Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

13.16 CHAPTER THIRTEEN
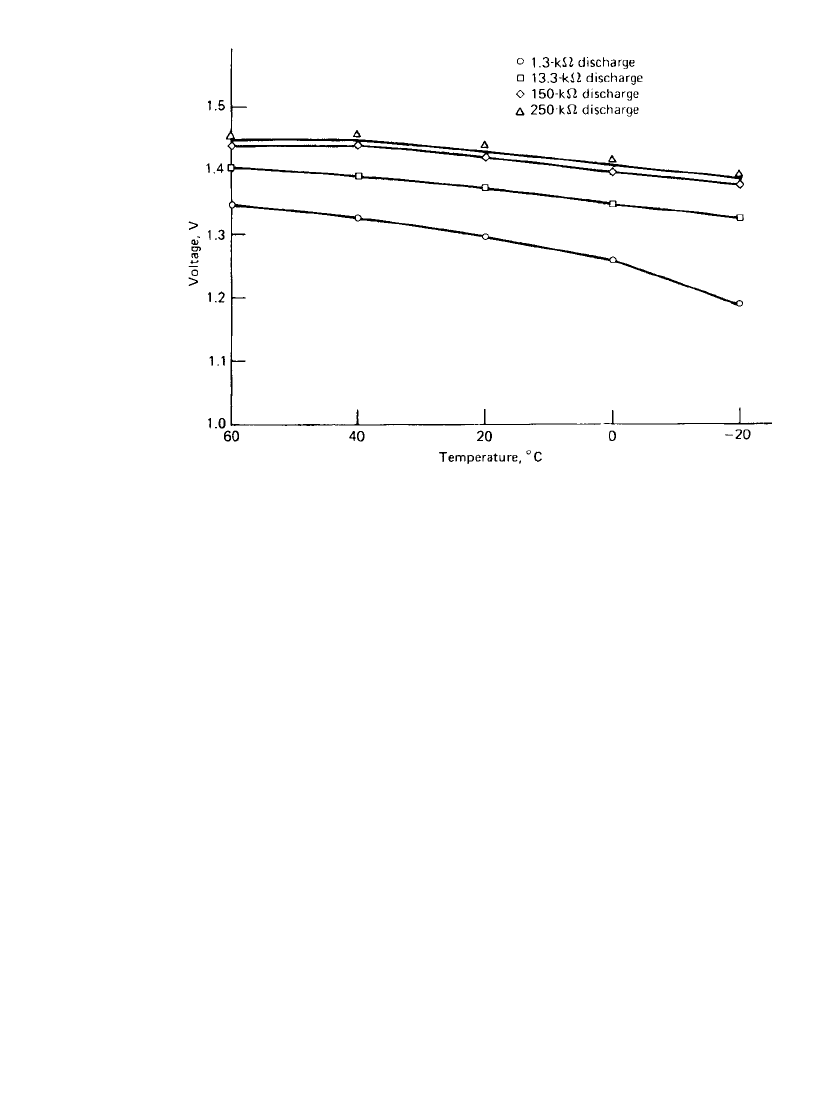
FIGURE 13.15 Effect of temperature on voltage—DA675 zinc / air battery. (Courtesy
of Duracell, Inc.)
13.4.9 Storage Life
Four principal mechanisms affect the capacity of zinc /air batteries during storage and op-
erating service. One mechanism, self-discharge of the zinc (corrosion), is an internal reaction;
the other three are caused by gas transfer. The gas transfer mechanisms are direct oxidation
of the zinc anode, carbonation of the electrolyte and electrolyte water gain or loss.
During storage the air access holes of a zinc/ air battery can be sealed to prevent gas
transfer decay. A typical material for sealing a battery is a polyester tape. Note that, unlike
conventional batteries, one of the zinc /air cell’s reactants, oxygen, is sealed outside the cell
during storage. This characteristic gives zinc /air batteries excellent shelf-life performance.
The primary mechanism affecting the shelf-life of a zinc /air battery is the self-discharge
reaction. Zinc is thermodynamically unstable in an alkaline solution (electrolyte) and reacts
to form zinc oxide (discharged zinc) and hydrogen gas. (An additional advantage of zinc/
air batteries is that hydrogen evolved during the reaction is vented through the sealing tape
to prevent the pressure buildup that can cause cell deformation in conventional batteries.)
This reaction is controlled by additives to the zinc. Results of shelf-life evaluation of DA675
zinc/ air batteries at room ambient conditions over a 5-year storage period are presented in
Table 13.4. Capacity retention over this period is 85% of initial capacity, yielding an average
capacity loss per year of less than 3%.
Elevated temperatures will increase the rate of the self-discharge reaction dramatically.
The capacity loss after 28 days of storage at 54
⬚C, averaged about 3%. Storage life at high
temperatures can be optimized through tradeoffs between other performance parameters and
choice of cell and battery design components.

ZINC / AIR BATTERIES—BUTTON CONFIGURATION 13.17
TABLE 13.4 Capacity Retention vs. Storage Time at 20⬚C*—Zinc /Air
Battery, 675-Size
Storage, years
Average
capacity,
mAh
% change from
initial capacity
Average % change
per annum
0
1.8
5.0
523
497
452
0
⫺5.0
⫺13.5
0
⫺2.8
⫺2.7
* 1.8-year data are actual test data: 5-year data are estimates based on actual
studies conducted on larger cell sizes.
13.4.10 Factors Affecting Service Life
The combination of the effects of self-discharge and gas transfer degradation determines the
service life performance of a zinc/air battery. For most applications water transfer is the
dominant factor. However, under some conditions electrolyte carbonation and direct oxida-
tion mechanisms can adversely affect performance.
Carbonation of Electrolyte. Carbon dioxide, which is present in the atmosphere at a con-
centration of approximately 0.04%, reacts with an alkaline solution (electrolyte) to form an
alkali metal carbonate and bicarbonate. Zinc/air batteries can be satisfactorily discharged
using a carbonated electrolyte, but there are two disadvantages of extreme carbonation: (1)
vapor pressure of the electrolyte is increased, aggravating water vapor loss in low-humidity
conditions, and (2) crystals of carbonate formed in the cathode structure may impede air
access, eventually causing cathode damage with subsequent deterioration of cathode per-
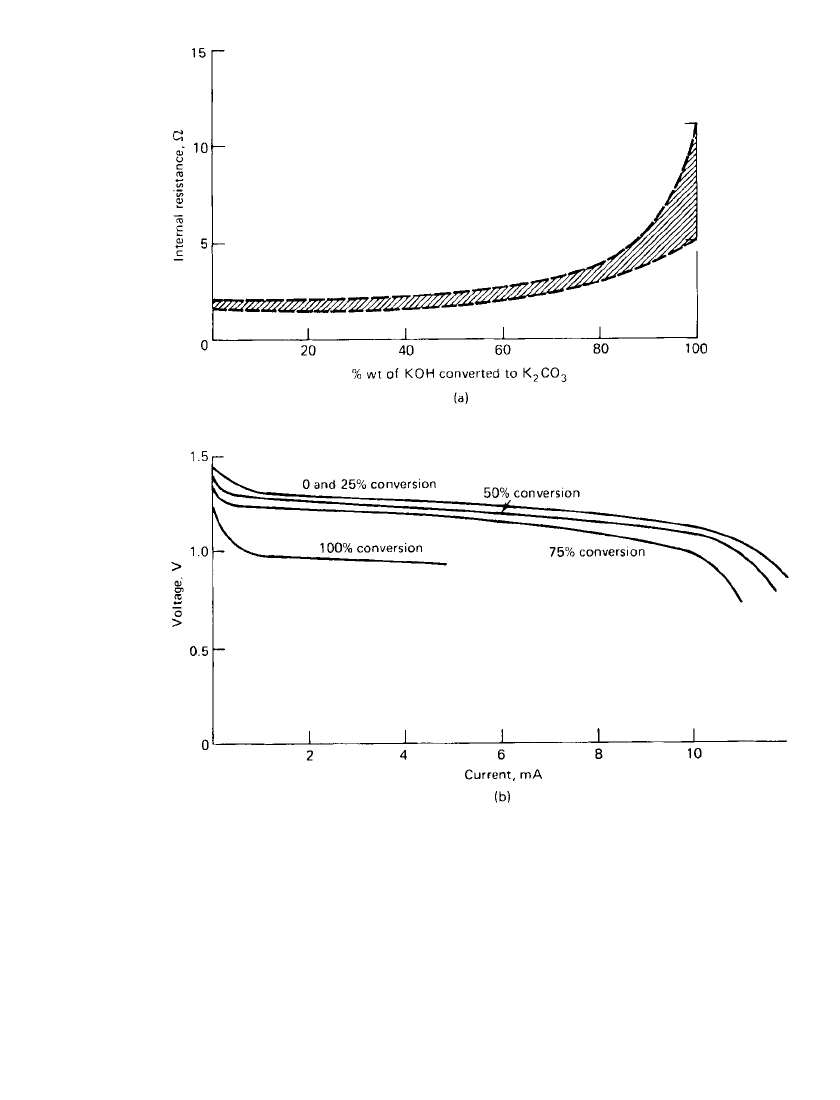
formance. As indicated in Fig. 13.16 carbonation must be extreme to be detrimental to cell
performance in most applications.
16
Direct Oxidation. The zinc anode of a zinc /air battery can be oxidized directly by oxygen
which enters the cell and dissolves in and diffuses through the electrolyte. Measurements of
the effect of direct oxidation on cell capacity have been predicted experimentally,
17
using
advanced microcalorimetry techniques to predict direct oxidation effects on DA675 type cells
(cells open, not sealed). This research indicates a capacity loss of less than 1.5% of rated
capacity per year at 25
⬚C and less than 5% per year at 37.5⬚C.
The combined effect of oxidation and self-discharge on fresh batteries, open to the am-
bient, yields a predicted capacity loss of less than 4% per year. However, the typical useful
service life for a zinc/air battery as presented in Table 13.2a is about 3 months.
Effect of Water Vapor Transfer on Service Life. The decay mechanism determining the
useful service life is water vapor transfer. It occurs when a partial pressure difference exists
between the vapor pressure of the electrolyte and the surrounding environment. As indicated
previously, a cell with a typical electrolyte, consisting of 30% concentration of potassium
hydroxide, will lose water when the humidity at room temperature is below 60% and will
gain water at humidities above 60%. Excessive water loss increases the concentration of the
electrolyte and can eventually cause the cell to fail because of inadequate electrolyte to
13.18 CHAPTER THIRTEEN
FIGURE 13.16 Effect of electrolyte carbonation in 675-size zinc/ air cell (KOH electrolyte).
(a) On internal impedance at 20⬚C (4000 Hz). (b) On voltage-current profile at 20⬚C. (From
Ref. 8; courtesy of Duracell, Inc.)
maintain the discharge reaction. Excessive water gain dilutes the electrolyte, which also
reduces conductivity. Furthermore, the catalyst layer of the air cathode will flood under
sustained water gain conditions, reducing electrochemical activity and eventually causing
cell failure.
The design of a zinc/air battery can be optimized to compensate for water transfer for a
specific set of operating conditions. Tradeoffs can be made between the volume and com-
position of the electrolyte, the amount of zinc, and the degree of gas diffusion regulation to
maximize service life. For most applications and cell types, diffusion control is the most
important variable.
ZINC / AIR BATTERIES—BUTTON CONFIGURATION 13.19
The balance between performance and service life as related to water transfer rates is
addressed in Table 13.5. This table compares the weight loss and impedance changes of four
commercially available 312-size zinc/air batteries, typically used in hearing aids. The bat-
teries were exposed to 14 and 28 days of ambient air storage after which time their water
weight loss and 1-kHz AC impedance were measured. The variation in this table is primarily
related to battery design. Battery A, for example, was designed with a high air diffusion
cathode for better power capability. However, the low barrier to gas diffusion allows for
more rapid water loss from the cell. Subsequently the A battery is not designed for a low-
rate discharge since it will reach end of life earlier due to increased water loss and internal
impedance. On the other hand, battery D is designed for lower gas diffusion rates, which
allows for more of a medium power capability, but will deliver longer service under low-
rate drain conditions as a result of its lower rate of water loss. Batteries B and C in Table
13.5 lie in the midrange of A and D, achieving neither the highest power capability nor the
longest operating life. The specific performance characteristics for the 312-size zinc /air bat-
teries are useful in matching power capabilities to the amplification requirements in common
hearing aids to achieve the best performance level.
TABLE 13.5 Water Weight and Battery Impedance
Changes for Four Commercialy Available 312-Size Zinc /
Air Button Batteries Exposed to Ambient Conditions for
14–28 Days
Cumulative water weight loss, mg
ABCD
Storage time*
14 days
28 days
9.1
12.2
5.9
7.5
7.3
9.3
0.3
1.0
Impedance,
⍀†
Initial
Final (28 days)
2.3
70.8
4.7
18.0
3.8
15.1
4.3
8.8
Relative change
⫻30 ⫻4 ⫻4 ⫻2
* Relative Humidity during storage—55 Ⳳ 5%.
† AC impedance measured at 1000 Hz and 10 mA.
The effect of diffusion control on water vapor transfer rates for three zinc/air battery
sizes is presented in Table 13.6. Actual instantaneous water transfer rates at a given envi-
ronment will vary throughout the life of the battery, depending on the length of exposure.
As a battery gains or loses water, transfer rates will decrease because the electrolyte equi-
librium relative humidity moves toward the ambient relative humidity. The figures presented
in Table 13.6 represent average rates computed over the indicated exposure period. Actual
average rates over the life of the battery would be lower.
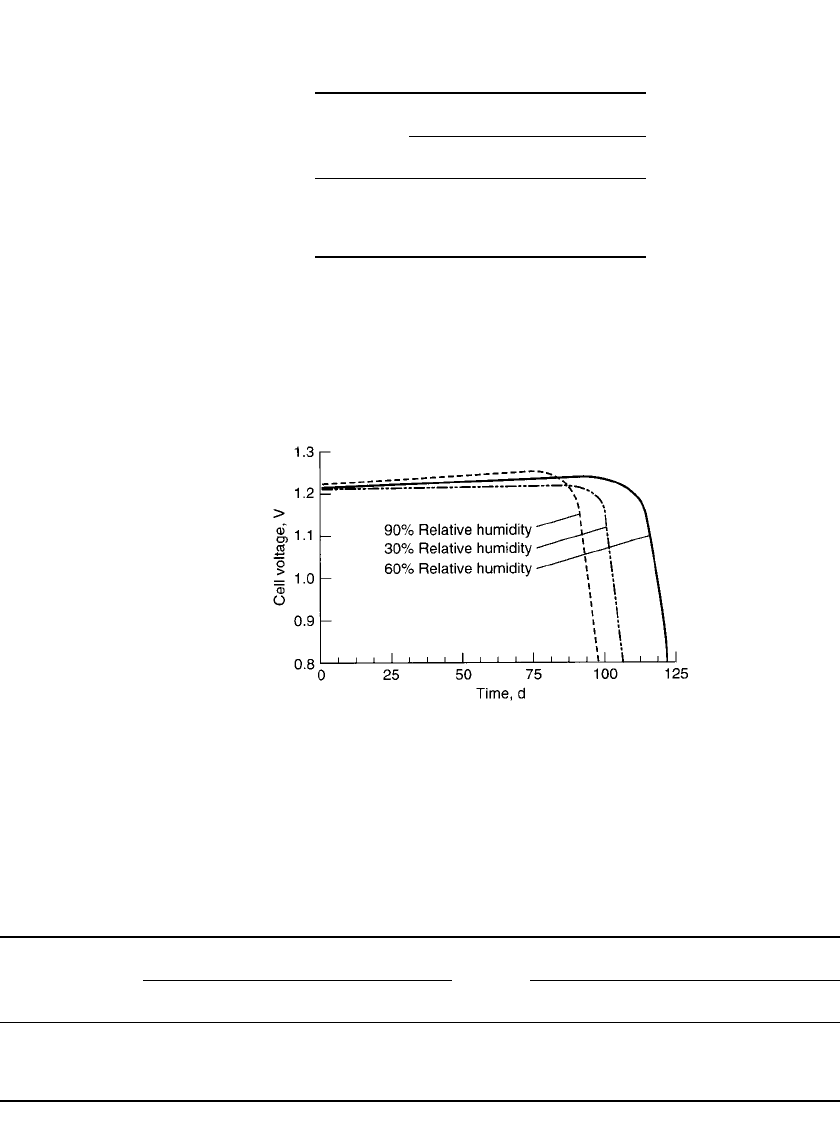
The effect of continued moisture loss or gain from the electrolyte is illustrated in Fig.
13.17. This figure shows an intermittent discharge of a zinc/ air battery at 30, 60, and 90%
relative humidity. The least amount of moisture transfer occurs at the 60% relative humidity
condition and the battery delivers the maximum service.
Table 13.7 lists data for zinc /air 675-size battery tested after 14 and 28 days of storage,
unsealed and exposed to various relative humidity conditions. The effects of electrolyte loss
at each of these conditions is presented. The batteries had not been discharged prior to the
test. It is evident that high relative humidity is more detrimental to performance retention,
leading to battery capacity degradation.
13.20 CHAPTER THIRTEEN
TABLE 13.6 Relative Degree of
Environmental Tolerance
Relative
humidity
Ⳳ5%, %
Zinc/ air battery type*
230-size 312-size 675-size
20
60
90
NA
⫺0.047
NA
⫺0.057
⫺0.036
NA
⫺0.084
⫺0.027
⫹0.018
* Storage periods 230-Size—21 days; 312-size—28
days; 675-size—30 days.
FIGURE 13.17 Zinc / air battery discharged at 21⬚C at various
levels of relative humidity. (Courtesy of Duracell, Inc.)
TABLE 13.7 Weight and Capacity Changes as a Function of Storage at Normal and Extreme Relative Humidity
Conditions—675-size Zinc/ Air Battery
Relative
humidity,
%
Weight loss or gain, mg
0 days 10 days 30 days
Delivered capacity, mAh
0 days 10 days 30 days
20
60
90
0
0
0
⫺152
0.99
3.28
⫺2.51
1.56
3.54
500
500
500
465
490
176
283
410
30

ZINC / AIR BATTERIES—BUTTON CONFIGURATION 13.21
The conditions of use of a battery also affect its performance. Batteries enclosed in a
sealed battery compartment (with allowance for air access) or other enclosure undergo less
performance loss due to water vapor transmission than batteries exposed in the open air.
Batteries exposed to daily indoor and outdoor relative humidity conditions will undergo water
vapor transmission cycling which tends to average out the effects of both environments.
Indoor relative humidities tend to be closer to the conditions for recommended use (40 to
60% RH).
Note that the larger batteries such as the 675-size have greater tolerance to the detrimental
effects of water gain or loss. This is typical in a comparison of batteries of different sizes.
Larger batteries have more electrolyte and also a greater anode-free volume, making them
more tolerant to water loss and gain conditions, respectively.
The operational life of zinc /air battery is most dependent on the control of gas transmis-
sion into and out of the cell. It is evident that water vapor transmission is the key factor in
extending the service life for these batteries. A key area for research and development relating
to zinc /air batteries is focused on membrane technology. A selectively permeable gas dif-
fusion membrane, which allows air diffusion into the cell but excludes or greatly reduces
water vapor transmission, will greatly broaden the range of application for zinc/ air batteries.
Papers and patent disclosures over the last few years have discussed a series of new materials
under study.
18–20
REFERENCES
1. G. W. Elmore and H. A. Tanner, U.S. Patent 3,419,900.
2. A. M. Moos, U.S. Patent 3,267,909.
3. R. G. Biddick, U.S. Patent 4,129,633.
4. E. Yeager, ‘‘Electrochemical Catalysis for Oxygen Electrodes,’’ Rep. LBL-25817, Lawrence Berkeley
Lab., Calif. 1988.
5. C. Warde and A. D. Glasser, U.S. Patent 3935027.
6. B. Szczesniak, et al., Abstract Number 280, Joint International Meeting of ECS and ISE, Paris, 1997.
7. M. Ohashi, H. Watabe, and H. Ogata, Japanese Patent Kokai 9-289045.
8. T. Saeki, T. Watabe, and S. Kobayashi, Japanese Patent Kokai 8-338836 and 8-315870.
9. K. Yoshida and M. Watabe, U.S. Patent 4,380,567.
10. R. Dopp, J. Ottman and J. Passanti, U.S. Patent 5,650,246.
11. A. Borbely and J. Molla, U.S. Patent 4,894,296.
12. A. Ohta, Y. Morita, et al., ‘‘Manganese Oxide as a Catalyst for Zinc-Air Cells,’’ Proc. Battery
Material Symp., 1985.
13. J. Passanti and R. Dopp, U.S. Patent 5,308,711.
14. J. Ottman, B. Dopp and J. Burns, U.S. Patent 5,567,538.
15. H. Konishi and T. Yokoyama, ‘‘Air Cells for Pagers,’’ National Tech. Rep., vol. 37, no. 1, JEC Press,
Cleveland, Ohio, 1991.
16. J. W. Cretzmeyer, H. R. Espig, and J. C. Hall, ‘‘Commercial Zinc-Air Batteries,’’ Power Sources,
Vol. 6, Oriel Press, Newcastle-upon-Tyne, U.K., 1977.
17. S. Bender, J. W. Cretzmeyer, and J. C. Hall, ‘‘Long Life Zinc-Air Cells as a Power Source for
Consumer Electronics, in Progress in Batteries and Solar Cells, vol. 2, JEC Press, Cleveland, Ohio,
1979.
18. T. Takamura, Y. Sato, M. Susuki, T. Nakamura, and K. Sasaki, ‘‘High Performance Zn-Air Cell
Using Gas-Selective Membranes,’’ Proc. Int. Power Sources Symp., Brighton, U.K., The Paul Press,
London, 1985.
19. M. Yoshino, S. Noya, and M. Yanagihara, Japanese patednt Kokai 2-216755 and 2-216756.
20. N. Yoshino, S. Noya, A. Hanabusa, and N. Yangihara, Japanese Patent Kokai 3-108255, 3-108256,
and 3-108257.

14.1
CHAPTER 14
LITHIUM BATTERIES
David Linden and Thomas B. Reddy
14.1 GENERAL CHARACTERISTICS
Lithium metal is attractive as a battery anode material because of its light weight, high
voltage, high electrochemical equivalence, and good conductivity. Because of these outstand-
ing features, the use of lithium has dominated the development of high-performance primary
and secondary batteries during the last two decades. (See Chap. 34 and 35 covering lithium
secondary batteries.)
1
Serious development of high-energy-density battery systems was started in the 1960s and
concentrated on nonaqueous primary batteries using lithium as the anode. The lithium bat-
teries were first used in the early 1970s in selected military applications, but their use was
limited as suitable cell structures, formulations, and safety considerations had to be resolved.
Lithium primary cells and batteries have since been designed, using a number of different
chemistries, in a variety of sizes and configurations. Sizes range from less than 5 mAh to
10,000 Ah; configurations range from small coin and cylindrical cells for memory backup
and portable applications to large prismatic cells for standby power in missile silos.
Lithium primary batteries, with their outstanding performance and characteristics, are
being used in increasing quantities in a variety of applications, including cameras, memory
backup circuits, security devices, calculators, watches, etc. Nevertheless, lithium primary
batteries have not attained a major share of the market as was anticipated, because of their
high initial cost, concerns with safety, the advances made with competitive systems and the
cost-effectiveness of the alkaline/manganese battery. World-wide sales of lithium primary
batteries for 1999 have been estimated at $1.1 billion.
2
14.1.1 Advantages of Lithium Cells
Primary cells using lithium anodes have many advantages over conventional batteries. The
advantageous features include the following:
1. High voltage: Lithium batteries have voltages up to about 4 V, depending on the cathode
material, compared with 1.5 V for most other primary battery systems. The higher voltage
reduces the number of cells in a battery pack by a factor of at least 2.
2. High specific energy and energy density: The energy output of a lithium battery (over
200 Wh /kg and 400 Wh /L) is 2 to 4 or more times better than that of conventional zinc
anode batteries.

14.2 CHAPTER FOURTEEN
3. Operation over a wide temperature range: Many of the lithium batteries will perform
over a temperature range from about 70 to
⫺40⬚C, with some capable of performance to
150
⬚C or as slow as ⫺80⬚C.
4. Good power density: Some of the lithium batteries are designed with the capability to
deliver their energy at high current and power levels.
5. Flat discharge characteristics: A flat discharge curve (constant voltage and resistance
through most of the discharge) is typical for many lithium batteries.
6. Superior shelf life: Lithium batteries can be stored for long periods, even at elevated
temperatures. Storage of up to 10 years at room temperature has been achieved and
storage of 1 year at 70
⬚C has also been demonstrated. Shelf lives over 20 years have been
projected from reliability studies.
The performance advantages of several types of lithium batteries compared with conven-
tional primary and secondary batteries, are shown in Secs. 6.4 and 7.3. The advantage of
the lithium cell is shown graphically in Figs. 7.2 to 7.9, which compare the performance of
the various primary cells. Only the zinc/air, zinc/mercuric oxide, and zinc/silver oxide cells,
which are noted for their high energy density, approach the capability of the lithium systems
at 20
⬚C. The zinc /air cell, however, is very sensitive to atmospheric conditions; the others
do not compare as favorably on a specific energy basis nor at lower temperatures.
14.1.2 Classification of Lithium Primary Cells
Lithium batteries use nonaqueous solvents for the electrolyte because of the reactivity of
lithium in aqueous solutions. Organic solvents such as acetonitrile, propylene carbonate, and
dimethoxyethane and inorganic solvents such as thionyl chloride are typically employed. A
compatible solute is added to provide the necessary electrolyte conductivity. (Solid-state and
molten-salt electrolytes are also used in some other primary and reserve lithium cells; see
Chaps. 15, 20, and 21.) Many different materials were considered for the active cathode
material; sulfur dioxide, manganese dioxide, iron disulfide, and carbon monofluoride are now
in common use. The term ‘‘lithium battery,’’ therefore, applies to many different types of
chemistries, each using lithium as the anode but differing in cathode material, electrolyte,
and chemistry as well as in design and other physical and mechanical features.
Lithium primary batteries can be classified into several categories, based on the type of
electrolyte (or solvent) and cathode material used. These classifications, examples of mate-
rials that were considered or used, and the major characteristics of each are listed in Table
14.1.
Soluble-Cathode Cells. These use liquid or gaseous cathode materials, such as sulfur di-
oxide (SO
2
) or thionyl chloride (SOCl
2
), that dissolve in the electrolyte or are the electrolyte
solvent. Their operation depends on the formation of a passive layer on the lithium anode
resulting from a reaction between the lithium and the cathode material. This prevents further
chemical reaction (self-discharge) between anode and cathode or reduces it to a very low
rate. These cells are manufactured in many different configurations and designs (such as
high and low rate) and with a very wide range of capacities. They are generally fabricated
in cylindrical configuration in the smaller sizes, up to about 35 Ah, using a bobbin construc-
tion for the low-rate cells and a spirally wound (jelly-roll) structure for the high-rate designs.
Prismatic containers, having flat parallel plates, are generally used for the larger cells up to
10,000 Ah in size. Flat or ‘‘pancake-shaped’’ configurations have also been designed. These
soluble cathode lithium cells are used for low to high discharge rates. The high-rate designs,
using large electrode surface areas, are noted for their high power density and are capable
of delivering the highest current densities of any active primary cell.

14.3
TABLE 14.1 Classification of Lithium Primary Batteries*
Cell
classification
Typical
electrolyte
Power
capability Size, Ah
Operating
range,
⬚C
Shelf
life,
years
Typical
cathodes
Nominal
cell
voltage,
V
Key
characteristics
Soluble cathode
(liquid or gas)
Organic or
inorganic
(w/ solute)
Moderate to
high power,
W
0.5 to 10,000
⫺80 to 70 5–20 SO
2
SOCl
2
SO
2
Cl
2
3.0
3.6
3.9
High energy
output, high power
output, low-
temperature
operation, long
shelf life
Solid cathode Organic
(w/ solute)
Low to
moderate
power, mW-W
0.03 to 33
⫺40 to 50 5–8 V
2
O
5
AgV
2
O
5.5
Ag
2
CrO
4
MnO
2
Cu
4
O(PO
4
)
2
(CF)
n
CuS
FeS
2
FeS
CuO
3.3
3.2
3.1
3.0
3.0
2.6
1.7
1.5
1.5
1.5
High energy output
for moderate
power
requirements,
nonpressurized
cells
Solid
electrolyte (see
Chap. 15)
Solid state Very low
power,
W
0.003 to 0.5 0 to 100 10–25 PbI
2
/PbS/Pb
I
2
(P2VP)
1.9
2.8
Excellent shelf life,
solid state—no
leakage, long-term
microampere
discharge
*For reserve batteries; see Chaps. 20 and 21.
14.4 CHAPTER FOURTEEN
Solid-Cathode Cells. The second type of lithium anode primary cells uses solid rather than
soluble gaseous or liquid materials for the cathode. With these solid cathode materials, the
cells have the advantage of not being pressurized or necessarily requiring a hermetic-type
seal, but they do not have the high-rate capability of the soluble-cathode systems. They are
designed, generally, for low- to medium-rate applications such as memory backup, security
devices, portable electronic equipment, photographic equipment, watches and calculators,
and small lights. Button, flat, and cylindrical-shaped cells are available in low-rate and the
moderate-rate jelly-roll configurations. A number of different solid cathodes are being used
in lithium primary cells, as listed in Table 14.1. The discharge of the solid-cathode cells is
not as flat as that of the soluble-cathode cells, but at the lower discharge rates and ambient
temperature their capacity (energy density) may be higher than that of the lithium/ sulfur
dioxide cell.
Solid-Electrolyte Cells. These cells are noted for their extremely long storage life, in excess
of 20 years, but are capable of only low-rate discharge in the microampere range. They are
used in applications such as memory backup, cardiac pacemakers, and similar equipment
where current requirements are low but long life is critical (see Chap. 15).
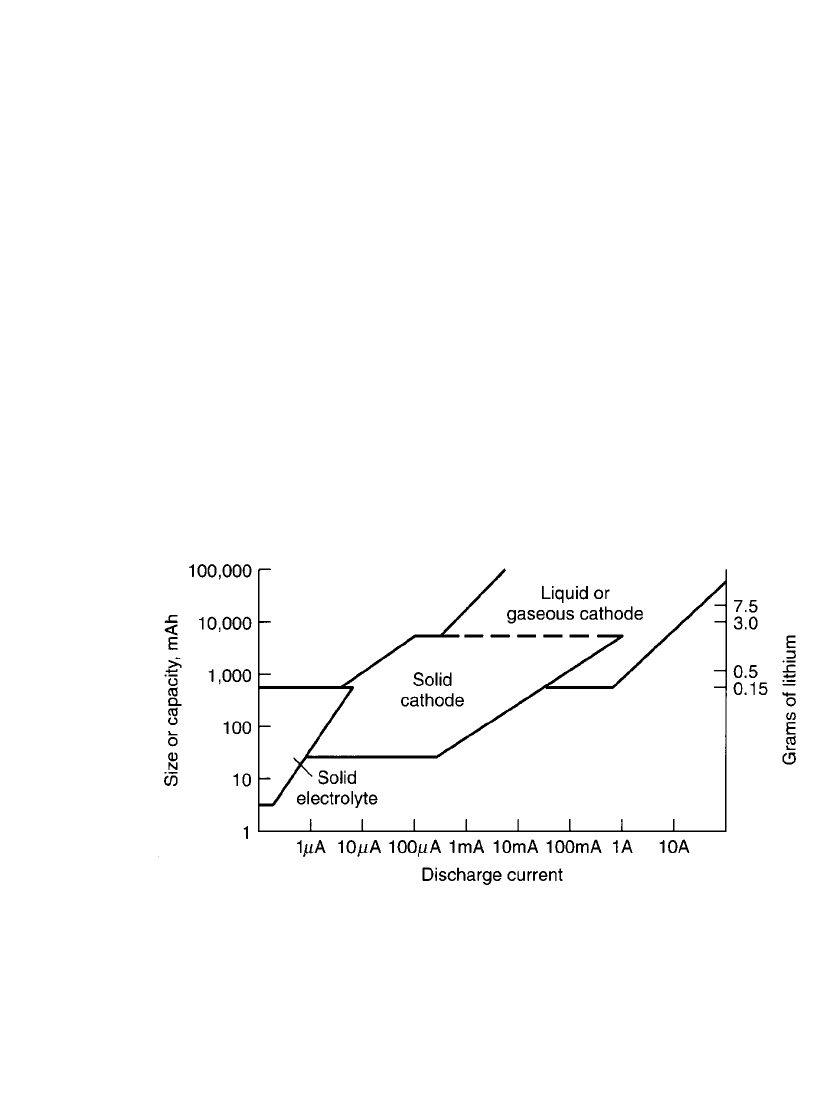
In Fig. 14.1 the size or capacity of these three types of lithium cells (up to the 30-Ah
size) is plotted against the current levels at which they are typically discharged. The ap-
proximate weight of lithium in each of these cells is also shown.
FIGURE 14.1 Classification of lithium primary cell types.
