high
concentration of oxidizers the corrosion rate starts to increase rapidly as the
behavior enters the transpassive zone. It is not fully understood why metals
exhibit
active-passive-transpassive
transitions, although it is thought that it is
a special case of activation polarization. This is due to the formation of an inert
surface film or a protective barrier, which fails in very strong oxidizing solutions.
There are various theories on how passive films are formed; however, there
are two commonly accepted theories. One theory is called the
oxide
film
theory
and states that the passive film is a diffusion-barrier layer of reaction products
(i.e., metal oxides
or
other compounds). The barriers separate the metal from
the hostile environment and thereby slow the rate of reaction. Another theory
is the
adsorption theory
of
passivity.
This states that the film is simply adsorbed
gas that forms a barrier to diffusion of metal ions from the substrata.
Considering Figure
4-421
we can conclude that metals that exhibit passivity
can be used in moderately to strongly oxidizing environments. In highly oxidizing
environments they will lose their corrosion-resistant properties and, therefore,
cannot be used. It is, however, possible to passivate some metals by exposing
them to passivating environments (i.e., iron in chromate or nitrite solutions)
or by anodic polarization at sufficiently high-current densities (i.e., in
H,SO,).
The desired passivity can be achieved by appropriate alloy additions to the metal.
Figure
4-422
illustrates the corrosion behavior of iron (Fe) and chromium
stainless steel. Introducing
18%
chromium to iron reduces the amount of oxidizer
necessary to achieve passivity. The addition of chromium to iron also reduces
the corrosion rate in the passive state. Proper alloying is an effective way to
improve the corrosion resistance of a metal.
Forms of Corrosion
Attack
Corrosion may take various forms and may combine other forms of damage
(erosion, wear, fatigue, etc.) to cause equipment failure. The forms of corrosion
most encountered in drilling equipment are
uniJorm
corrosion
and
galvanic corrosion.
Uniform Corrosion
All homogeneous metals without differences in potential between any points
on their surfaces are subject to this type of general attack under some condi-
tions. Uniform corrosion is usually characterized by a chemical
or
electrochemical
attack over the entire exposed surface, Figure
4-423.
Metal corrodes in an even
Original surface
Figure
4-423.
Uniform corrosion.
(From
Ref.
[785].)

Corrosion and Scaling
1269
and regular manner becoming thinner, and consequently leads to failure due
to reduction of the material's load-carrying capabilities. The rate of penetration
or the thinning
of
a structural member can be used directly to predict the
service life of a given component. Therefore, the expression mils penetration
per year (mpy) is used to express corrosion resistance directly in terms of
penetration. This expression can be calculated by the following
[
185,1861:
534w
mpy
=
-
DAT
(4-349)
where
W
=
weight loss in mg
D
=
density of specimen in g/cm3
A
=
area of specimen in
T
=
exposure time in hr
Using the value of mpy, we can classify metals into three categories according
to the corrosion rates and intended applications. The categories are the following:
<
5
mpy-Metals in this group have good resistance to corrosion and are quite
5
to 50 mpy-Metals in this group have an acceptable corrosion resistance if a
suitable for use as critical equipment parts.
higher rate of corrosion is tolerable.
50
mpy-Metals of this group are usually not acceptable for service use.
This form of corrosion is not of great concern from the engineering aspect, as
the service life can be estimated within reasonable accuracy. Uniform corrosion can
be prevented or reduced by using the following methods singly or in combination:
proper selection of materials for the types of service environments and
conditions
proper selection
of
inhibitor
proper selection of coatings
cathodic protection
These methods will be discussed in more detail.
Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals or alloys come into
electronic contact in a conductive solution. The severity
of
galvanic corrosion
depends primarily on the difference in solution potentials between the two
materials and the relative sizes of the cathode and the anode areas. The farther
apart these metals are from each other in the galvanic series, the greater the
possible corrosion
of
the anodic member of the galvanic couple. The galvanic
series for some commercial metals and alloys in seawater is shown in Table 4170.
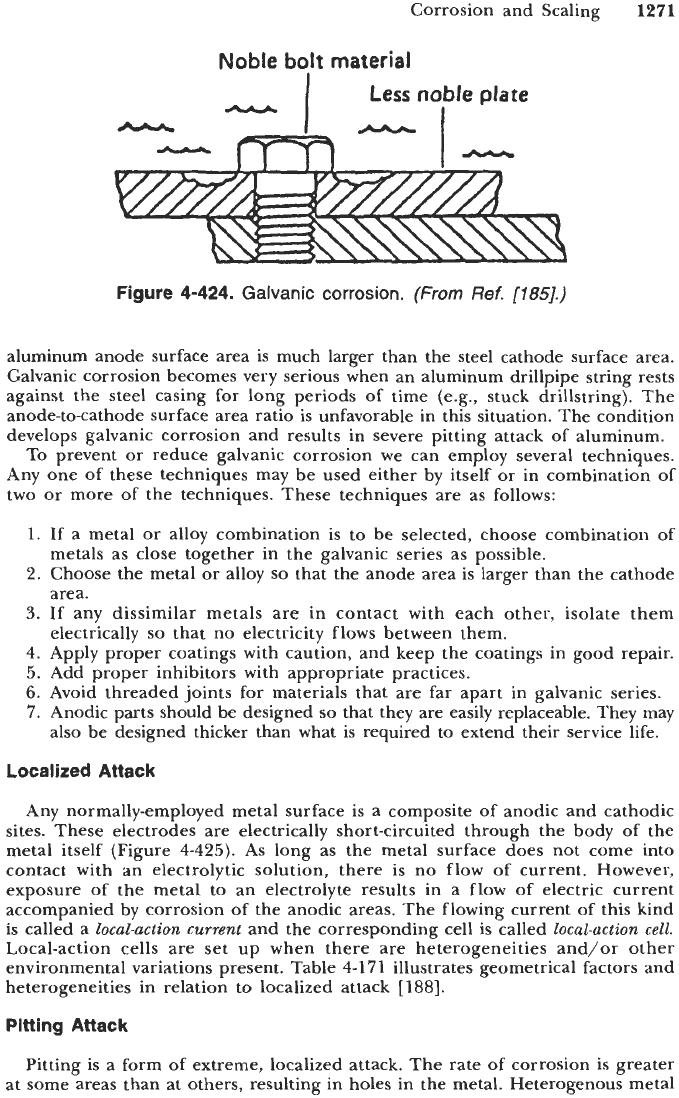
Figure
4-424
shows schematically an example of galvanic corrosion
[
186,1871.
Species with more positive corrosion potential, located toward the bottom of the
series, are called
noble
or
cathodic
metals and alloys. Those species with more
negative corrosion potential located toward the top of the series are referred to as
active
or
anodic
metals and alloys.
Conductive films such
as
"magnetite" (Fe,O,) or "mill scale" on steel, and conduc-
tive nonmetals such
as
carbon can function
as
cathodes when
in
contact with anodes

1270
Drilling and Well Completions
Table 4-1
70
Galvanic Series
of
Some Commercial Metals and Alloys in Seawater
Active or Anodic
(-)
Noble or Cathodic
(+)
Magnesium
Magnesium Alloys
Zinc
Galvanized Steel
Aluminum 1100
Aluminum 2024 (4.5 Cu, 1.5 Mg, 0.6 Mn)
Mild Steel
Wrought Iron
Cast Iron
13% Chromium Stainles Steel Type 410 (Active)
18-8 Stainless Steel Type 304 (Active)
Lead-Tin Solders
Lead
Tin
Muntz Metal
Manganese Bronze
Naval Brass
Nickel (Active)
76 Ni-16 Cr-7 Fe Alloy (Active)
60 Ni-30 Mo-6 Fe-1
Mn
Yellow Brass
Admirality Brass
Red Brass
Copper
Silicon Bronze
70:30 Cupro Nickel
G-Bronze
Silver Solder
Nickel (Passive)
76 Ni-16 Cr-7 Fe Alloy (Passive)
13% Chromium Stainless Steel Type 410 (Passive)
Titanium
18-8 Stainless Steel Type 304 (Passive)
Silver
Graphite
Gold
Platinum
Source: From
Ref.
[187].
of clean metal. Aluminum drillpipes can experience localized galvanic corrosion
attack resulting in pitting. The attack generally occurs immediately adjacent to the
steel tool joints. Although not very severe, the extent
of
attack depends on salt
concentration of the drilling fluid and exposure time. One reason the attack is not
very severe is the favorable anode-to-cathode surface area ratio. That is, the
Corrosion and Scaling
1271
Noble bolt material
Less
noble
plate
Figure
4-424.
Galvanic corrosion.
(From
Ref.
[185].)
aluminum anode surface area is much larger than the steel cathode surface area.
Galvanic corrosion becomes very serious when an aluminum drillpipe string rests
against the steel casing for long periods of time (e.g., stuck drillstring). The
anode-to-cathode surface area ratio is unfavorable in this situation. The condition
develops galvanic corrosion and results in severe pitting attack of aluminum.
To prevent or reduce galvanic corrosion we can employ several techniques.
Any one of these techniques may be used either by itself or in combination of
two or more of the techniques. These techniques are as follows:
1.
2.
3.
4.
5.
6.
7.
If a metal or alloy combination is to be selected, choose combination of
metals as close together in the galvanic series as possible.
Choose the metal or alloy
so
that the anode area is larger than the cathode
area.
If any dissimilar metals are in contact with each other, isolate them
electrically
so
that no electricity flows between them.
Apply proper coatings with caution, and keep the coatings in good repair.
Add proper inhibitors with appropriate practices.
Avoid threaded joints for materials that are far apart in galvanic series.
Anodic parts should be designed
so
that they are easily replaceable. They may
also be designed thicker than what is required to extend their service life.
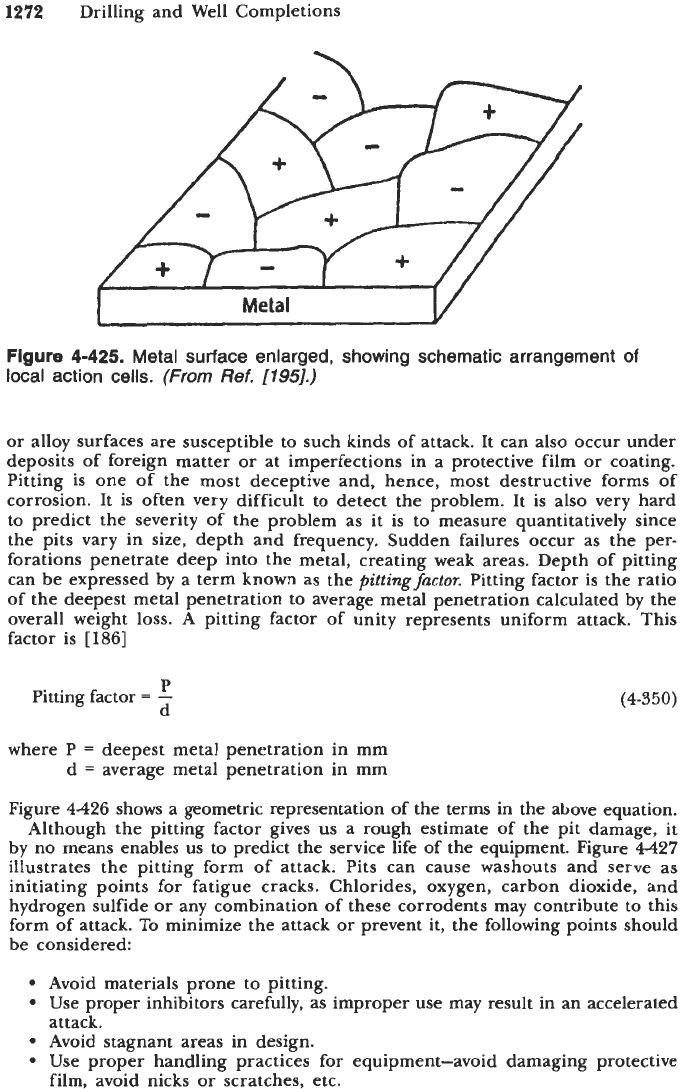
Localized Attack
Any normally-employed metal surface is a composite of anodic and cathodic
sites. These electrodes are electrically short-circuited through the body of the
metal itself (Figure
4-425).
As long as the metal surface does not come into
contact with an electrolytic solution, there is no flow of current. However,
exposure of the metal to an electrolyte results in a flow of electric current
accompanied by corrosion of the anodic areas. The flowing current of this kind
is
called a
local-action current
and the corresponding cell
is
called
local-action cell.
Local-action cells are set up when there are heterogeneities and/or other
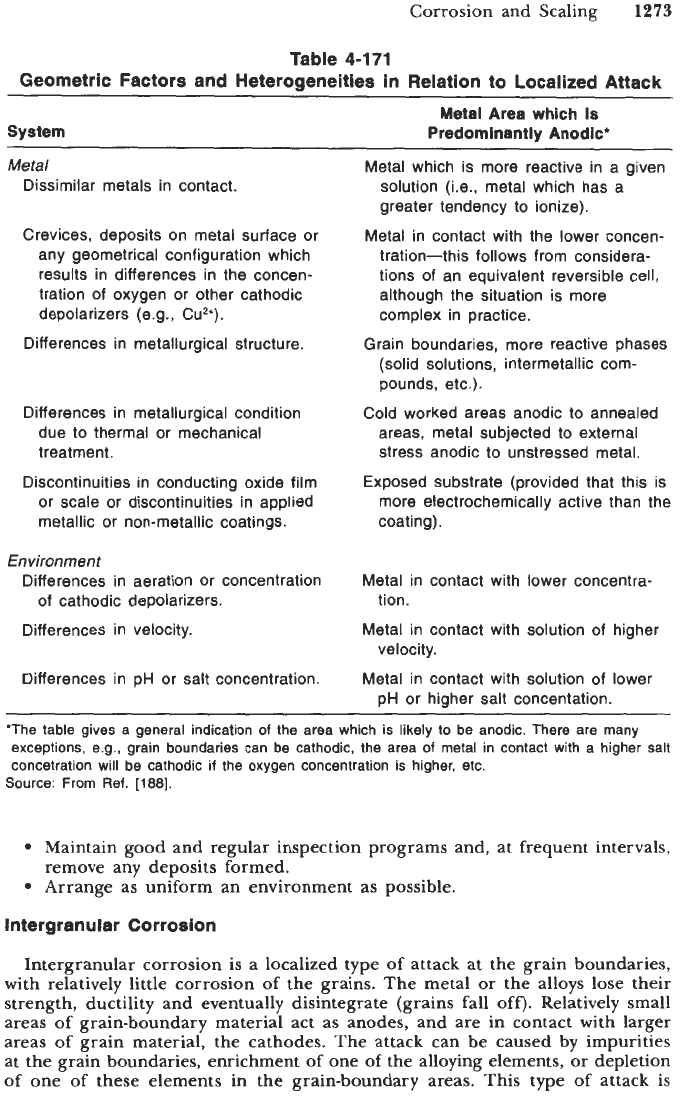
environmental variations present. Table
4-171
illustrates geometrical factors and
heterogeneities in relation to localized attack
[
1881.
Pitting
Attack
Pitting
is
a form of extreme, localized attack. The rate of corrosion is greater
at some areas than at others, resulting in holes in the metal. Heterogenous metal
1272
Drilling and Well Completions
I
Metal
V
Figure
4-425.
Metal surface enlarged, showing schematic arrangement
of
local action cells.
(From
Ref.
[795].)
or alloy surfaces are susceptible to such kinds of attack. It can also occur under
deposits of foreign matter or at imperfections in a protective film or coating.
Pitting
is
one of the most deceptive and, hence, most destructive forms of
corrosion. It is often very difficult to detect the problem. It is also very hard
to predict the severity of the problem as it is to measure quantitatively since
the pits vary in size, depth and frequency. Sudden failures occur as the per-
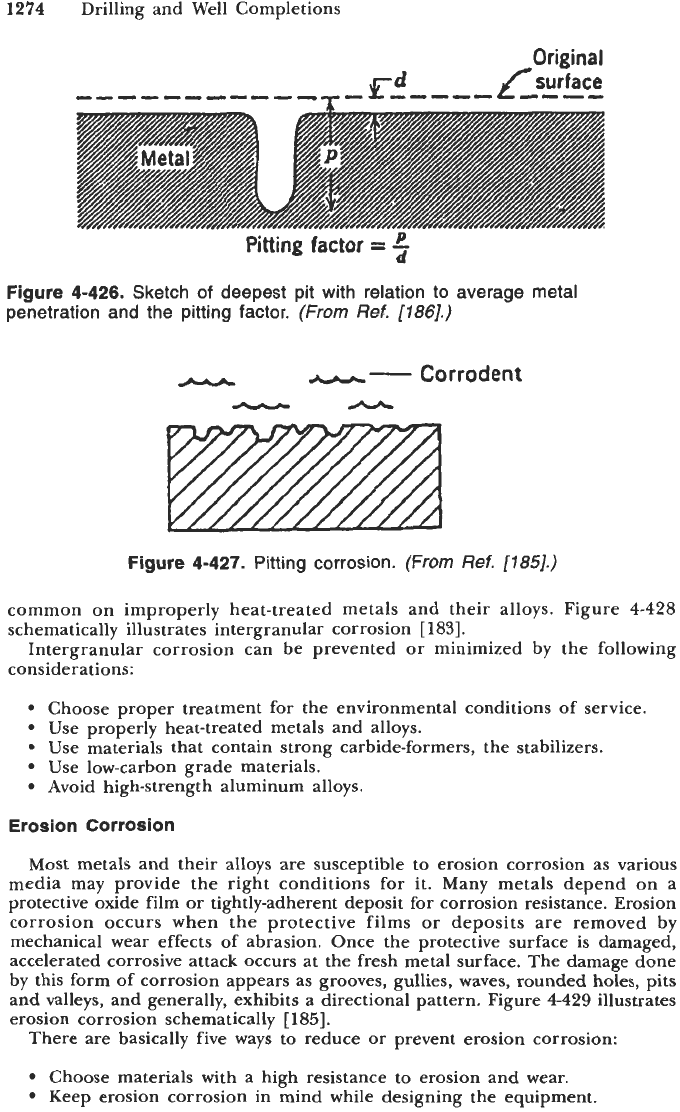
forations penetrate deep into the metal, creating weak areas. Depth of pitting
can be expressed by a term known as the
pittingfuctor.
Pitting factor is the ratio
of the deepest metal penetration to average metal penetration calculated by the
overall weight loss. A pitting factor of unity represents uniform attack. This
factor is
[186]
P
Pitting factor
=
-
d
(4-350)
where P
=
deepest metal penetration in mm
d
=
average metal penetration in mm
Figure
4426
shows a geometric representation of the terms in the above equation.
Although the pitting factor gives us a rough estimate of the pit damage, it
by no means enables us to predict the service life of the equipment. Figure
4-427
illustrates the pitting form of attack. Pits can cause washouts and serve as
initiating points for fatigue cracks. Chlorides, oxygen, carbon dioxide, and
hydrogen sulfide or any combination
of
these corrodents may contribute to this
form of attack.
To
minimize the attack or prevent it, the following points should
be considered:
Avoid materials prone to pitting.
Use proper inhibitors carefully, as improper use may result in an accelerated
attack.
Avoid stagnant areas in design.
Use proper handling practices for equipment-avoid damaging protective
film, avoid nicks or scratches, etc.
Corrosion and Scaling
1273
Table
4-171
Geometric Factors and Heterogeneities in Relation to Localized Attack
Metal Area
which
Is
System Predominantly Anodic'
Metal
Dissimilar metals in contact.
Crevices, deposits on metal surface or
any geometrical configuration which
results in differences in the concen-
tration
of
oxygen or other cathodic
depolarizers (e.g., Cu2+).
Differences in metallurgical structure.
Differences in metallurgical condition
due to thermal or mechanical
treatment.
Discontinuities in conducting oxide film
or scale or discontinuities in applied
metallic or non-metallic coatings.
Environment
Differences in aeration or concentration
Differences in velocity.
of cathodic depolarizers.
Differences in pH or salt concentration.
Metal which is more reactive in a given
solution (Le., metal which has a
greater tendency to ionize).
Metal in contact with the lower concen-
tration-this follows from considera-
tions of an equivalent reversible cell,
although the situation is more
complex in practice.
(solid solutions, intermetallic com-
pounds, etc.).
Cold worked areas anodic to annealed
areas, metal subjected to external
stress anodic to unstressed metal.
Exposed substrate (provided that this is
more electrochemically active than the
coating).
Grain boundaries, more reactive phases
Metal in contact with
Metal in contact with
Metal in contact with
tion.
velocity.
lower concentra-
solution of higher
solution of lower
pH or higher salt concentation
"The table gives a general indication of the area which is likely
to
be anodic. There are many
exceptions, e.g., grain boundaries can
be
cathodic, the area of metal in contact with
a
higher
salt
concetration will be cathodic
if
the oxygen concentration is higher, etc.
Source: From Ref.
[188].
Maintain good and regular inspection programs and, at frequent intervals,
remove any deposits formed.
Arrange as uniform an environment as possible.
intergranular Corrosion
Intergranular corrosion is a localized type of attack at the grain boundaries,
with relatively little corrosion of the grains. The metal
or
the alloys lose their
strength, ductility and eventually disintegrate (grains fall off). Relatively small
areas of grain-boundary material act as anodes, and are in contact with larger
areas of grain material, the cathodes. The attack can be caused by impurities
at the grain boundaries, enrichment of one of the alloying elements,
or
depletion
of one of these elements in the grain-boundary areas. This type of attack is
1274
Drilling and Well Completions
Original
-----------y-E-----
d
[surface
e---
Pitting
factor
=
f
Figure 4-426. Sketch of deepest pit with relation to average metal
penetration and the pitting factor.
(From
Ref.
11861.)
---
Corrodent
)cLLA.
u
*
Figure 4-427. Pitting corrosion.
(From
Ref.
11851.)
common on improperly heat-treated metals and their alloys. Figure
4-428
schematically illustrates intergranular corrosion
[
1831.
Intergranular corrosion can be prevented
or
minimized by the following
considerations:
Choose proper treatment for the environmental conditions of service.
Use properly heat-treated metals and alloys.
Use materials that contain strong carbide-formers, the stabilizers.
Use low-carbon grade materials.
Avoid high-strength aluminum alloys.
Erosion
Corrosion
Most metals and their alloys are susceptible
to
erosion corrosion as various
media may provide the right conditions for it. Many metals depend on a
protective oxide film or tightly-adherent deposit for corrosion resistance. Erosion
corrosion occurs when the protective films or deposits are removed by
mechanical wear effects of abrasion. Once the protective surface is damaged,
accelerated corrosive attack occurs at the fresh metal surface. The damage done
by this form
of
corrosion appears as grooves, gullies, waves, rounded holes, pits
and valleys, and generally, exhibits a directional pattern. Figure
4-429
illustrates
erosion corrosion schematically
[
1851.
There are basically five ways to reduce
or
prevent erosion corrosion:
Choose materials with a high resistance to erosion and wear.
Keep erosion corrosion in mind while designing the equipment.
