Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

LITHIUM BATTERIES 14.25
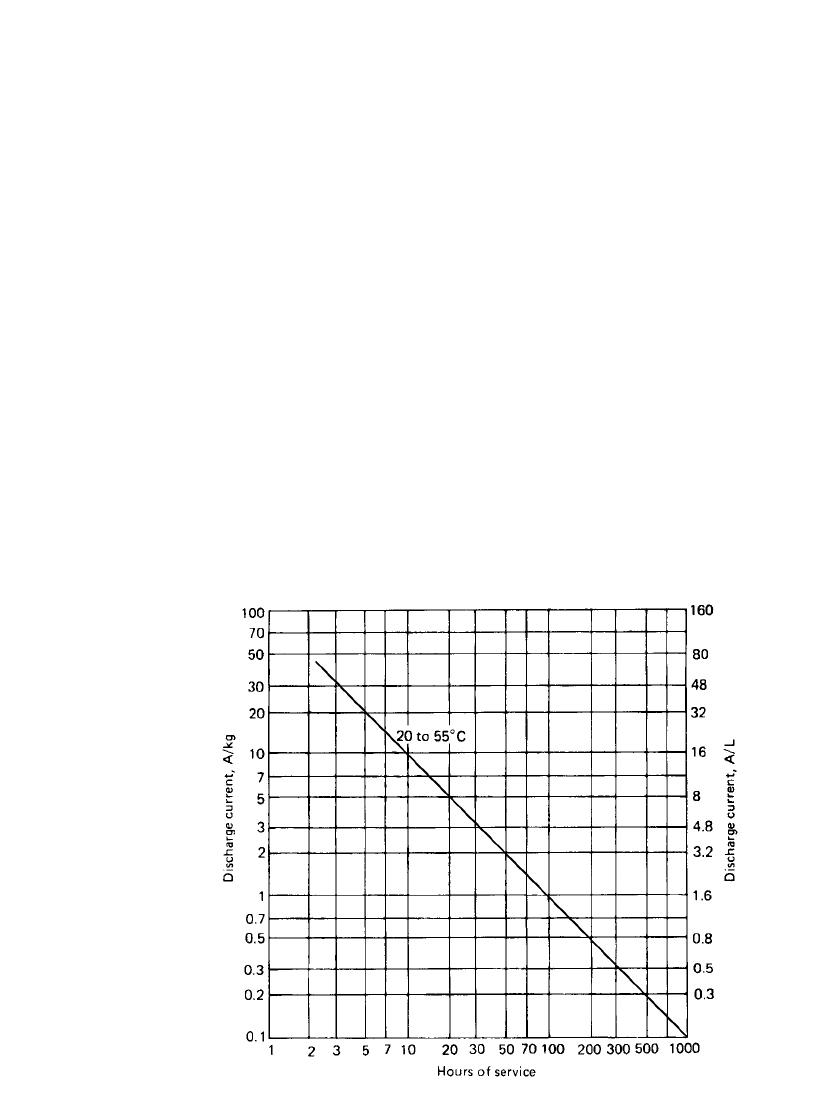
FIGURE 14.13 Service life of high-rate Li / SO
2
batteries; 2.0-V end voltage.
Service Life. The capacity or service life of the Li /SO
2
battery at various discharge rates
given in Fig. 14.13. The data are normalized for a 1-kg or 1-L size battery and presented in
terms of hours of service at various discharge rates. The linear shape of this curve is again
indicative of the capability of the Li/ SO
2
battery to be efficiently discharged at these extreme
conditions. This data can be used in several ways to calculate the approximate performance
of a given battery or to select a Li/SO
2
battery of suitable size for a particular application,
recognizing that the specific energy of the larger-size batteries is higher than that of the
smaller ones.
The service life of a battery at a given current load can be estimated by dividing the
current (in Amperes) by the weight or volume of the battery. This value is located on the
ordinate, and the service life, at a specific current and temperature, is read of the abscissa.
The weight or volume of a battery needed to deliver a required number of hours of service
at a specified current load can be estimated by locating a point on the curve corresponding
to the required service hours and discharge temperature. The battery weight or volume is
calculated by dividing the value of the specified current (in Amperes) by the value of Am-
peres per kilogram or Amperes per liter obtained from the ordinate.
Shelf Life. The Li/ SO
2
battery is noted for its excellent storage characteristics, even at
temperatures as high as 70
⬚C. Most primary batteries lose capacity while idle or on stand
due to anode corrosion, side chemical reactions, or moisture loss. With the exception of the
magnesium battery, most of the conventional primary batteries cannot withstand temperatures
in excess of 50
⬚C and should be refrigerated if stored for long periods. The Li /SO
2
battery,
however, is hermetically sealed and protected during storage by the formation of a film on
the anode surface. Capacity losses during stand are minimal.
14.26 CHAPTER FOURTEEN
Recent data
18
on two-year old BA-5590 batteries consisting of 10 Li/SO
2
D-size cells
discharged in series at 2 amps at
⫹21 and ⫺30⬚C showed a 6.5% capacity loss at the higher
temperature but no loss at the lower temperature. Fourteen-year storage data was also ob-
tained on BA-5598 batteries consisting of five ‘‘squat’’ D-size cells in series. These batteries
showed only an 8% capacity loss when discharged at room temperature at 2 amps, but
virtually no loss at cold temperature. In both cases, a lower operating voltage was observed
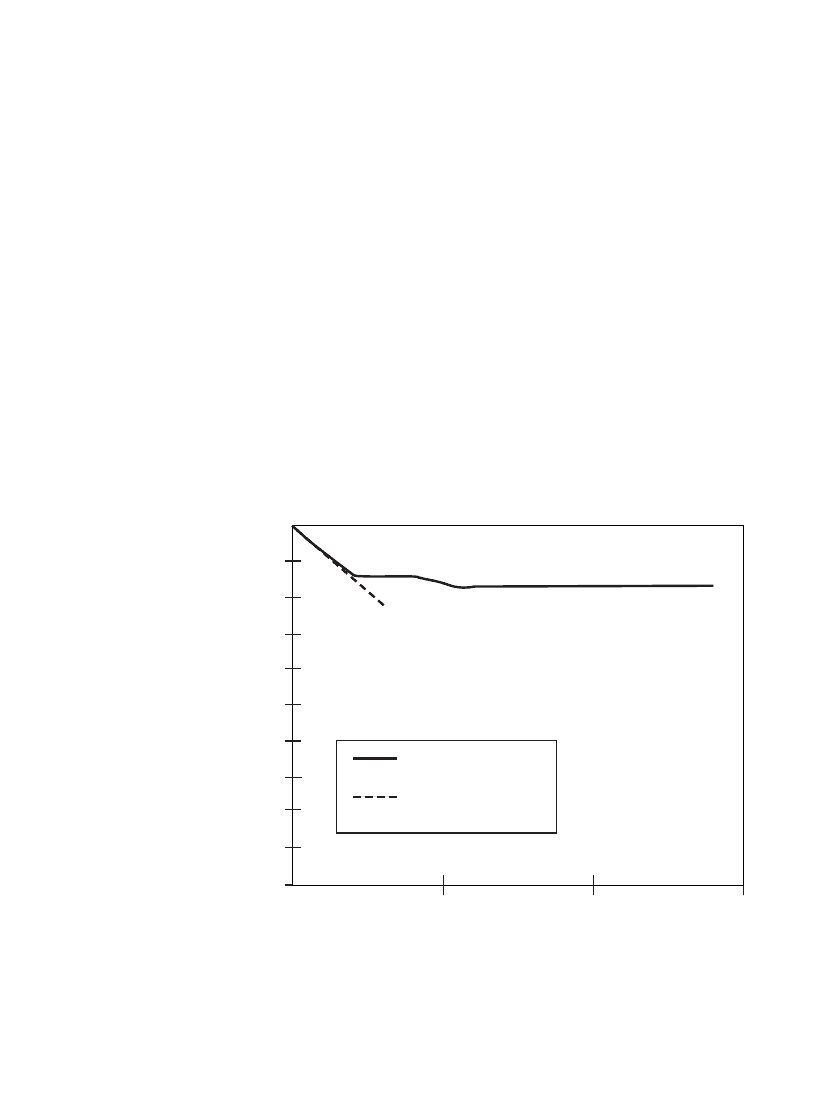
after storage. Using multiple groups of batteries, stored for 4, 6 and 14 years under ambient
conditions, the data shown in Fig. 14.14 was obtained. The capacity loss for the first two
years is approximately 3%/year, but the rate of loss decreases significantly after that period.
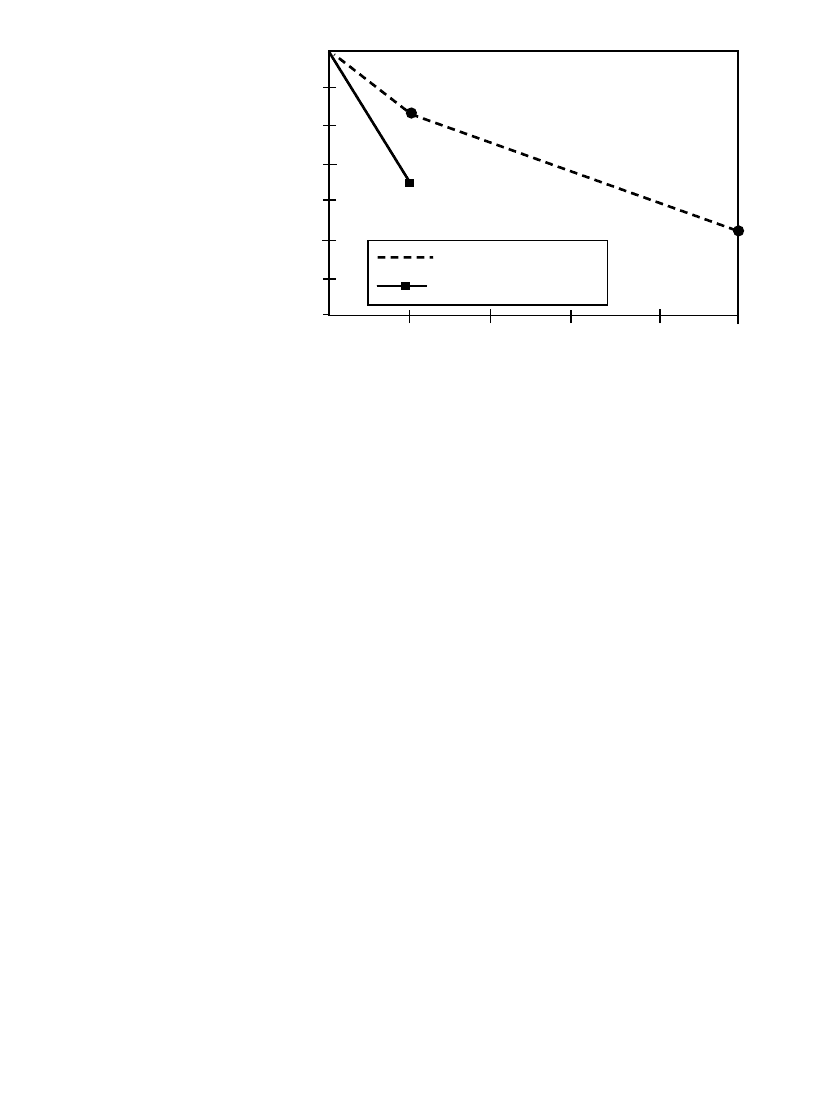
High-temperature storage of batteries was also carried out at
⫹70 and ⫹85⬚C, as shown in
Fig. 14.15. At 70
⬚C, these batteries showed 92% capacity retention after 1 month and 77%
capacity retention after five months. At 85
⬚C, 82% capacity retention was observed after
one-month’s storage. This study concluded that there was no obvious benefit to making long-
term storage predictions based on accelerated aging tests at high temperature.
STORAGE TIME (Years)
0 5 10 15
100%
95%
90%
85%
80%
75%
70%
65%
60%
55%
50%
% of ORIGINAL CAPACITY
ACTUAL DATA
3%/YEAR LOSS
PREDICTION
FIGURE 14.14 Capacity retention of Li/ SO
2
batteries after ambient storage and
discharge at the 2 amp rate.
LITHIUM BATTERIES 14.27
100%
95%
90%
85%
80%
75%
70%
65%
70 Deg C
85 Deg C
0 1 2 3 4 5
TIME (Months)
% ORIGINAL
CAPACITY
FIGURE 14.15 Effect of storage time / temperature on capacity of Li / SO
2
bat-
teries.
Voltage Delay. After extended long-term storage at elevated temperatures, the Li/SO
2
bat-
tery may exhibit a delay in reaching its normal operating voltage when placed on discharge,
especially at high current loads and low temperatures. This start-up or voltage delay is caused
by the protective film formed on the lithium anode, the characteristic responsible for the
excellent shelf life of the cell. The specific delay time for a battery depends on such factors
as the history of the battery, the specific cell design and components, the storage time and
temperature, discharge load and temperature. Typically, the voltage delay is minimal or
nonexistent for discharges at moderate to low rates at temperatures above
⫺20⬚C. No delay
is evident on discharge at 20
⬚C, even after storage at 70⬚C for 1 year. On discharge at ⫺30⬚C,
the delay time is less than 200 ms after 8 weeks of storage at 70
⬚C on discharges lower than
the 40-h rate. At higher rates, the voltage delay increases with increasing storage temperature
and time. At the 2-h discharge rate, for example, the maximum start-up time is about
80 s after 8 weeks of storage at 70
⬚C; it is 7 s after 2 weeks of storage.
19
The start-up
voltage delay can be eliminated by preconditioning with a short discharge at a higher rate
to depassivate the anode until the operating voltage is reached since the delay will return
only after another extended storage period.
14.5.4 Cell and Battery Types and Sizes
The Li/SO
2
batteries are manufactured in a number of cylindrical cell sizes, ranging in
capacity to 35 Ah. Some of the cells are manufactured in standard ANSI cell sizes in
dimensions of popular conventional zinc primary batteries. While these single batteries may
be physically interchangeable, they are not electrically interchangeable because of the higher
cell voltage of the lithium cell (3.0 V for lithium, 1.5 V for the conventional zinc cells).
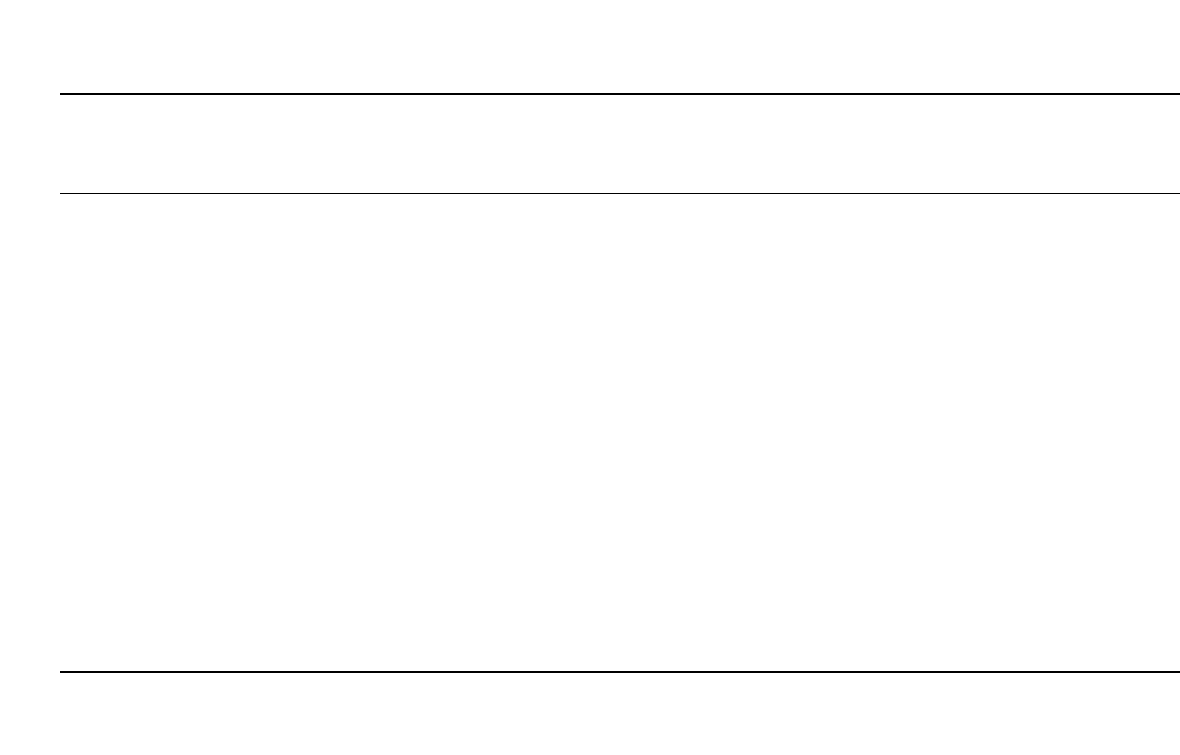
Table 14.9 lists some of the sizes and rated capacities of Li /SO
2
batteries that are currently
manufactured.
14.28
TABLE 14.9 Typical Lithium/ Sulfur Dioxide Cylindrical Cells (SAFT American, Inc.).*
Model
number Size
Nominal
capacity
(drain)
Maximum
recommended
continuous
current
Pulse
capability
Standard
operating
range Max. OD Max. H Weight
LO 34 SX 1 /3 R14
1/3C
0.86 Ah
(30 mA)
0.25 A 0.50 A ⫺60 /⫹70⬚C 25.6 mm 20.3 mm 16 g
LO 35 SX 2 /3 R14
2/3 C
2.00 Ah
(100 mA)
2.0 A 10 A
⫺60/ ⫹70⬚C 25.6 mm 35.9 mm 30 g
LO 26 SX R20
D
7.5 Ah
(240 mA)
3.0 A 10 A
⫺60/ ⫹70⬚C 33.8 mm 59.3 mm 85 g
LO 25 SX ‘‘Fat’’
D
8.00 Ah
(270 mA)
3.0 A 10 A
⫺60/ ⫹70⬚C 38.4 mm 50.3 mm 93 g
LO 52 SX 2 R20
DD
16.0 Ah
(500 mA)
6.0 A 20 A
⫺60/ ⫹70⬚C 33.7 mm 115.6 mm 163 g
LO 38 SHX ‘‘long’’
A
1.50 Ah
(250 mA)
1.0 A 3.0 A
⫺60/ ⫹70⬚C 16.3 mm 57.2 mm 21 g
LO 29 SHX R14
C
3.50 Ah
(120 mA)
2.0 A 30 A
⫺60/ ⫹70⬚C 25.6 mm 50.4 mm 40 g
LO 43 SHX 5 / 4 R14
5/4 C
4.50 Ah
(500 mA)
2.0 A 30 A
⫺60/ ⫹70⬚C 25.6 mm 59.3 mm 53 g
LO 40 SHX 2 / 3 ‘‘Thin’’
D
3.50 Ah
(120 mA)
2.0 A 10 A
⫺60/ ⫹70⬚C 28.8 mm 41.6 mm 40 g
LO 30 SHX ‘‘Thin’’ D 5.75 Ah
(200 mA)
3.0 A 30 A
⫺60/ ⫹70⬚C 29.1 mm 59.8 mm 63 g
LO 26 SHX R20
D
7.20 Ah
(200 mA)
4.0 A 30 A
⫺60/ ⫹70⬚C 34.2 mm 59.8 mm 85 g
LO 39 SHX F 11.0 Ah
(200 mA)
8.0 A 60 A
⫺60/ ⫹70⬚C 30.7 mm 99.4 mm 125 g
* Pulse capability: 1 sec./ min pulses over 2.0 volts.

LITHIUM BATTERIES 14.29
14.5.5 Use and Handling of Li/ SO
2
Cells and Batteries—Safety
Considerations
The Li/ SO
2
battery is designed as a high-performance system and is capable of delivering
a high capacity at high discharge rates. The cell should not be physically or electrically
abused, safety features should not be bypassed, and manufacturers’ instructions should be
followed.
Abusive conditions could adversely affect the performance of the Li/SO
2
battery and
result in cell venting, rupture, explosion, or fire. Preventative measures are discussed in Sec.
14.4.
The Li/ SO
2
battery is pressurized and contains materials that are toxic or flammable.
Properly designed batteries are hermetically sealed so that there will be no leakage or out-
gassing, and they are equipped with safety vents which release if the batteries reach exces-
sively high temperatures and pressures, thus preventing an explosive condition.
The Li/SO
2
batteries can deliver very high currents. Because high internal temperatures
can develop from continuous high current drain and short circuit, batteries must be protected
by electrical fusing and thermal cutoffs. Charging of Li/ SO
2
batteries may result in venting,
rupture, or even explosion and should never be attempted. Cells or groups of cells connected
in parallel should be diode-protected to prevent one group from charging another. The bal-
anced Li /SO
2
cell is designed to handle forced discharges or cell reversal and will perform
safely within the specified bounds, but design limits should not be exceeded in any appli-
cation.
Proper battery design, using the Li/ SO
2
cell, should follow these guidelines:
1. Use electrical fusing and/ or current-limiting devices to prevent high currents or short-
circuits.
2. Protect with diodes if cells are paralleled or connected to a possible charging source.
3. Minimize heat buildup by adequate heat dissipation and protect with thermal cutoff de-
vices.
4. Do not inhibit cell vents in battery construction.
5. Do not use flammable materials in the construction of batteries.
6. Allow for release of vented gases.
7. Incorporate resistor and switch to activate it to ensure complete depletion of active ma-
terials after normal discharge.
8. In certain cases, a diode is placed in parallel with the cell to limit the voltage excursion
in reversal.
Currently special procedures govern the transportation, shipment, and disposal of Li/SO
2
batteries as well as other lithium batteries.
12–15
Procedures for the use, storage, and handling
of these batteries also have been recommended. The latest issue of these regulations should
be consulted for the most recent procedures.
14.5.6 Applications
The desirable characteristics of the Li /SO
2
battery and its ability to deliver a high energy
output and operate over a wide range of temperatures, discharge loads, and storage conditions
have opened up applications for this primary battery that, heretofore, were beyond the ca-
pability of primary battery systems (see Sec. 6.4).
Major applications for the Li /SO
2
battery are in military equipment, such as radio trans-
ceivers and portable surveillance devices, taking advantage of its light weight and wide-
temperature operation. Table 14.10 lists the most common types of military Li/SO
2
batteries,
14.30
TABLE 14.10 U.S. Millitary Lithium/ Sulfur Dioxide Batteries (Per MIL-B-49430)
Battery type
Open circuit voltage
(series/ parallel)(V)
Nominal voltage
(series/ parallel)(V)
Nominal capacity
(series/ parallel)(Ah)
Weight
(g) Typical applications
Ba-5112/ U 12.0 11.2 1.8 180 Rescue radio/ beacon
BA-5567/U 3.0 2.6 0.8 20.0 Night vision equipment
BA-5599/U 9.0 7.2 7.2 454 Test equipment
Night vision equipment
BA-5600/U 9.0 8.4 7.2 363 Data terminals
BA-5800/U 6.0 5.6 7.2 220 Chemical agent monitors
Global positioning equipment
BA-5847/U 6.0 5.6 7.2 240 Test equipment
Antennas
Night vision equipment
BA-5598 15.0
(with 3.0 Volt tap)
14.0 8.0 631 Radios (PRC-77, PRC-25),
Scramblers
BA-5588 15.0 14.0 3.9 295 Handheld radios
Gas masks
BA-5557 30.0/ 15.0 26.0/13.0 2.25/ 4.5 500 Digital message device
BA-5590 30.0/ 15.0 24.0/12.0 7.2/ 14.4 1021 Radios (SINCGARS)
Satellite radios
Scramblers
Radar
Loudspeakers
UHF radios
Range finders
Counter measures
Weather instruments
Jammers
Cooling systems

LITHIUM BATTERIES 14.31
their characteristics and applications. Other military applications, such as sonobuoys and
munitions, have long shelf-life requirements, and the active Li /SO
2
primary battery can
replace reserve batteries used earlier. Some industrial applications have developed, particu-
larly to replace secondary batteries and eliminate the need for recharging. Consumer appli-
cations have been limited to date because of restrictions in shipment and transportation and
concern with its hazardous components.
20
14.6 LITHIUM/THIONYL CHLORIDE (Li/SOCl
2
) BATTERIES
The lithium/thionyl chloride (Li /SOCl
2
) battery has one of the highest cell voltages (nominal
voltage 3.6 V) and energy densities of the practical battery systems. Specific energy and
energy densities range up to about 590 Wh /kg and 1100 Wh /L, the highest values being
achieved with the low-rate batteries. Figures 7.8, 7.9, and 14.2 illustrate some of the advan-
tageous characteristics of the Li /SOCl
2
cell.
Li/ SOCl
2
batteries have been fabricated in a variety of sizes and designs, ranging from
wafer or coin cells with capacities as low as 400 mAh, cylindrical cells in bobbin and spirally
wound electrode structures, to large 10,000-Ah prismatic cells, plus a number of special sizes
and configurations to meet particular requirements. The thionyl chloride system originally
suffered from safety problems, especially on high-rate discharges and overdischarge, and a
voltage delay that was most evident on low-temperature discharges after high-temperature
storage.
21
Low-rate batteries have been used commercially for a number of years for memory backup
and other applications requiring a long operating life. The large prismatic batteries have been
used in military applications as an emergency back-up power source. Medium- and high-
rate batteries have also been developed as power sources for a variety of electric and elec-
tronic devices. Some of these batteries contain additives to the thionyl chloride and other
oxyhalide electrolytes to enhance certain performance characteristics. These are covered in
Sec. 14.7.
14.6.1 Chemistry
The Li /SOCl
2
cell consists of a lithium anode, a porous carbon cathode, and a nonaqueous
SOCl
2
:LiAlCl
4
electrolyte. Other electrolyte salts, such as LiGaCl
4
have been employed for
specialized applications. Thionyl chloride is both the electrolyte solvent and the active cath-
ode material. There are considerable differences in electrolyte formulations and electrode
characteristics. The proportions of anode, cathode, and thionyl chloride will vary depending
on the manufacturer and the desired performance characteristics. Significant controversy
exists as to the relative safety of anode-limited vs. cathode-limited designs.
22
Some cells
have one or more electrolyte additives. Catalysts, metallic powders, or other substances have
been used in the carbon cathode or in the electrolyte to enhance performance.
The generally accepted overall reaction mechanism
4Li
⫹ 2SOCl → 4LiCl ↓ ⫹ S ⫹ SO
22
The sulfur and sulfur dioxide are initially soluble in the excess thionyl chloride electrolyte,
and there is a moderate buildup of pressure due to the generation of sulfur dioxide during
the discharge. The lithium chloride, however, is not soluble and precipitates within the porous
carbon black cathode as it is formed. Sulfur may precipitate in the cathode at the end of
discharge. In most cell designs and discharge conditions, this blocking of the cathode is the
factor that limits the cell’s service or capacity. Formation of sulfur as a discharge product
can also present a problem because of a possible reaction with lithium which may result in
a thermal runaway condition.

14.32 CHAPTER FOURTEEN
TABLE 14.11 Characteristics of Typical High-Capacity and Wafer-Type Cylindrical Bobbin-Type Li / SOCl
2
Batteries
AA
1
–
2
AA
2
–
3
AA C
D
1
–
6
D
Rated capacity at C /1000 rate, Ah 1.20 1.65 2.40 8.5 1.7 19.0
Dimensions (max)
Diameter, mm 14.5 14.5 14.5 26.2 32.9 32.9
Height, mm 25.2 33.5 50.5 50 10.0 61.5
Volume, cm
3
4.16 5.53 8.34 27.0 8.50 52.3
Weight, g 9.2 11.8 17.6 50.5 21.5 92.5
Maximum current for continuous
use, mA
50 75 100 230 230
Specific Energy Wh/ kg 456 490 475 590 275 720
Energy Density Wh/ L 1010 1045 1010 1100 700 1270
Source: Tadiran, Ltd.
The lithium anode is protected by reacting with the thionyl chloride electrolyte during
stand forming a protective LiCl film on the anode as soon as it contacts the electrolyte. This
passivating film, while contributing to the excellent shelf life of the cell, can cause a voltage
delay at the start of a discharge, particularly on low-temperature discharges after long stands
at elevated temperatures. The presence of trace qualities of moisture leads to the formation
of HCl which increases passivation, as does the presence of ppm levels of iron. Some prod-
ucts have special anode treatments or electrolyte additives to overcome or lower this voltage
delay.
The low freezing point of thionylchloride (below
⫺110⬚C) and its relatively high boiling
point (78.8⬚C) enable the cell to operate over a wide range of temperature. The electrical
conductivity of the electrolyte decreases only slightly with decreasing temperature. Some of
the components of the Li /SOCl
2
systems are toxic and flammable; thus exposure to open or
vented cells or cell components should be avoided.
14.6.2 Bobbin-Type Cylindrical Batteries
Li/ SOCl
2
bobbin batteries are manufactured in a cylindrical configuration, some in sizes
conforming to ANSI standards. These batteries are designed for low- to moderate-rate dis-
charge and are not typically subjected to continuous discharge at rates higher than the
C/ 100 rate. They have a high energy density. For example, the D-size cell delivers 19.0-Ah
at 3.5 V, compared with 15 Ah at 1.5 V for the conventional zinc-alkaline cells (see Tables
7.5 and 14.11).
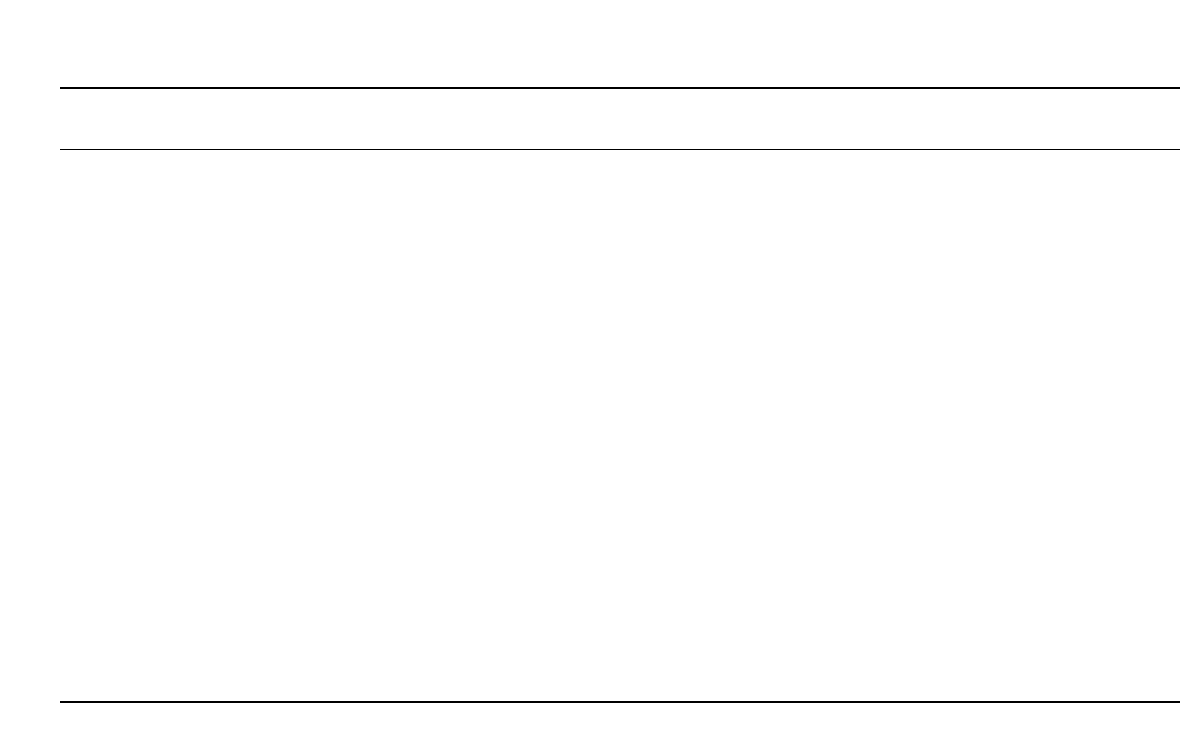
Construction. Figure 14.16 shows the constructional features of the cylindrical Li/SOCl
2
cell, which is built as a bobbin-type construction. The anode is made of lithium foil which
is swaged against the inner wall of a stainless or nickel-plated steel can; the separator is
made of nonwoven glass fibers. The cylindrical, highly porous cathode, which takes up most
of the cell volume, is made of Teflon-bonded acetylene black. The cathode also incorporates
a current collector which is a metal cylinder in the case of the larger cells and a pin in the
case of smaller cells which do not have an annular cavity.
LITHIUM BATTERIES 14.33
FIGURE 14.16 Cross section of bobbin-type Li / SOCl
2
battery.
(From Ref. 23.)
Performance. The open-circuit voltage of the Li /SOCl
2
cell is 3.65 V; typical operating
voltages range between 3.3 and 3.6 V with an end voltage of 3.0 V. Typical discharge curves
for the Li/SOCl
2
battery are shown in Fig. 14.17a for the D-size cell. The Li /SOCl
2
cell
discharges are characterized by a flat profile with good performance over a wide range of
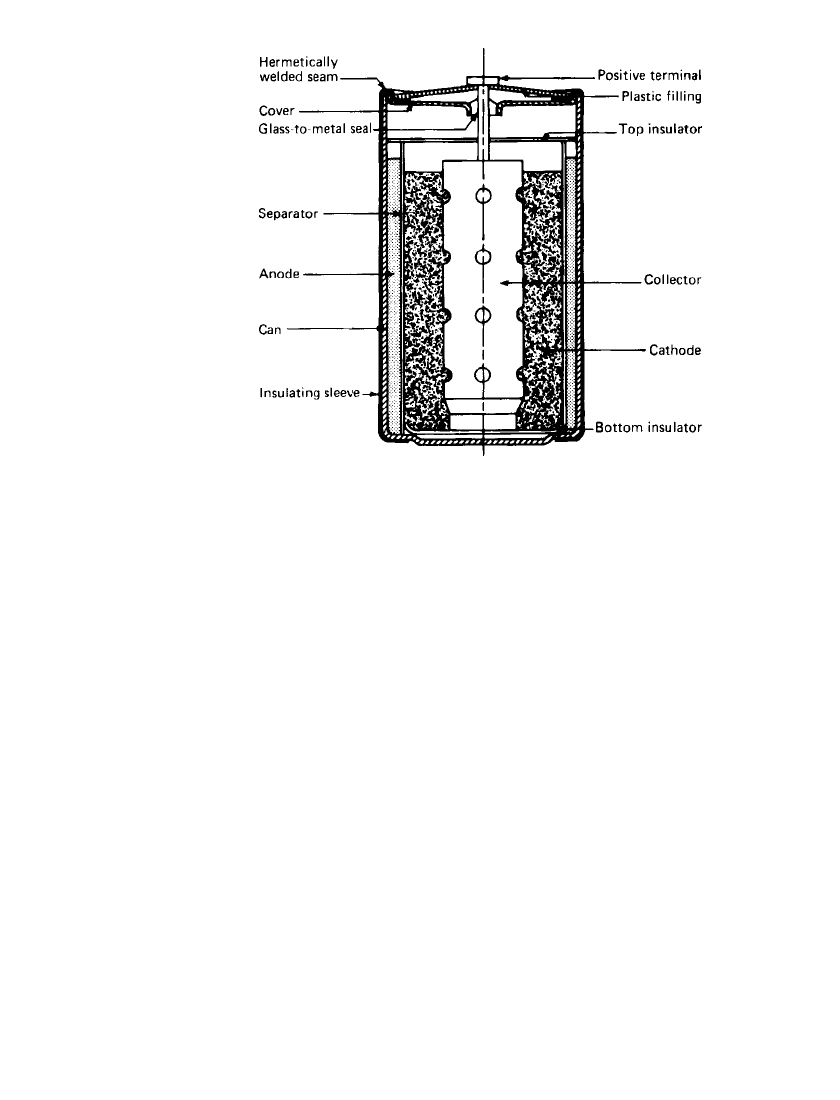
temperatures and low- to moderate-rate discharges. Figure 14.17b shows the operating volt-
age of the bobbin D-cell at various drain rates and temperatures. The relationship of capacity
with current is given in Fig. 14.18, showing the performance from
⫺30 to 72⬚C. The Li/
SOCl
2
cell is capable of performance at unusually high temperatures. At 145⬚C, (Fig. 14.19)
the cells deliver most of their capacity at high rates and up to 70% at low discharge rates
(20 days of discharge).
22
Li/ SOCl
2
cells are used to build battery packs that are employed
in oil exploration and most withstand temperatures to 150
⬚C as well as high levels of shock
and vibration.
Figure 14.20 shows the behavior of AA cells on continuous low-rate discharge at 25
⬚C.
The discharge curve is very flat at these low-current drains, but capacity loss below the 2.4
Ah rating occurs below the 1000-hour rate due to parasitic self-discharge.
The capacity or service life of the high-capacity bobbin-type Li/SOCl
2
cell, normalized
for a 1-kg and 1-L size cell, at various discharge temperatures and loads, is summarized in
Fig. 14.21.
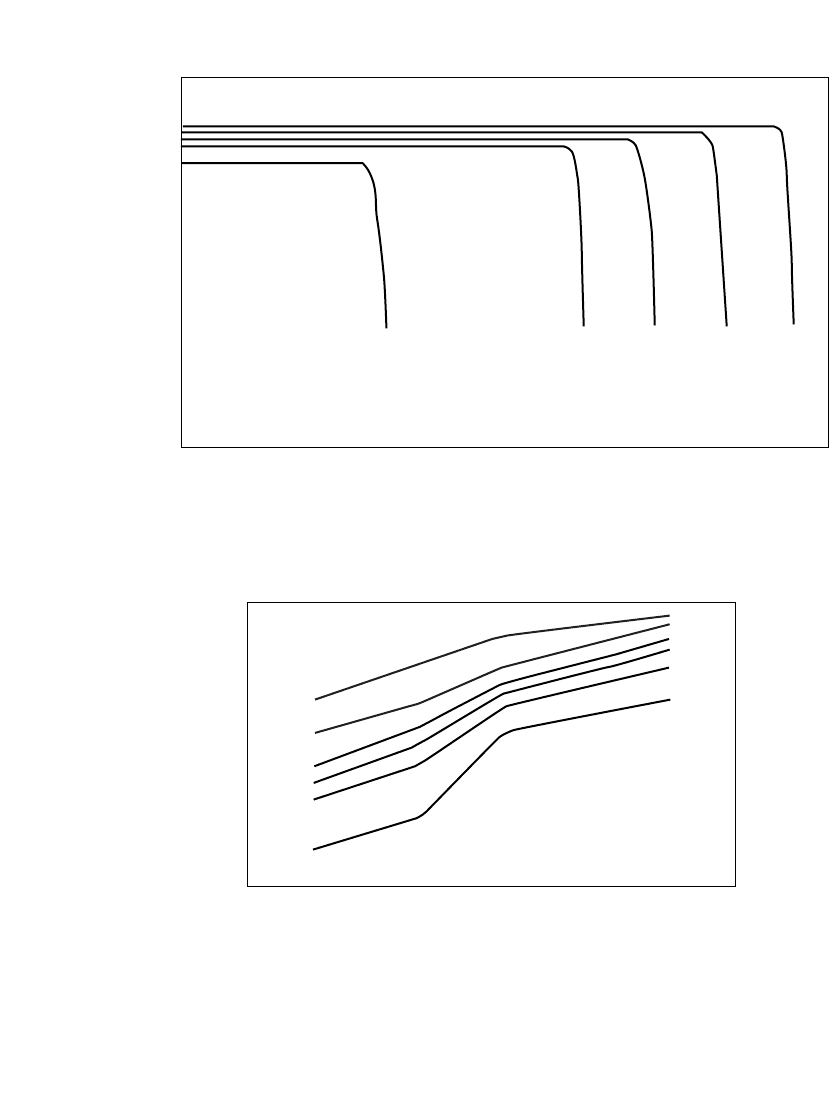
The long shelf life of the Li /SOCl
2
battery is due to the stability of the lithium anode in
contact with the electrolyte, as a result of a protective LiCl film that forms on the lithium
surface. The long shelf life can also be attributed to the stability of other cell components.
For example, the can and cover are cathodically protected by the lithium, and the carbon,
stainless-steel collector, and glass separator are all inert in the electrolyte. Figure 14.22 shows
the loss of capacity after 3 years at 20
⬚C, a loss of about 1 to 2% per year. Storage at 70⬚C
results in a capacity loss of about 5% per year. Cells should preferably be stored in an
upright position; storage on the side or upside down may result in higher capacity loss.
14.34 CHAPTER FOURTEEN
DISCHARGE CHARACTERISTICS @ + 25 C
O
Volts
56.2 Ω
60 mA
(10.0 Ah)
698 Ω
5 m A
(19.0 Ah)
2.0 KΩ
1.8 mA
(18.5 AH)
5.9 KΩ
0.6 mA
(18.5 Ah)
18.2 KΩ
200 µA
(16.6 Ah)
10 100 1000 10000 100000
Hours
4.0
3.5
3.0
2.5
2.0
1.5
1.0
(a)
VOLTAGE VS. TEMPERATURE
Temperature
Volts
-50 -30 -10 10 30 50 70 90
C
o
3.7
3.6
3.5
3.4
3.3
3.2
3.1
3.0
2.9
0.2mA
2mA
6mA
10mA
20mA
60mA
(b)
FIGURE 14.17 (a) Discharge characteristics of high-capacity Li / SOCl
2
cylindrical
D-size bobbin battery at ⫹25⬚C. (b) Operating (plateau) voltage of the same battery
as a function of temperature at various drain rates. (Ref. 23.)
