Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM BATTERIES 14.55
A recent study
37
has evaluated the effect of ambient temperature storage for up to 6 years.
The interrelation of voltage stability, capacity retention, self-discharge and voltage delay has
been studied. This source should be consulted to obtain detailed data on this system.
14.8 LITHIUM/MANGANESE DIOXIDE (Li/MnO
2
) BATTERIES
The lithium /manganese dioxide (Li/MnO
2
) battery was one of the first lithium/solid-cathode
systems to be used commercially and is now the most widely used primary lithium battery.
It is available in many configurations (including coin, bobbin, spirally wound cylindrical,
and prismatic configurations in multicell batteries, and in designs for low, moderate, and
moderately high drain applications. The capacity of batteries available commercially ranges
up to 2.5 Ah. Larger sized batteries are available for special applications and have been
introduced commercially. Its attractive properties include a high cell voltage (nominal voltage
3 V), specific energy above 230 Wh/ kg and an energy density above 535 Wh/ L, depending
on design and application, good performance over a wide temperature range, long shelf life,
storability even at elevated temperatures, and low cost.
The Li/ MnO
2
battery is used in a wide variety of applications such as long-term memory
backup, safety and security devices, cameras, many consumer devices and in military elec-
tronics. It has gained an excellent safety record during the period since it was introduced.
The performance of a Li/MnO
2
battery is compared with comparable mercury, silver
oxide, and zinc batteries in Sec. 7.3 illustrating the higher energy output of the Li /MnO
2
battery.
14.8.1 Chemistry
The Li /MnO
2
cell uses lithium for the anode, and electrolyte containing lithium salts in a
mixed organic solvent such as propylene carbonate and 1,2-dimethoxyethane, and a specially
prepared heat-treated form of MnO
2
for the active cathode material.
The cell reactions for this system are
⫹
xLi → Li ⫹ eAnode
IV
⫹
III
Cathode
Mn O ⫹ x Li ⫹ e → Li Mn O
2 x 2
IV III
Overall
xLi ⫹ Mn O → Li Mn O
2 x 2
Manganese dioxide, an intercalation compound, is reduced from the tetravalent to the tri-
valent state producing Li
x
MnO
2
as the Li
⫹
ion enters into the MnO
2
crystal lattice.
1,38
The theoretical voltage of the total cell reaction is about 3.5 V, but an open circuit voltage
of a new cell is typically 3.3 V. Cells are typically predischarged to lower the open circuit
voltage to reduce corrosion.
14.8.2 Construction
The Li /MnO
2
electrochemical system is manufactured in several different designs and con-
figurations to meet the range of requirements for small, lightweight, portable power sources.
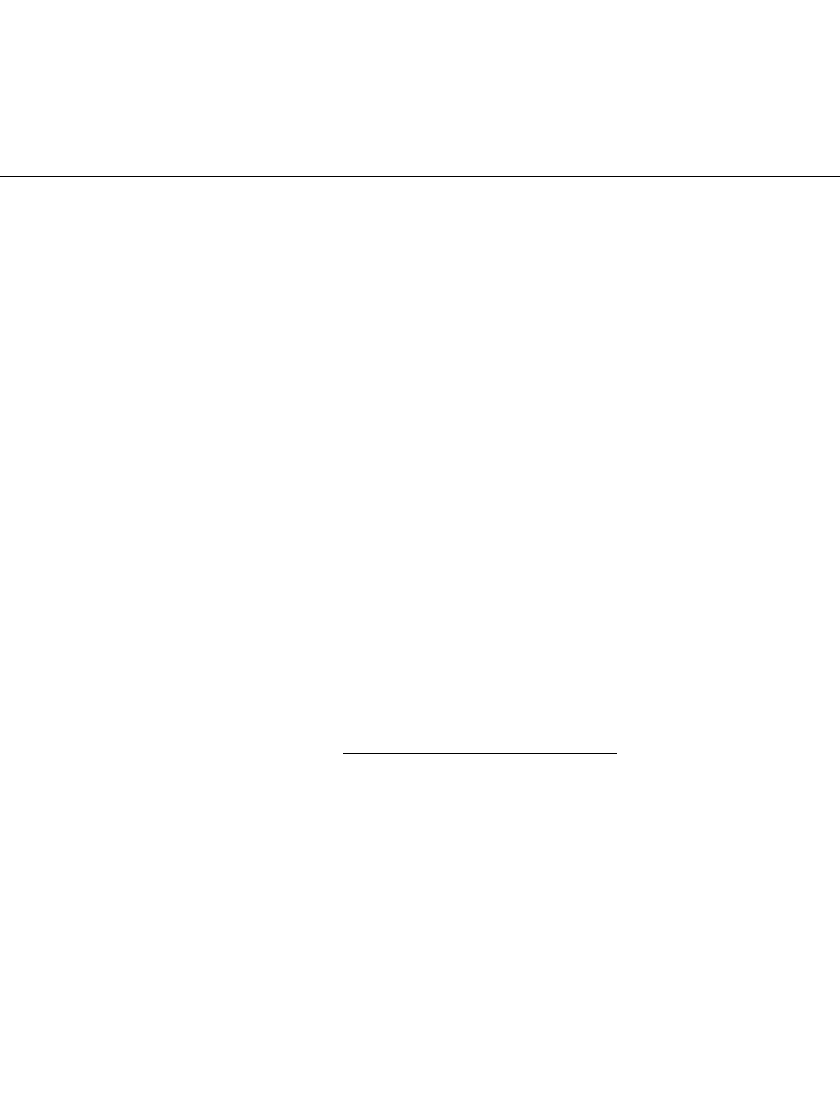
Coin Cells. Figure 14.38 shows a cutaway illustration of a typical coin cell. The manganese
dioxide pellet faces the lithium anode disk and is separated by a nonwoven polypropylene
separator impregnated with the electrolyte. The cell is crimped-sealed, with the can serving
as the positive terminal and the cap as the negative terminal.
14.56 CHAPTER FOURTEEN
FIGURE 14.38 Cross-sectional view of Li /MnO
2
coin-type bat-
tery. (Courtesy of Duracell, Inc.)
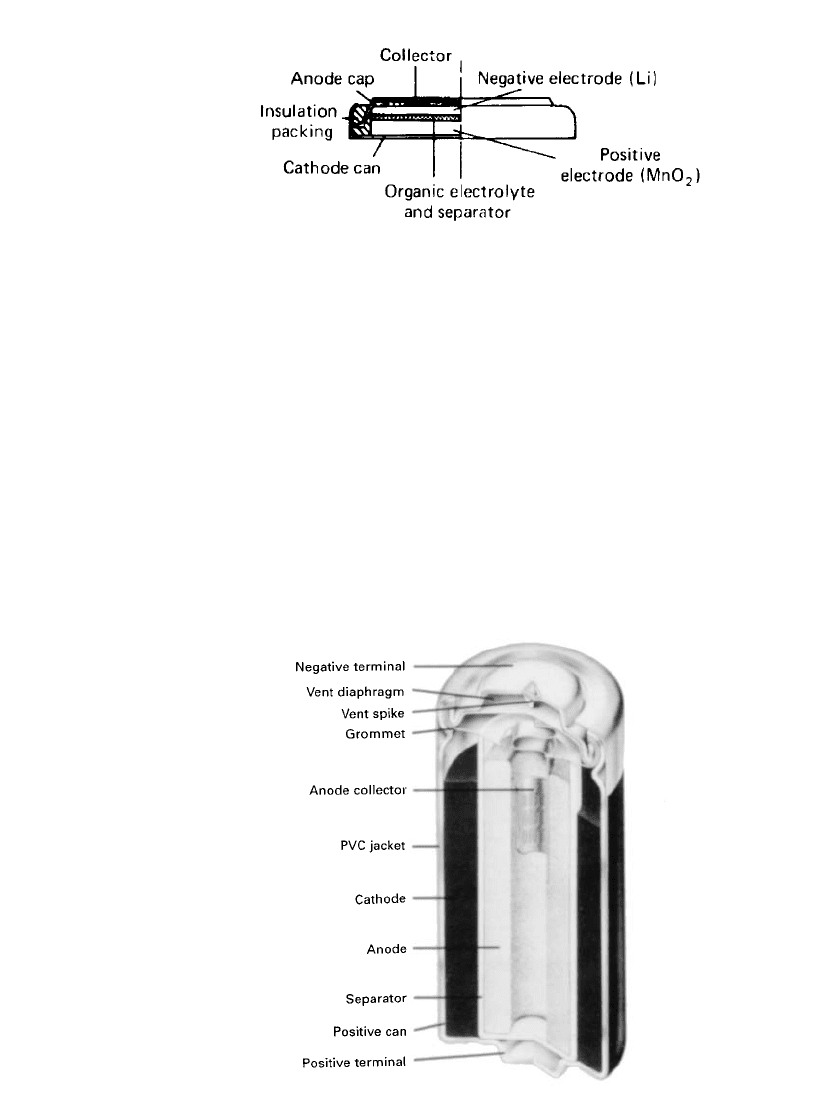
FIGURE 14.39 Cross-sectional view of Li /MnO
2
bob-
bin battery. (Courtesy of Duracell, Inc.)
Bobbin-Type Cylindrical Cells. The bobbin-type cell is one of the two Li/ MnO
2
cylindrical
cells. The bobbin design maximizes the energy density due to the use of thick electrodes
and the maximum amount of active materials, but at the expense of electrode surface area.
This limits the rate capability of the cell and restricts its use to low-drain applications.
A cross section of a typical cell is shown in Fig. 14.39. The cells contain a central lithium
anode core surrounded by the manganese dioxide cathode, separated by a polypropylene
separator impregnated with the electrolyte. The cell top contains a safety vent to relieve
pressure in the event of mechanical or electrical abuse. Welded-sealed cells are manufactured
in addition to the crimped-seal design. These cells, which have a 10-year life, are used for
memory backup and other low-rate applications.
Spirally Wound Cylindrical Cells. The spirally wound cell, illustrated in Fig. 14.40, is
designed for high-current pulse applications as well as continuous high-rate operation. The
lithium anode and the cathode (a thin, pasted electrode on a supporting grid structure) are
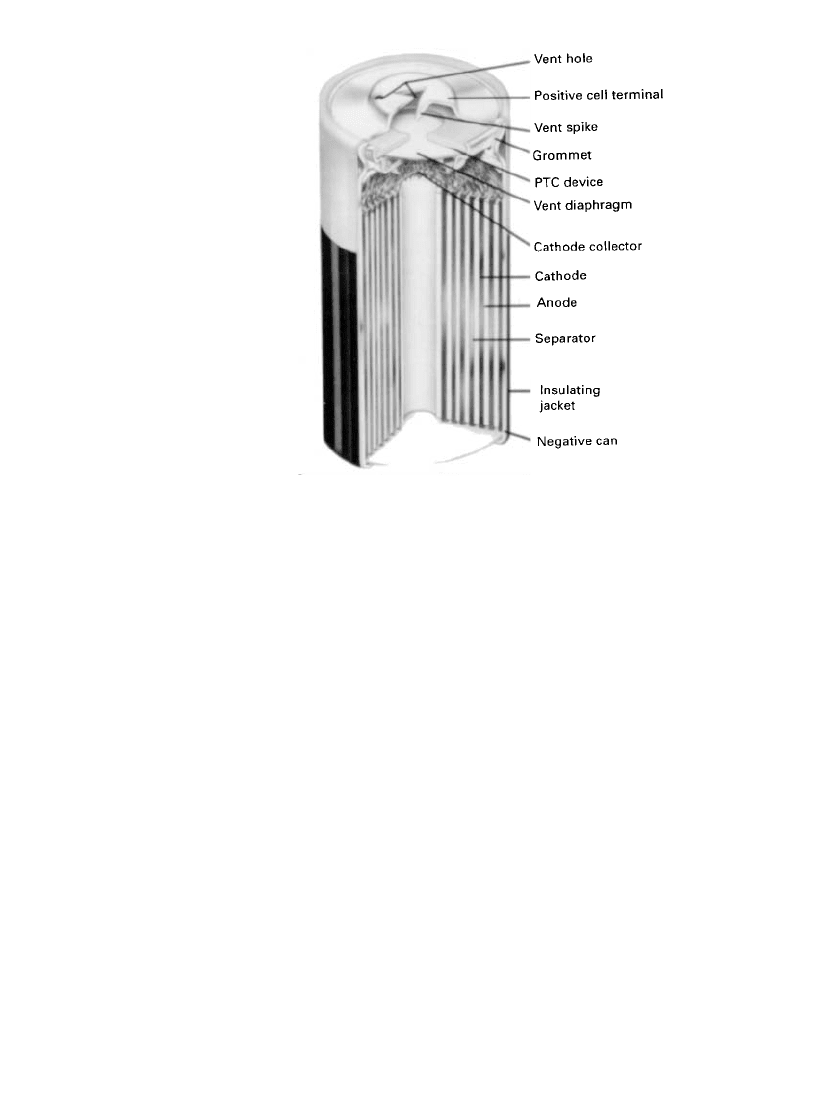
LITHIUM BATTERIES 14.57
FIGURE 14.40 Cross-sectional view of Li / MnO
2
spi-
rally wound electrode battery. (Courtesy of Duracell,
Inc.)
wound together with a microporous polypropylene separator interspaced between the two
thin electrodes to form the jelly-roll construction. With this design a high electrode surface
area is achieved and the rate capability increased.
High-rate spirally wound cells contain a safety vent to relieve internal pressure in the
event the cell is abused. Many of these cells also contain a resettable positive temperature
coefficient (PTC) device which limits the current and prevents the cell from overheating if
short-circuited accidentally (see also Sec. 14.8.5).
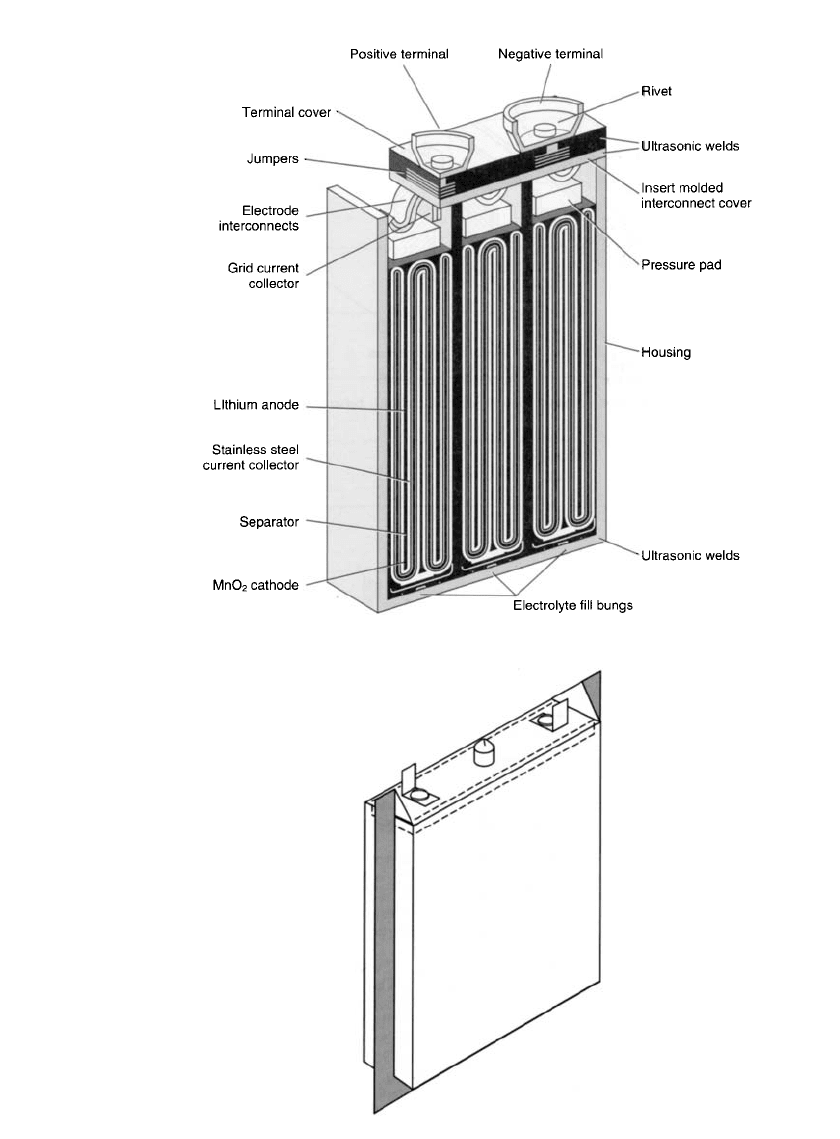
Multicell 9-V Battery. The Li /MnO
2
system has also been designed in a 9-V battery with
1200 mAh capacity in the ANSI 1604 configuration as a replacement for the conventional
zinc battery. The battery contains three prismatic cells, using an electrode design that utilizes
the entire interior volume, as shown in Fig. 14.41. An ultrasonically sealed plastic housing
is used for the battery case.
Foil Cell Designs. Other cell design concepts are being used to reduce the weight and cost
of batteries by using lightweight cell packaging. One of these approaches is the use of heat-
sealable thin foil laminates, in a prismatic cell configuration in place of metal containers.
The design of a cell with a capacity of about 16 Ah is illustrated in Fig. 14.42. The cell
contains 10 anode and 11 cathode plates in a parallel plate array.
39
14.58 CHAPTER FOURTEEN
FIGURE 14.41 Cross-sectional view of three-cell 9-V Li / MnO
2
battery. (Courtesy
of Ultralife Batteries, Inc.)
–
+
H = 7.1 cm
L = 6.1 cm
Seams: 0.32 cm
Thickness:
1.4 cm
FIGURE 14.42 Foil cell design. (From Ref. 39.)
LITHIUM BATTERIES 14.59
14.8.3 Performance
Voltage The open-circuit voltage of the Li /MnO
2
battery is typically 3.1 to 3.3 V after
pre-discharge. The nominal voltage is 3.0 V. The operating voltage during discharge ranges
from about 3.1 to 2.0 V and is dependent on the cell design, state of charge, and the other
discharge conditions. The end or cutoff voltage, the voltage by which most of the capacity
has been expended, is 2.0 V, except under high-rate, low-temperature discharges, when a
lower end voltage may be specified.
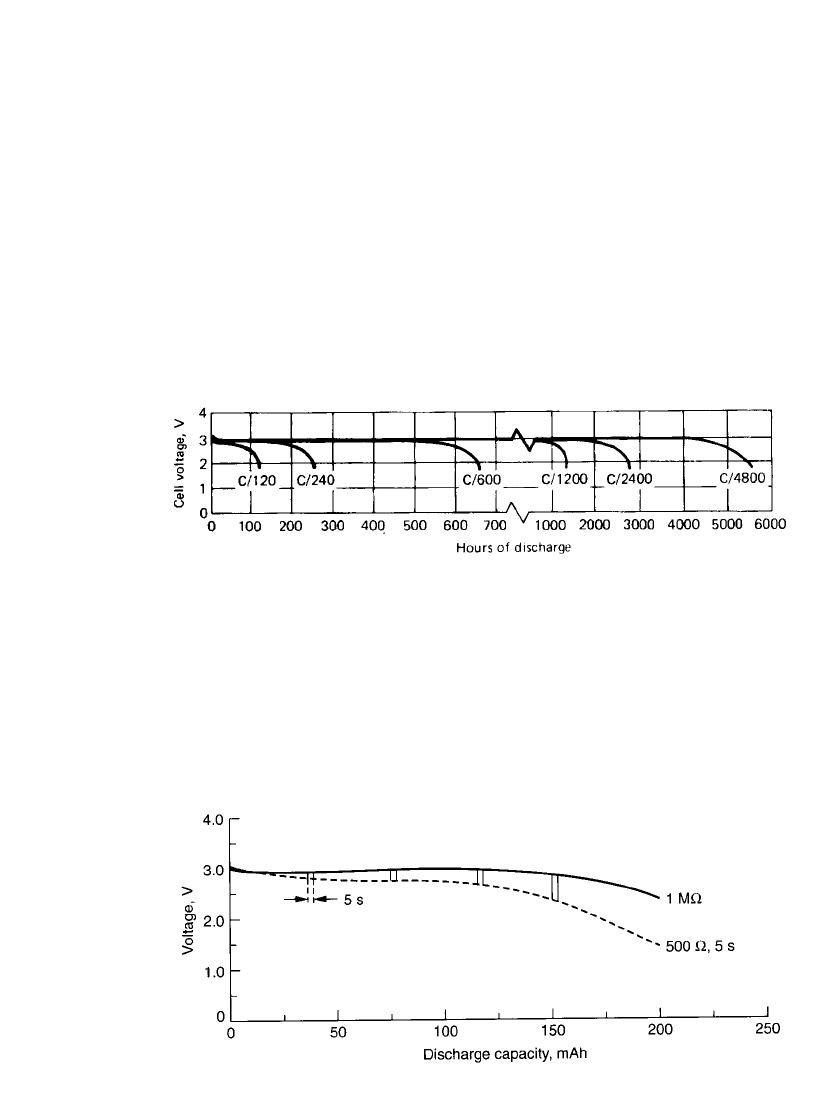
Discharge Characteristics of Coin-Type Batteries. Typical discharge curves for the Li/
MnO
2
coin cells are presented in Fig. 14.43. The discharge profile is fairly flat at these low
to moderate discharge rates throughout most of the discharge, with a gradual drop near the
end of life. This gradual drop in voltage can serve as a state-of-charge indicator to show
when the battery is approaching the end of its useful life.
FIGURE 14.43 Typical discharge curves of Li / MnO
2
coin-type batteries. (Courtesy of Dur-
acell, Inc.)
Some applications (such as an LED watch with backlight) require a high pulse load
superimposed on a low background current. The performance of a coin-type battery under
these conditions is shown in Fig. 14.44, illustrating good voltage regulation even at the higher
discharge rates.
FIGURE 14.44 Pulse characteristics of Li/ MnO
2
coin-type battery (190-mAh size) at
20⬚C. Test conditions: continuous load—1 M⍀ ⬇ 3
A; pulse load—500 ⍀ ⬇ 5.5 mA;
duration—5 s; pulses—4; time between pulses—3 h. (Courtesy of Duracell, Inc.)
14.60 CHAPTER FOURTEEN
The Li/MnO
2
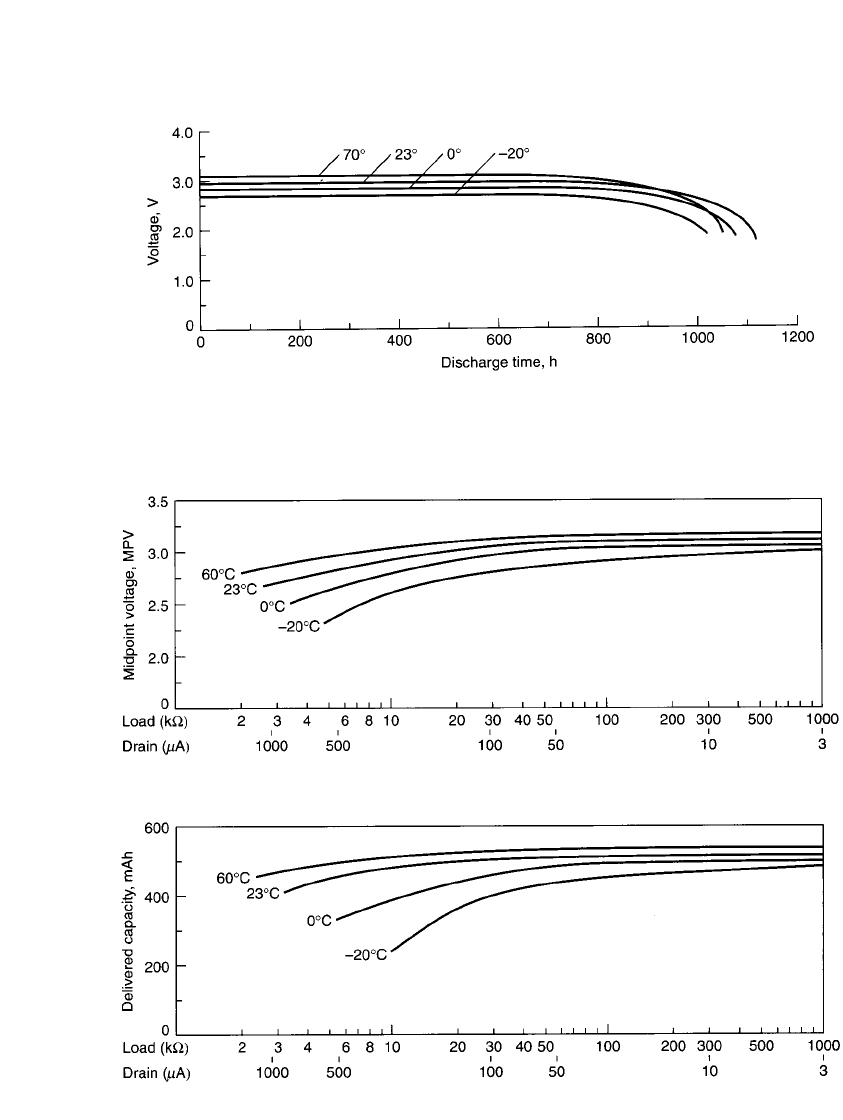
coin-type battery is capable of performing over a wide temperature range,
from about
⫺20 to 55⬚C, as shown in Fig. 14.45. The midpoint voltages, when discharged
at various loads and temperatures, are plotted in Fig. 14.46.
FIGURE 14.45 Typical discharge performance of Li/ MnO
2
coin-type battery at various tempera-
tures. (Courtesy of Duracell, Inc.)
The discharge characteristics of the Li/MnO
2
battery are summarized in Fig. 14.47, which
shows the percent capacity delivered at various temperatures and discharge loads.
FIGURE 14.46 Midpoint voltage during discharge of Li / MnO
2
coin-type (280-mAh size). (Courtesy of
Duracell, Inc.)
FIGURE 14.47 Delivered capacity of Li / MnO
2
coin-type (280-mAh size) at various temperatures and loads.
(Courtesy of Duracell, Inc.)
LITHIUM BATTERIES 14.61
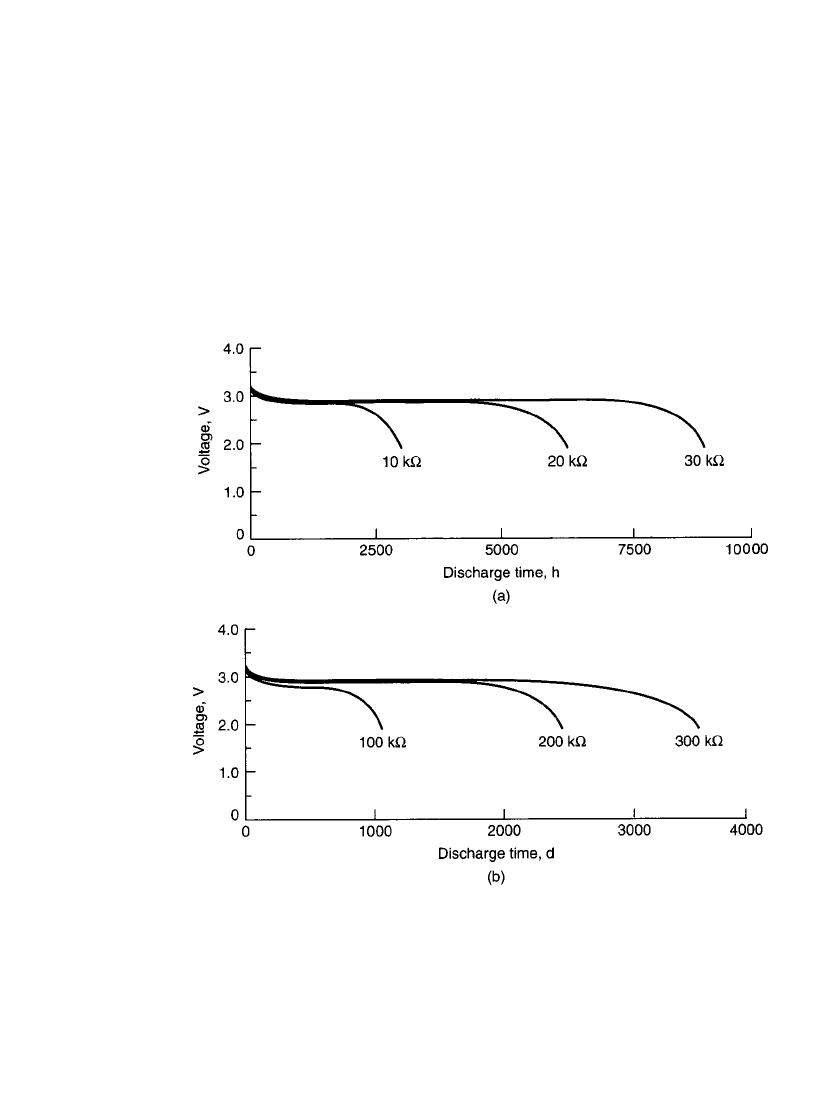
Discharge Characteristics of Cylindrical Bobbin Batteries. Typical discharge curves for
the Li /MnO
2
cylindrical bobbin batteries are given in Fig. 14.48. These bobbin electrode
batteries are designed for use at low to moderate discharge rates, delivering higher capacities
at these discharge rates than the spirally wound electrode batteries of the same size (see
Table 14.19). The discharge profile is fairly flat at these low rates throughout most of the
discharge, with the typical gradual slope near the end of the discharge. The effect of a high
pulse load superimposed on a low background current is shown in Fig. 14.49.
The performance of the Li/MnO
2
cylindrical bobbin battery at temperatures from ⫺20
to 60
⬚C is shown in Fig. 14.50. Operation of the coin-type and cylindrical bobbin electrode
batteries at the lower temperatures is limited to the lower discharge rates.
FIGURE 14.48 Discharge characteristics of Li / MnO
2
cylindrical bobbin battery (850-
mAh size) at 20⬚C. (a) Discharge time in hours. (b) Discharge time in days. (Courtesy
of Duracell, Inc.)
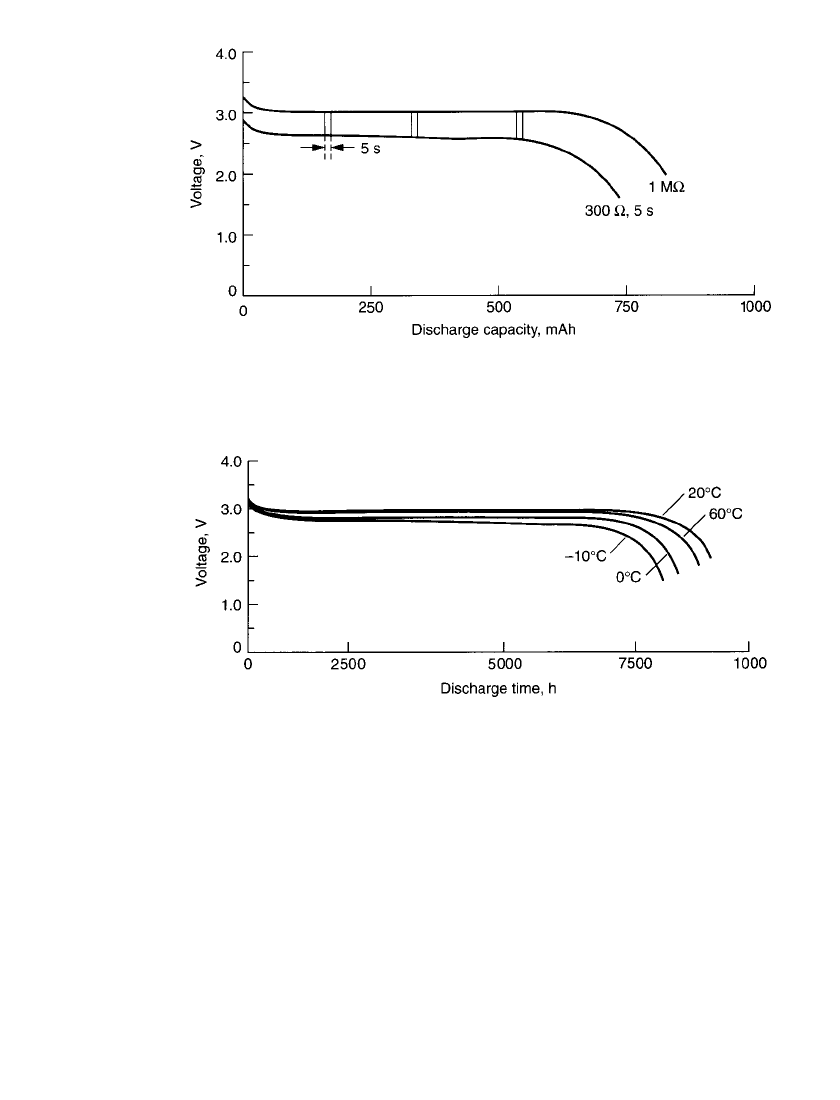
14.62 CHAPTER FOURTEEN
FIGURE 14.49 Pulse discharge characteristics of Li / MnO
2
cylindrical bobbin cell
(850-mAh size) at 20⬚C. Test conditions: continuous load—1 M⍀ ⬇ 2.9
A; pulse load—
300 ⍀ ⬇ 10 mA; duration—5 s; pulses—3; time between pulses—3 h. (Courtesy of
Duracell, Inc.)
FIGURE 14.50 Discharge performance of Li / MnO
2
cylindrical bobbin cell (850-mAh
size) at various temperature; 30-k⍀ discharge rate. (Courtesy of Duracell, Inc.)
Discharge Characteristics of Cylindrical Spirally Wound Batteries. Typical discharge
curves for Li/MnO
2
cylindrical spirally wound batteries at various constant-current discharge
loads and temperatures are given in Fig. 14.51. These batteries are designed for operation
at fairly high rates and low temperatures. Their discharge profile is flat under most of these
discharge conditions. The midpoint voltage when discharged at various loads and tempera-
tures, is plotted in Fig. 14.52.
The characteristics of the batteries under constant power discharge are shown in Fig.
14.53. These data are expressed in terms of E-rate, which is calculated in a manner similar
to calculating the C rate, but based on the rated Watt-hour capacity. For example, the E/5
rate for a cell rated at 4 Wh is 800 mW.
The discharge characteristics of the cylindrical spirally wound Li/ MnO
2
battery at various
temperatures and loads are summarized in Fig. 14.54. Figure 14.54a shows the percent
capacity delivered on constant-resistance loads, Fig. 14.54b the percent capacity delivered
on constant-current loads. The good performance of the Li/ MnO
2
battery at the lower-rate
discharges is evident, and it still delivers a higher percentage of its capacity at relatively
high discharge rates compared to conventional aqueous primary cells.
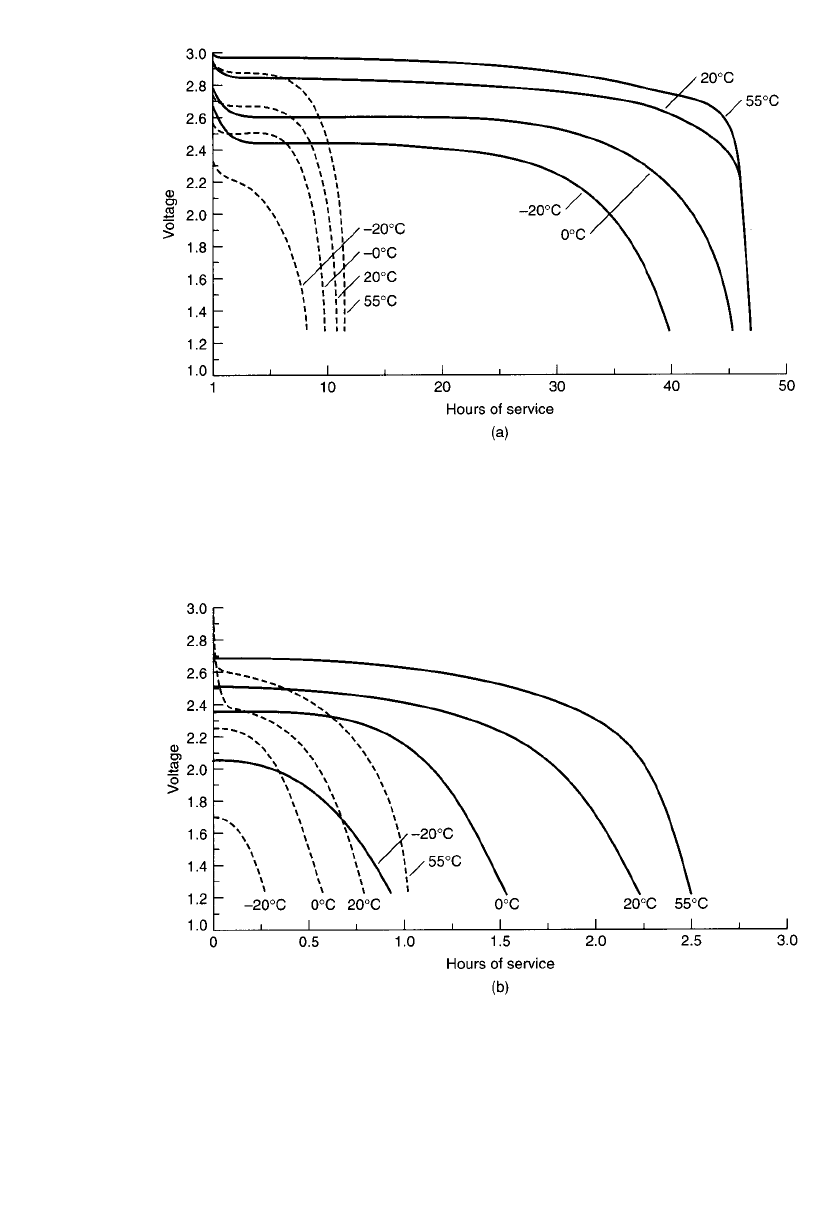
LITHIUM BATTERIES 14.63
FIGURE 14.51 Discharge characteristics of cylindrical (spirally wound electrode) Li / MnO
2
battery (CR123A-size). (a) Discharge at 30 and 125 mA. Broken line—125 mA; solid line—30
mA. (b) Discharge at 500 and 1000 mA. Broken line—1 A; solid line—0.5 A. (b) Discharge at
500 and 1000 mA. Broken line—1 A; solid line—0.5 A.
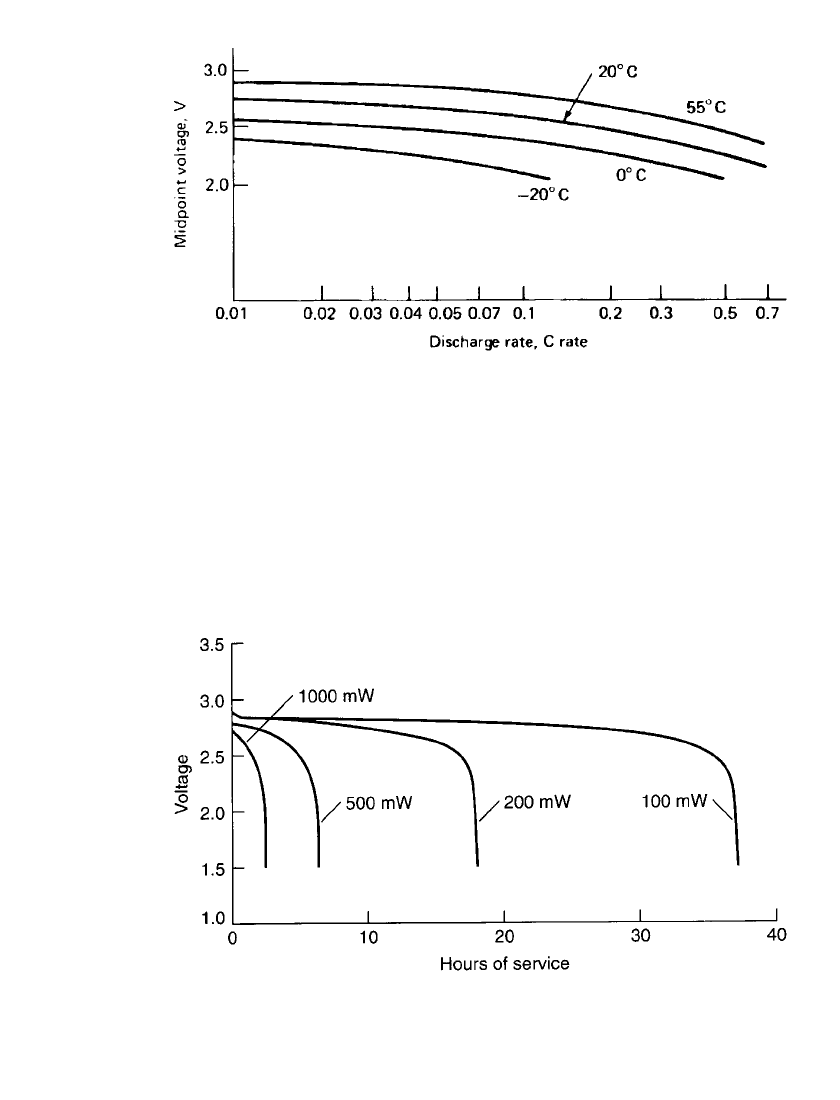
14.64 CHAPTER FOURTEEN
FIGURE 14.52 Midpoint voltage of cylindrical (spirally wound) Li / MnO
2
batteries during
discharge; 2-V end voltage.
FIGURE 14.53 Discharge characteristics of cylindrical (spirally wound electrode) Li / MnO
2
cells (CR123A-size) under constant-power mode at 20⬚C.
