Lyons W.C. (ed.). Standard handbook of petroleum and natural gas engineering.2001- Volume 1
Подождите немного. Документ загружается.

Corrosion
and
Scaling 1285
0
0
Crock
depth4
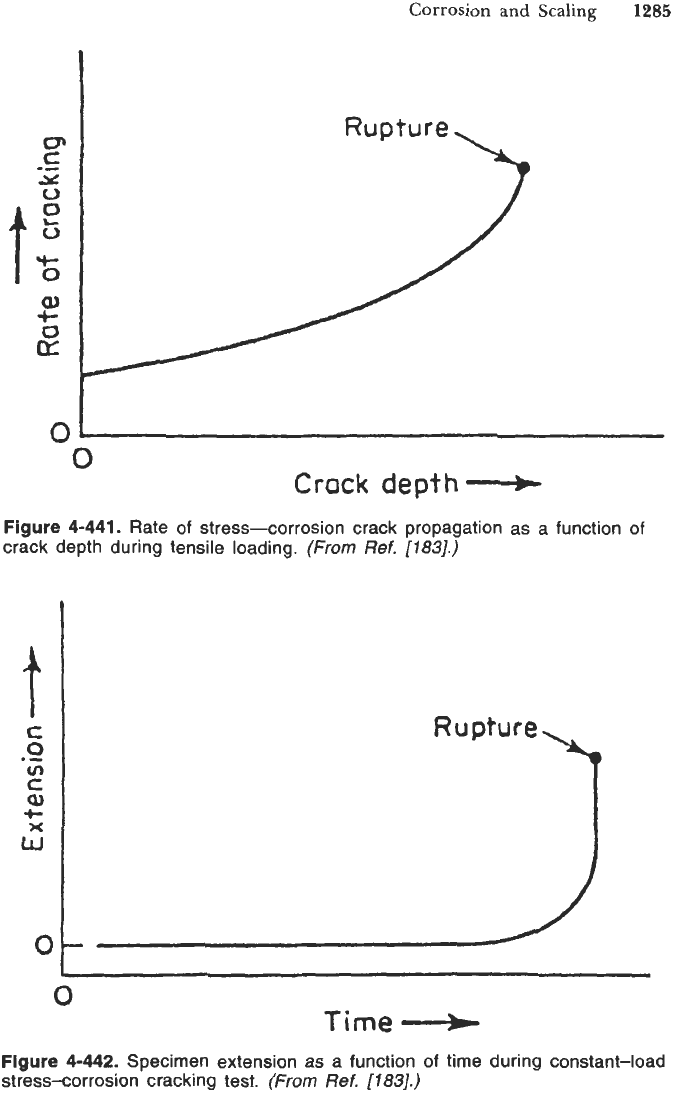
Figure 4-441.
Rate
of
stress-corrosion crack propagation as a function of
crack depth during tensile loading.
(From
Ref.
[183].)
t
C
0
u,
C
Q,
t
x
.-
Rupture
t
C
0
u,
C
Q,
t
x
W
.-
0-
0
A
Time
+
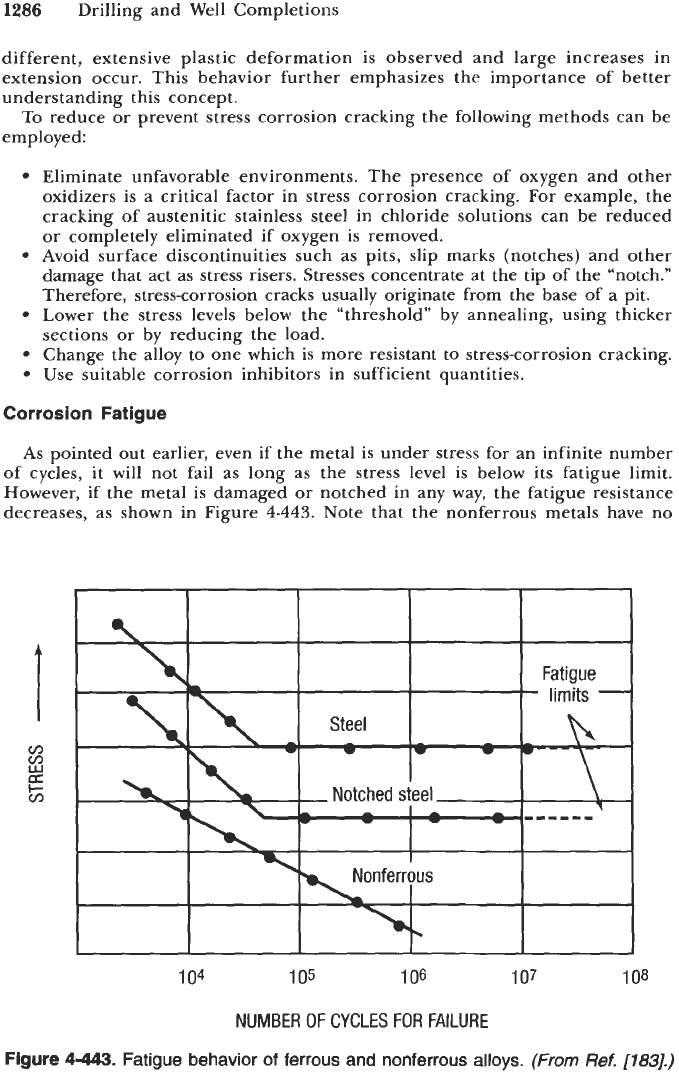
Figure 4-442.
Specimen extension
as
a function of time during constant-load
stress-corrosion cracking test.
(From
Ref.
[183].)
0
Time
+
Figure 4-442.
Specimen extension
as
a function of time during constant-load
stress-corrosion cracking test.
(From
Ref.
[183].)
1286
Drilling and Well Completions
different, extensive plastic deformation is observed and large increases in
extension occur. This behavior further emphasizes the importance of better
understanding this concept.
To reduce or prevent stress corrosion cracking the following methods can be
employed:
Eliminate unfavorable environments. The presence of oxygen and other
oxidizers is a critical factor in stress corrosion cracking. For example, the
cracking
of
austenitic stainless steel in chloride solutions can be reduced
or
completely eliminated if oxygen is removed.
Avoid surface discontinuities such as pits, slip marks (notches) and other
damage that act as stress risers. Stresses concentrate at the tip of the “notch.”
Therefore, stress-corrosion cracks usually originate from the base of a pit.
Lower the stress levels below the “threshold” by annealing, using thicker
sections or by reducing the load.
Change the alloy to one which is more resistant to stress-corrosion cracking.
Use suitable corrosion inhibitors in sufficient quantities.
Corrosion Fatigue
As
pointed out earlier, even if the metal is under stress for an infinite number
of cycles, it will not fail
as
long as the stress level is below its fatigue limit.
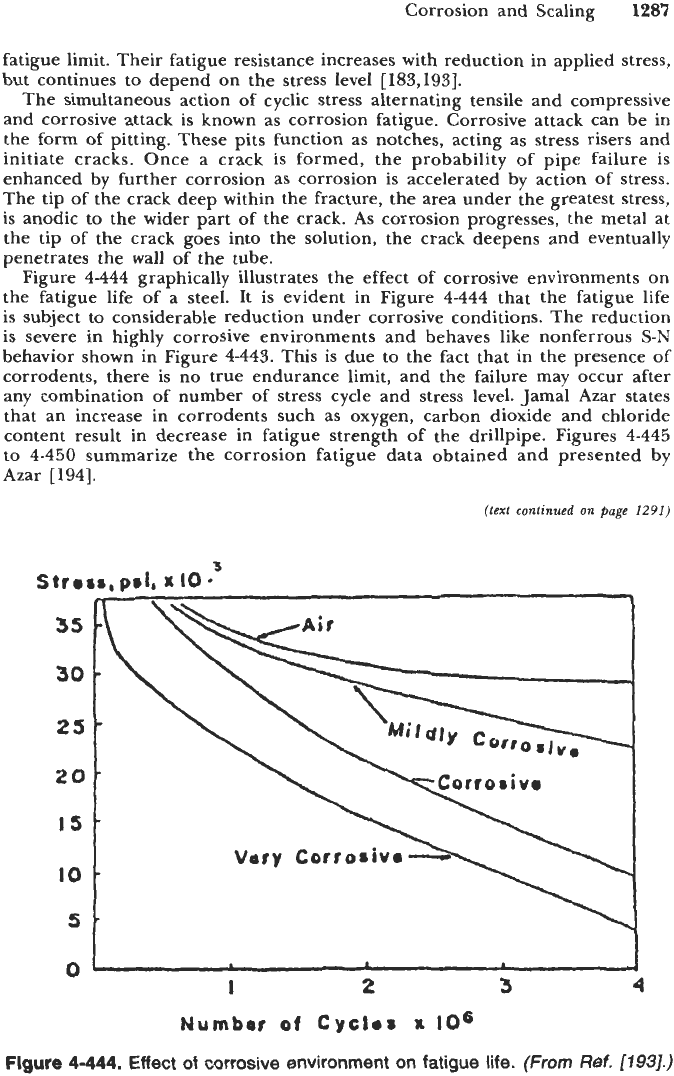
However, if the metal is damaged or notched in any way, the fatigue resistance
decreases, as shown in Figure
4-443.
Note that the nonferrous metals have no
104
105
106
107
108
NUMBER
OF
CYCLES
FOR
FAILURE
Flgure
4-443.
Fatigue behavior of ferrous and nonferrous alloys.
(From
Ref.
[183].)
Corrosion and Scaling
1287
fatigue limit. Their fatigue resistance increases with reduction in applied stress,
but continues to depend on the stress level
[183,193].
The simultaneous action of cyclic stress alternating tensile and compressive
and corrosive attack is known as corrosion fatigue. Corrosive attack can be in
the form
of
pitting. These pits function as notches, acting as stress risers and
initiate cracks. Once
a
crack is formed, the probability of pipe failure
is
enhanced by further corrosion as corrosion is accelerated by action of stress.
The tip of the crack deep within the fracture, the area under the greatest stress,
is
anodic to the wider part of the crack.
As
corrosion progresses, the metal
at
the tip of the crack goes into the solution, the crack deepens and eventually
penetrates the wall of the tube.
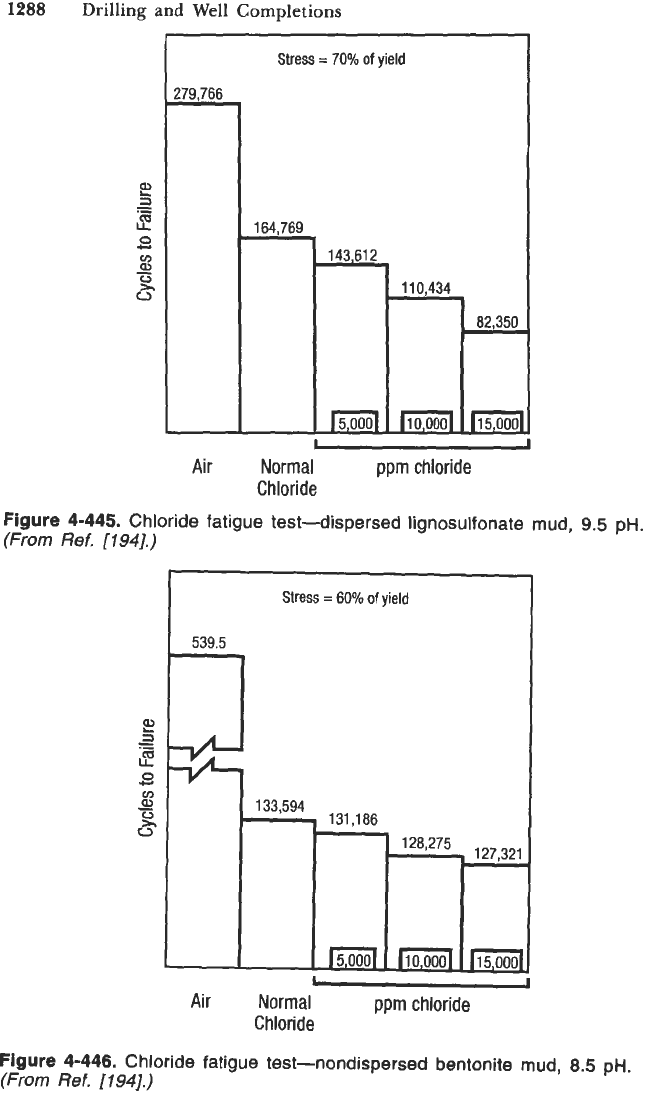
Figure
4-444
graphically illustrates the effect of corrosive environments on
the fatigue life
of
a steel. It
is
evident in Figure
4-444
that the fatigue life
is subject to considerable reduction under corrosive conditions. The reduction
is severe in highly corrosive environments and behaves like nonferrous
S-N
behavior shown in Figure
4443.
This is due to the fact that in the presence
of
corrodents, there is no true endurance limit, and the failure may occur after
any combination of number of stress cycle and stress level. Jamal Azar states
that an increase in corrodents such as oxygen, carbon dioxide and chloride
content result in decrease in fatigue strength of the drillpipe. Figures
4-445
to
4-450
summarize the corrosion fatigue data obtained and presented by
Azar
[194].
(text
continued
on
page
1291)
IO
-
5.
I
2
3
4
Numbor
of
Cyclor
x
lo6
Figure
4-444.
Effect
of
corrosive environment on fatigue life.
(From
Ref.
11931.)
1288
Drilling and
Well
Completions
279,766
-
Air
Stress
=
70%
of
yield
164,769
-
82,350
-
J-ZiFj
I
Normal ppm chloride
Chloride
Figure
4-445.
Chloride fatigue test-dispersed lignosulfonate mud,
9.5
pH.
(From
Ref.
[194].)
Stress
=
60%
of
yield
L
Air Normal ppm chloride
Chloride
Figure
4-446.
Chloride fatigue test-nondispersed bentonite mud,
8.5
pH.
(From
Ref.
[194].)
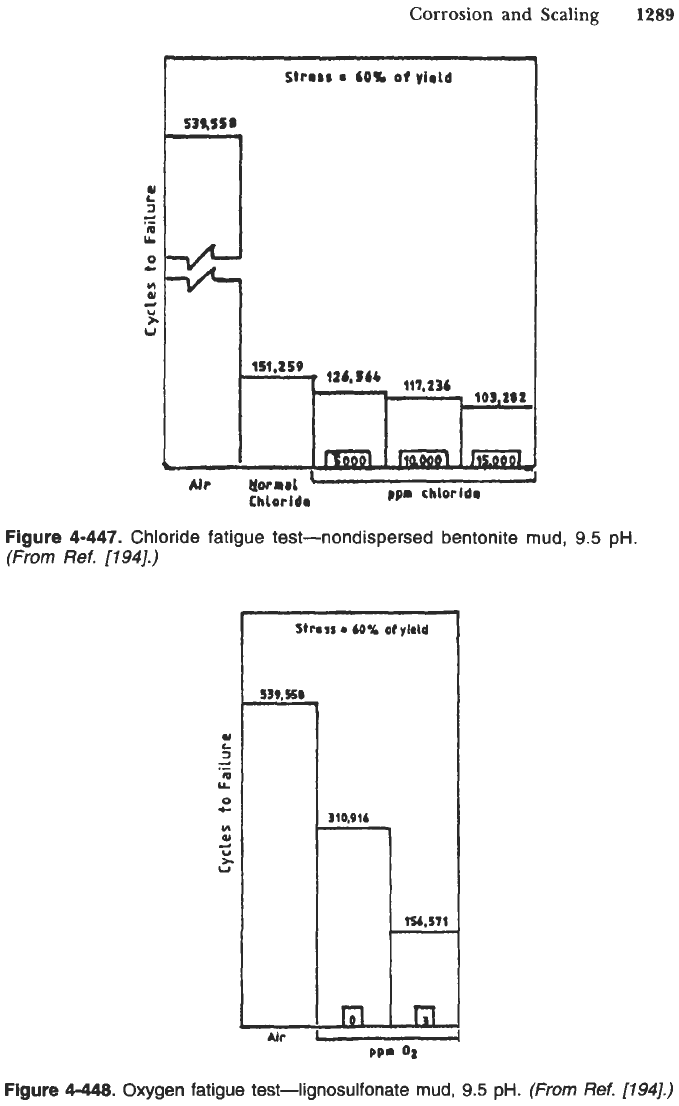
Corrosion and Scaling
1489
Y
L
a
r=
Io
(L
0
c
H
Y
*
U
w
U
539,551
151.2
5
0
ut’
uW8d
#pm
thlorldr
Chlor
Ida
Figure
4-447.
Chloride fatigue test-nondispersed bentonite mud,
9.5
pH.
(From
Ref.
17941.)
PP.
02
Figure
4-448.
Oxygen fatigue test-lignosulfonate mud,
9.5
pH.
(From
Ref.
(1941.)
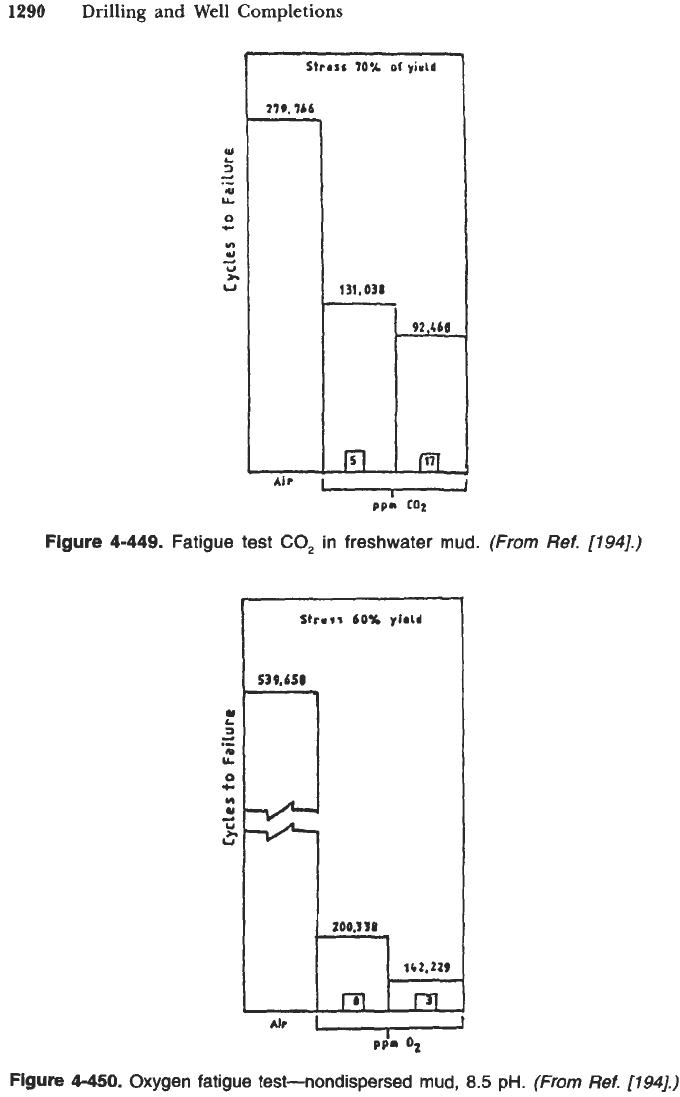
1290
Drilling and Well Completions
Figure
4-449.
Fatigue test
CO,
in freshwater
mud.
(From
Ref.
[194].)
Strrir
60%
yhld
I
Figure
4-450.
Oxygen fatigue test-nondispersed
mud,
8.5
pH.
(From
Ref.
[194].)

Corrosion and Scaling
1291
(text
continued
from page
1287)
Corrosion fatigue, therefore, is
a
special case of stress-corrosion cracking and
fatigue failure. Figure
4-451
shows an example of pipe failures due
to
corrosion
fatigue. Corrosion fatigue can be prevented or reduced by:
reducing the stress on the metal by altering the design, heat treatment;
use of proper corrosion inhibitors;
use of proper coatings;
keeping the drillstring under continuous tension.
Figure
4-451.
Corrosion-fatigue failure
of
drillpipe caused
by
internal pitting.
(From
Ref.
[218].)
1292
Drilling and Well Completions
Factors Influencing Corrosion Rate
The pH is one of the most important characteristics of an electrolyte,
commonly expressed as a number between zero and fourteen, and is the negative
logarithm of the hydrogen ion concentration
[
1951.
pH
=
-log[H']
The greater the concentration of hydrogen ions, the higher the acidity of the
solution and the lower the pH. Solutions with pH of
7
are neutral solutions.
Hydrogen ions (H') force the pH towards zero, and solutions with lower pH than
pH of
7
are acidic. Hydroxyl ions (OH-), on the other hand, make the solution
alkaline and the solutions have higher pH values than
7.
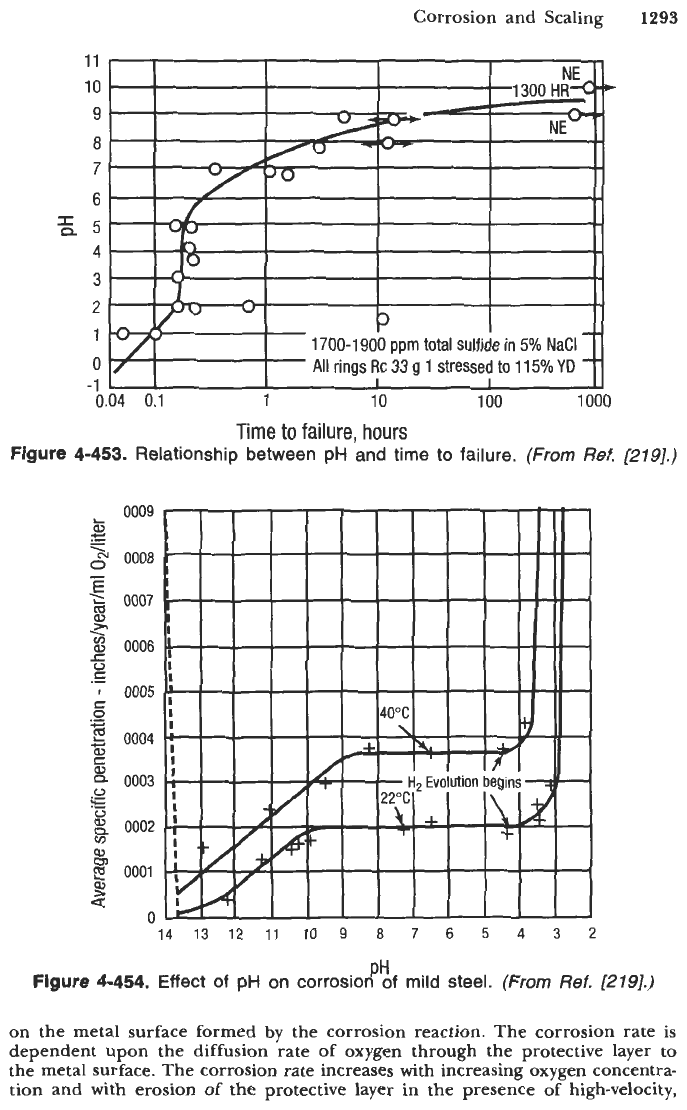
The pH variation affects
the
S-N
curve behavior of steel. Figure
4-452
shows that as the pH is increased
from
6.6
to
12.1
or
13,
the threshold or fatigue limit of steel is restored in
aerated saltwater systems. Figure
4-453
shows the relationship of failures by
embrittlement (sulfide cracking) and pH. The time to failure increases as the
pH rises. Below pH
7,
failure occurs in less than an hour in the presence of
hydrogen sulfide.
As
the pH rises above
7,
time to failure rapidly increases. Both
carbon dioxide and hydrogen sulfide lower the pH level to acidic regions and,
consequently, increase the corrosion rate. Figure
4-454
illustrates the effect of
pH on corrosion of mild steel. In high-pH range the corrosion reaction is
anodically controlled.
As
the pH values decrease, the corrosion reaction
gradually shifts to cathodic control. The resulting decrease in corrosion rate
can be explained by the formation of a protective layer
of
hydrous ferrous oxide
60
so
B
L
3
-0
40
c
c
-
30
20
-
0.1
1.0
2-
IO
Numbor
of
Cyclrr
X
10'
Figure
4-452.
Effect
of
pH
on corrosion fatigue
in
aerated saltwater.
(From
Ref.
[197].)
Corrosion and Scaling
1293
11
10
9
8
7
6
a5
4
I
c
w
3
-_
1700-1
900
ppm
total sulfide in
5%
NaCl
All
rings
Rc
33
g
1
stressed to
11
5%
YD
--
-1
-
I
I
1000
0.04
0.1
1
10 100
Time
to
failure, hours
Figure
4-453.
Relationship between pH and time to
failure.
(From
Ref.
[279].)
0009
b
c
T
0”
0008
8
-
E
2
0007
0006
I
0005
0004
5
0003
0002
>
.
c
0
c
c
0
.-
.-
w
0)
Ts
.-
!e
0
v)
W
g
0001
W
P
0
14131211
109
8
7
6
5
4
3
2
PH
figure
4-454.
Effect
of
pH
on corrosion
of
mild steel.
(From
Ref.
12791.)
on the metal surface formed by the corrosion reaction. The corrosion rate is
dependent upon the diffusion rate of oxygen through the protective layer to
the metal surface. The corrosion rate increases with increasing oxygen concentra-
tion and with erosion of the protective layer in the presence of high-velocity,
1294
Drilling and Well Completions
turbulent flow, which is often imposed on the drillstring.
As
the pH decreases
further, the protective film breaks down, the reaction shifts back under anodic
control, and
the
corrosion rate rises rapidly. During drilling operations it is very
important to maintain high-pH levels around the flat part of the curve shown
in Figure
4451.
However, it should be noted that aluminum alloys exhibit an
increased rate of corrosion at pH higher than
10.5.
Therefore, when aluminum
drillpipes are used the pH values are generally kept between
7
and
10.5.
Temperature
factors
[
1881:
The effect of temperature on corrosion rate is influenced by the following
1.
Increase in temperature increases the redox reaction.
2.
Solubility of gases in water decreases with increasing temperature.
3.
Change in viscosity may affect the circulation, diffusion and other properties
4.
Solubility of some reaction products can be affected by temperature
pertinent to the corrosion process.
variation.
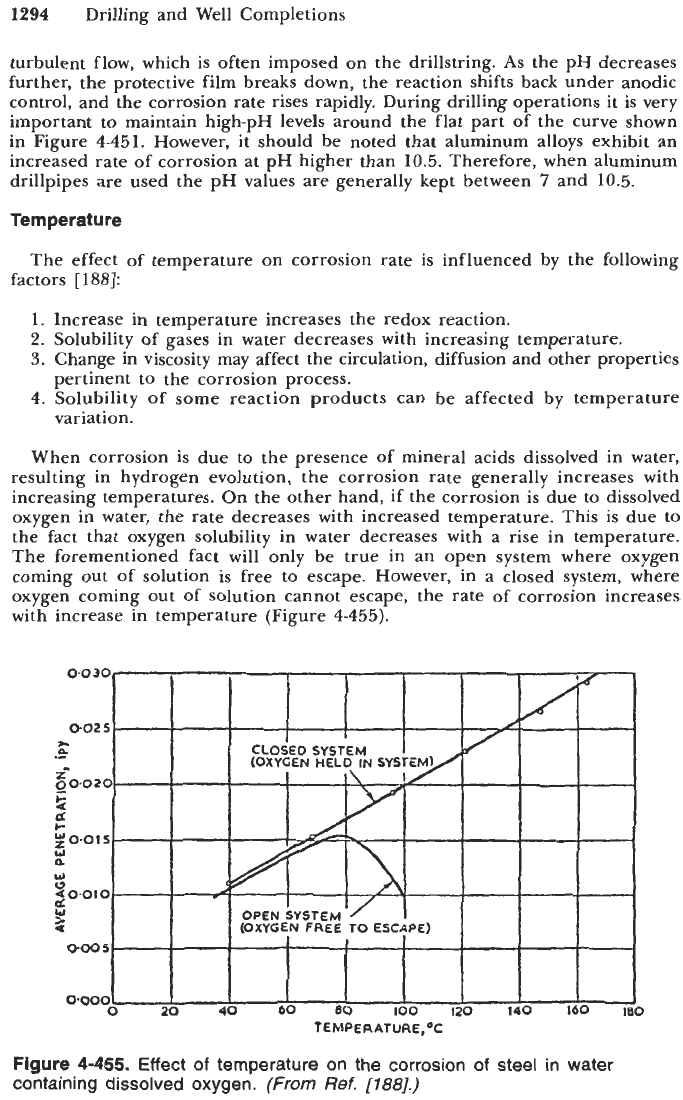
When corrosion is due
to
the presence of mineral acids dissolved in water,
resulting in hydrogen evolution, the corrosion rate generally increases with
increasing temperatures. On the other hand,
if
the corrosion is due to dissolved
oxygen in water, the rate decreases with increased temperature. This is due
to
the fact that oxygen solubility in water decreases with a rise in temperature.
The forementioned fact
will
only be true in an open system where oxygen
coming out of solution is free to escape. However, in a closed system, where
oxygen coming out of solution cannot escape, the rate of corrosion increases
with increase in temperature (Figure
4-455).
TEMPERATURE,%
Figure
4-455. Effect
of
temperature on the corrosion
of
steel in water
containing dissolved oxygen.
(From
Ref.
[l88].)
