Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


SILVER OXIDE BATTERIES 12.3
The oxidation of zinc in the anode is a complicated phenomenon. It is generally accepted
that the overall negative reactions are
3,4
⫺
0
Zn ⫹ 2OH → Zn(OH) ⫹ 2eE⫽⫹1.249 V
2
⫺
2
⫺
0
Zn ⫹ 4OH → ZnO ⫹ 2H O ⫹ 2eE⫽⫹1.215 V
22
Electrolyte gelling agents such as polyacrylic acid, potassium or sodium polyacrylate, sodium
carboxymethyl cellulose or various gums are generally blended into the zinc powder to
improve electrolyte accessibility during discharge.
12.2.2 Silver Oxide Cathode
Silver oxide can be prepared in three oxidation states:
2
monovalent (Ag
2
O), divalent (AgO),
and trivalent (Ag
2
O
3
). The trivalent silver oxide is very unstable and is not used for batteries.
The divalent form had been used in button cells, generally mixed with other metal oxides.
The monovalent silver oxide is the most stable and is the one now used for commercial
primary batteries.
The monovalent silver oxide is a very poor conductor of electricity. Without any additives,
a monovalent silver oxide cathode would exhibit a very high cell impedance and an unac-
ceptably low closed-circuit voltage (CCV). To improve the initial CCV, the monovalent silver
oxide is generally blended with 1 to 5% powdered graphite. However, as the cathode con-
tinues to discharge, the silver metal produced by the reaction helps to keep the internal cell
resistance low and the CCV high:
⫺
0
Ag O ⫹ HO⫹ 2e → 2Ag ⫹ 2OH E ⫽⫹0.342 V
22
The theoretical capacity of the monovalent silver oxide is 231 mAh /g by weight or 1640
Ah/ L by volume. The addition of graphite reduces the cathode capacity due to lower packing
density and lower silver oxide content.
Compared to the other silver oxides, the monovalent silver oxide is stable to decompo-
sition in alkaline solutions. Some decomposition to silver metal may occur due to the im-
purities brought into the cathode material by the graphite. The decomposition rate is depen-
dent upon the source of the graphite, the amount of graphite blended into the cathode, and
the cell storage temperature. Greater graphite impurities and higher cell storage temperatures
result in greater silver oxide decomposition rates.
5
To reduce the amount of silver in the cell or to alter the appearance of the discharge
curve, other cathode active additives may be blended into the monovalent silver oxide mix.
One common additive is manganese dioxide (MnO
2
). With increasing amounts of MnO
2
added to the cathode, the voltage curve can be altered from being constant throughout the
discharge to where the voltage will gradually decrease as the cathode nears depletion. This
gradual drop in voltage can serve as an indicator of silver oxide depletion as the cell is
nearing its end of life.
Another additive that can serve a dual function is silver nickel oxide (AgNiO
2
). Silver
nickel oxide is produced by the reaction of nickel oxyhydroxide (NiOOH) with monovalent
silver oxide in hot aqueous alkaline solution:
6,7
Ag O ⫹ 2NiOOH → 2AgNiO ⫹ HO
222
The dual feature of silver nickel oxide is that it is electrically conductive, like graphite,
as well as cathode active, like MnO
2
. Silver nickel oxide has a coulometric capacity (263
mAh/ g), higher than Ag
2
O, and will discharge against zinc at 1.5 V (Figs. 12.1 and 12.2).
Silver nickel oxide can replace both the graphite and part of the monovalent silver oxide,
reducing the cost of the cell.
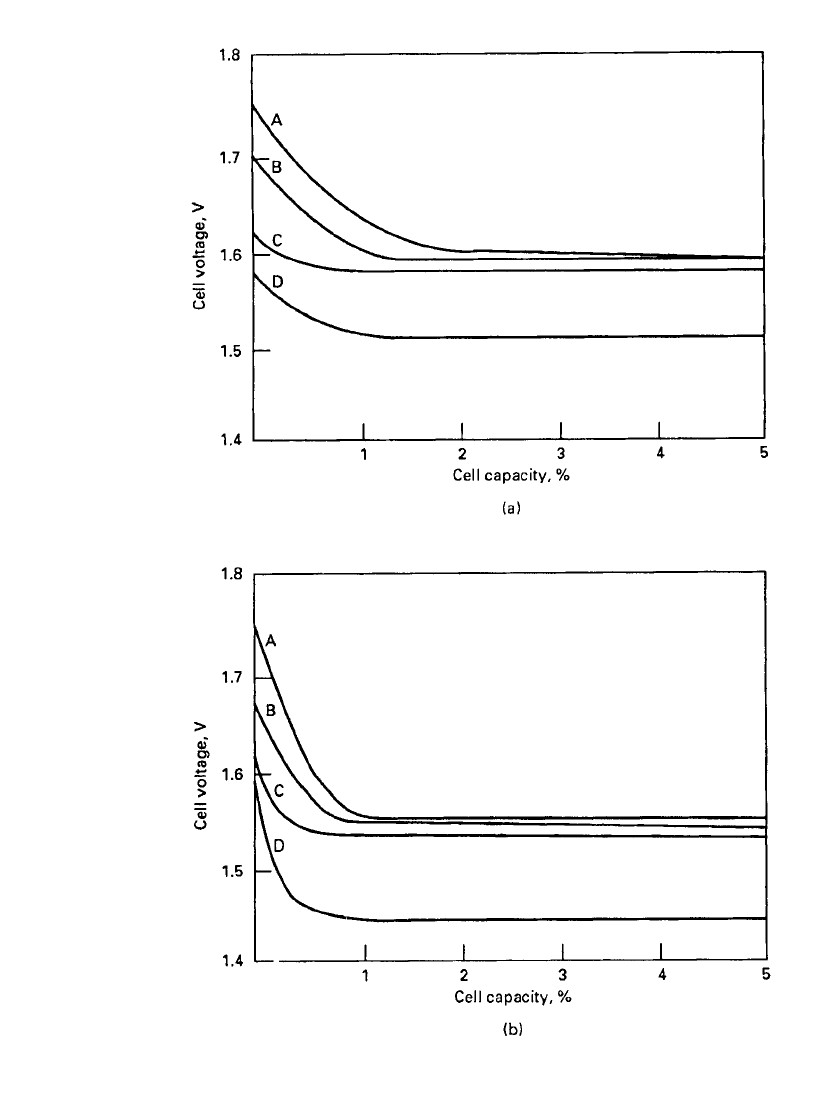
12.4 CHAPTER TWELVE
FIGURE 12.1 Closed-circuit voltage of various zinc / silver oxide chemistries, 7.8 ⫻
3.6 mm button type battery. A: Zn-‘‘double-treatment’’ AgO; B: Zn-Ag
2
O; C: Zn-AgO /
silver plumbate; D: Zn-AgNiO
2
.(a) Discharge on 150 ⍀,21⬚C. (b) Discharge on 15 k⍀,
21⬚C.
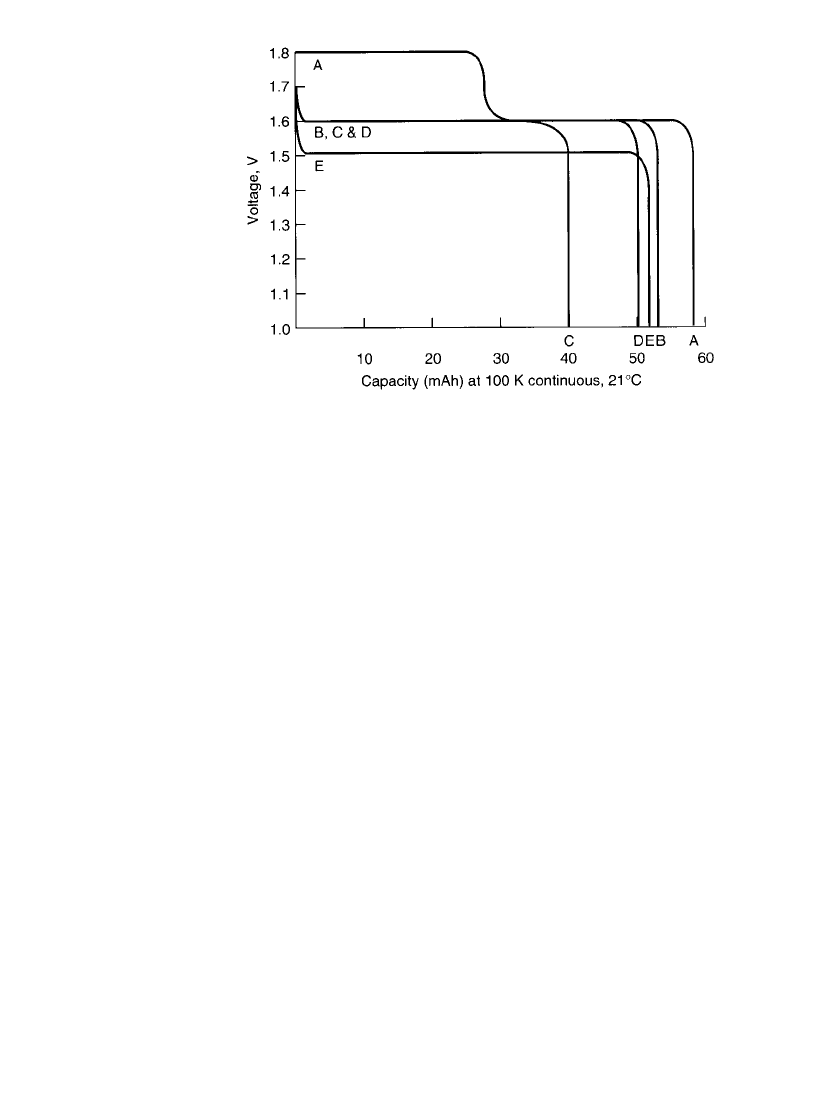
SILVER OXIDE BATTERIES 12.5
FIGURE 12.2 Comparison of performance of zinc / silver oxide button
batteries (various silver oxide cathodes). Size—7.8 ⫻ 3.6 mm; A—Zn /
AgO; B—double-treatment approach; C—Zn /Ag
2
O; D—Zn / plumbate;
E—Zn / AgNiO
2
.
Although the divalent silver oxide has a higher theoretical capacity (432 mAh/g by weight
or 3200 Ah /L by volume) than the monovalent silver oxide, the use of the divalent form in
button batteries has been limited and no longer marketed commercially.
2,8
This is due pri-
marily to its instability in alkaline solutions and the fact that it exhibits a two-step discharge
(Fig. 12.2).
Divalent silver oxide is unstable in alkaline solutions, decomposing to monovalent silver
oxide and oxygen gas:
4AgO
→ 2Ag O ⫹ O
22
This instability can be improved by the addition of lead or cadmium compounds
9–12
or by
the addition of gold to the divalent silver oxide.
13
The zinc /divalent silver oxide battery exhibits a two-step discharge curve. The first occurs
at 1.8 V, corresponding to the reduction of AgO to Ag
2
O:
⫺
0
2AgO ⫹ HO⫹ 2e → Ag O ⫹ 2OH E ⫽⫹0.607 V
22
As the discharge continues, the voltage drops to 1.6 V, corresponding to the reduction of
Ag
2
O to Ag:
⫺
0
Ag O ⫹ HO⫹ 2e → 2Ag ⫹ 2OH E ⫽⫹0.342 V
22
The overall electrochemical reaction of the Zn/AgO cell is
Zn
⫹ AgO → Ag ⫹ ZnO
This two-step discharge is not desirable for many electronic applications where tight voltage
regulation is required.
The elimination of the two-step discharge has been resolved by several methods.
11,14–16
One previously used commercial approach, shown schematically in Fig. 12.3, was to treat a
compressed pellet of AgO with a mild reducing agent such as methanol. The treatment forms
a thin outer layer of Ag
2
O around a core of AgO. The treated pellet is consolidated into a
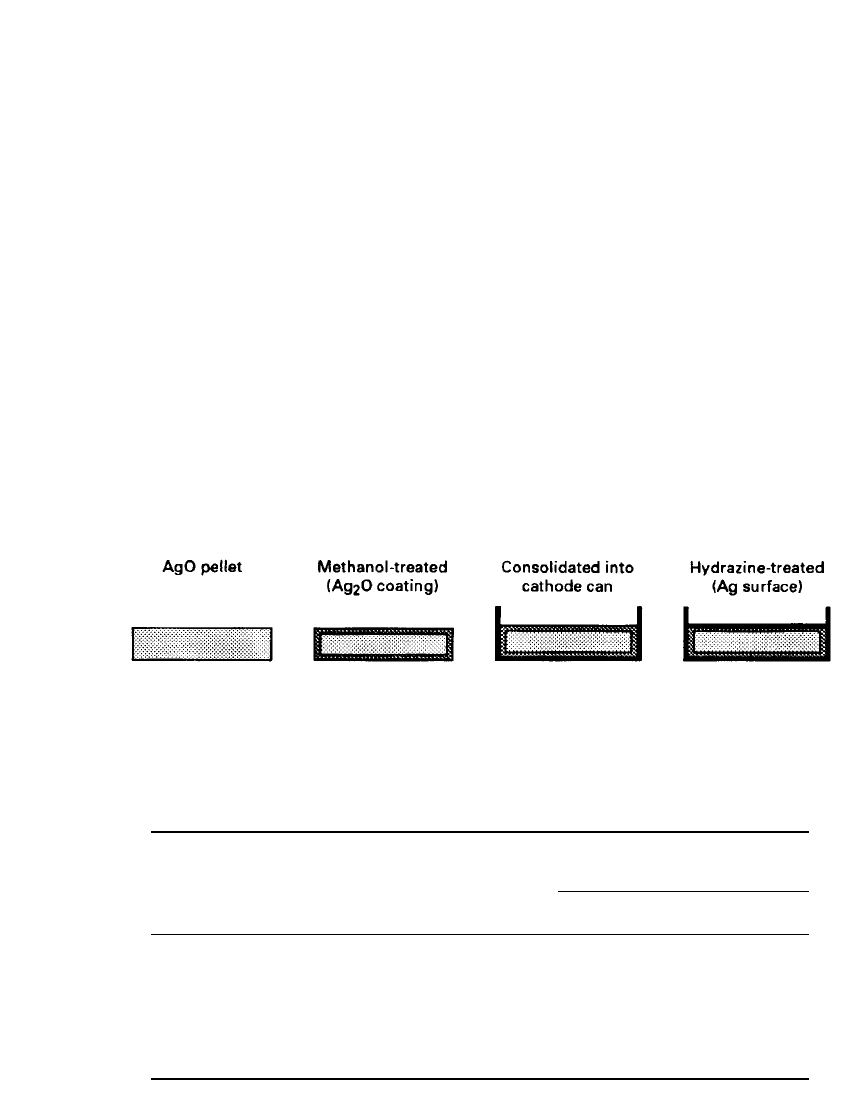
12.6 CHAPTER TWELVE
can and then reacted with a stronger reducing agent such as hydrazine. The hydrazine reduces
a thin layer of silver metal across the pellet surface. The cathode produced by this process
has only silver metal and Ag
2
O in contact with the cathode terminal. The layer of Ag
2
O
masks the higher potential of the AgO, while the thin, conductive silver layer reduces the
cell impedance as the Ag
2
O is resistive. In use, only the monovalent silver oxide voltage is
observed yet the cell delivers the greater capacity of the divalent silver oxide. Even with the
surface treatments, the cells delivered 20 to 40% more hours of service with the same weight
of silver than the standard zinc /monovalent silver oxide cells.
Cells produced by this ‘‘double-treatment’’ process are termed Ditronic
TM
cells. Figure
12.2 shows the benefit of the Ditronic
TM
design in a button cell. The treated cell has about
a 30% capacity advantage over a conventional Ag
2
O cathode at the same operating voltage.
The AgO cell, without this treatment, produces a two-step discharge.
This ‘‘double-treatment’’ method has the disadvantage that the control of the treatment
process is critical to the shelf life of the cell. Reducing the outer surface to either only
monovalent silver oxide or to only silver metal does not have the advantages of the dual
process (Fig. 12.4). The same is true if either coating is not sufficiently thick (Table 12.2).
In storage, the cells will eventually exhibit the phenomenon referred to as ‘‘voltage up’’ and
‘‘impedance up.’’ During storage, the silver layer is slowly oxidized by the divalent silver
oxide back to resistive monovalent silver oxide:
Ag
⫹ AgO → Ag O
2
FIGURE 12.3 ‘‘Double-treatment’’ method for divalent silver oxide.
TABLE 12.2
‘‘Double-Treatment’’ Method for Divalent Silver Oxide—Effect of Thickness
of Coating
Ag
2
O thickness
around each
pellet, mm
Ag thickness
on the
consolidation
surface, mm
Final cathode
capacity,
mAh/g
Voltage level at months
1 month 3 months 6 months
0.2
0.6
1.0
0.12
0.12
0.12
372
360
326
1.73
1.61
1.60
1.77
1.63
1.59
1.80
1.71
1.59
0.2
0.6
1.0
0.24
0.24
0.24
360
348
315
1.60
1.60
1.60
1.59
1.59
1.59
1.59
1.59
1.59
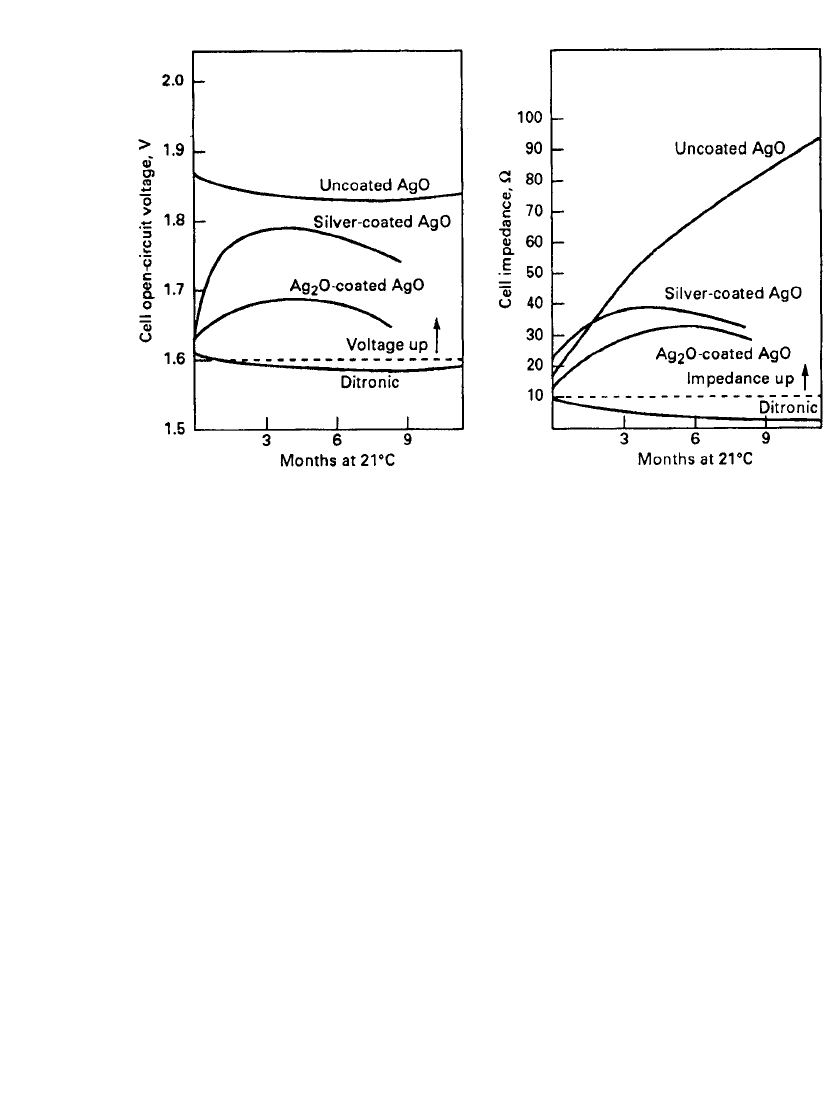
SILVER OXIDE BATTERIES 12.7
FIGURE 12.4 ‘‘Voltage-up’’ and ‘‘impedance-up’’ of Zn / AgO battery during 1 year storage at 21⬚C,
7.8 ⫻ 3.6 mm button type battery.
As the metallic silver layer is depleted, the cell demonstrates an increase in open-circuit
voltage and impedance. The high impedance, due to high internal resistance, results in a low
closed-circuit voltage and a nonfunctional cell.
A second approach to eliminate the two-step discharge was through the use of ‘‘silver
plumbate’’ as a cathode additive material.
17
Silver plumbate cathode material was prepared
by reacting an excess of divalent silver oxide powder with lead sulfide (PbS) in a hot alkaline
solution. The product of the reaction is a mixture of remaining divalent silver oxide (AgO),
monovalent silver oxide (Ag
2
O), and silver plumbate (Ag
5
Pb
2
O
6
). The sulfur is oxidized to
the sulfate and is washed from the reaction product:
2PbS
⫹ 19AgO ⫹ 4NaOH → Ag Pb O ⫹ 7Ag O ⫹ 2Na SO ⫹ 2H O
526 2 2 4 2
The AgO particles after the reaction retain a core of AgO but have an outer coating of
monovalent silver oxide and silver plumbate. The silver plumbate compound is conductive,
stable, and cathode active. The Ag
2
O serves to mask the AgO while the conductive Ag
5
Pb
2
O
6
improves the cell impedance. As the silver plumbate is not oxidized by the AgO, as is silver,
the cathode impedance remains low throughout the cell life.
Cathode material prepared by the silver plumbate process will discharge through the
following reactions (Fig. 12.5):
⫺
0
2AgO ⫹ HO⫹ 2e → Ag O ⫹ 2OH E ⫽⫹0.607 V
22
⫺
0
Ag O ⫹ HO⫹ 2e → 2Ag ⫹ 2OH E ⫽⫹0.342 V
22
⫺
0
Ag Pb O ⫹ 4H O ⫹ 8e → 5Ag ⫹ 2PbO ⫹ 8OH E ⫽⫹0.2 V
526 2
⫺
0
PbO ⫹ HO⫹ 2e → Pb ⫹ 2OH E ⫽⫺0.580 V
2
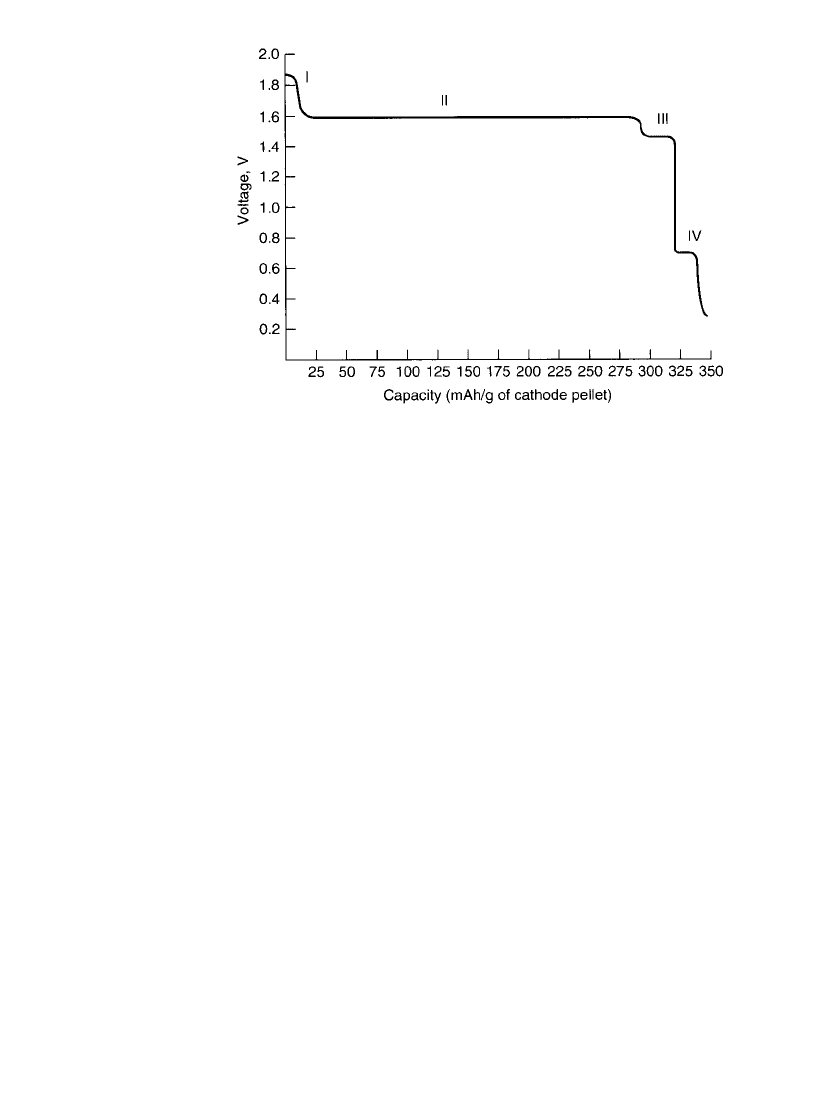
12.8 CHAPTER TWELVE
FIGURE 12.5 Discharge curve of cathode-limited zinc /plumbate sys-
tem. 300 ⍀ continuous in flooded beaker cell, 21⬚C. Cathode pellet
weight—0.12 g. Reaction I (1.8 V)—2AgO ⫹ H
2
O ⫹ 2e → Ag
2
O ⫹
2OH ; reaction II (1.6 V)—Ag
2
O ⫹ H
2
O ⫹ 2e → 2Ag ⫹ 2OH ; reaction
⫺ ⫺
III (1.4 V)—Ag
5
Pb
2
O
6
⫹ 4H
2
O ⫹ 8e
⫺
→ 5Ag ⫹ 2PbO ⫹ 8OH ; reaction
⫺
IV (0.7 V)—PbO ⫹ H
2
O ⫹ 2e → Pb ⫹ 2OH .
⫺
Not all of these steps are observed when the silver plumbate cathode material is used in
cells (Fig. 12.2). The open-circuit voltages (OCV) of these cells are found to be stable
at about 1.75 V. However, once placed on discharge, the cell voltage quickly drops to the
monovalent silver oxide operating voltage of about 1.6 V, eliminating the AgO plateau (Fig.
12.1). As button cells are anode limited, the cell is depleted in zinc capacity before the
Ag
5
Pb
2
O
6
and PbO reduction reactions can occur.
The silver plumbate approach has advantages over the dual-treatment process as the treat-
ment is simpler while still retaining a capacity advantage over monovalent silver oxide. The
product from the silver plumbate reaction (8% lead sulfide) has a coulometric capacity of
345 to 360 mAh/g.
The silver plumbate process has the disadvantage that the button cells do contain a small
amount of lead, 1 to 4% of the cell weight. An alternate approach is to use bismuth sulfide
in place of the lead sulfide in the material preparation reaction.
18
The product of the reaction
has the advantages of the silver plumbate material but without the toxicity of lead. Bismuth
is not considered toxic and has many medical and cosmetic applications, being used both
externally and internally in the body.
19
The product of the reaction of bismuth sulfide with divalent silver oxide is believed to
be silver bismuthate (AgBiO
3
):
Bi S
⫹ 28AgO ⫹ 6NaOH → 2AgBiO ⫹ 13Ag O ⫹ 3Na SO ⫹ 3H O
23 3 2 2 4 2
Like the silver plumbate compound, the silver bismuth compound is conductive and cathode
active. The monovalent silver oxide produced by the reaction coats the divalent silver oxide
particles while the conductive silver bismuthate reduces the cell impedance, maintaining a
high cell CCV. The silver bismuthate will discharge against zinc in alkaline solutions at
about 1.5 V. Therefore, in anode-limited button cells only the monovalent silver oxide voltage
is observed.
SILVER OXIDE BATTERIES 12.9
Unlike monovalent silver oxide systems, additives such as graphite or manganese dioxide
cannot be added to the divalent silver oxide. Graphite enhances the decomposition of AgO
to Ag
2
O and oxygen. Manganese dioxide is readily oxidized by AgO to alkali-soluble man-
ganate compounds.
12.2.3 Electrolyte
The electrolytes used for zinc/ silver oxide cells are based upon 20 to 45% aqueous solutions
of potassium hydroxide (KOH) or sodium hydroxide (NaOH). Zinc oxide (ZnO) is dissolved
in the electrolyte as the zincate to help control zinc gassing. The zinc oxide concentration
varies from a few percent to a saturated solution.
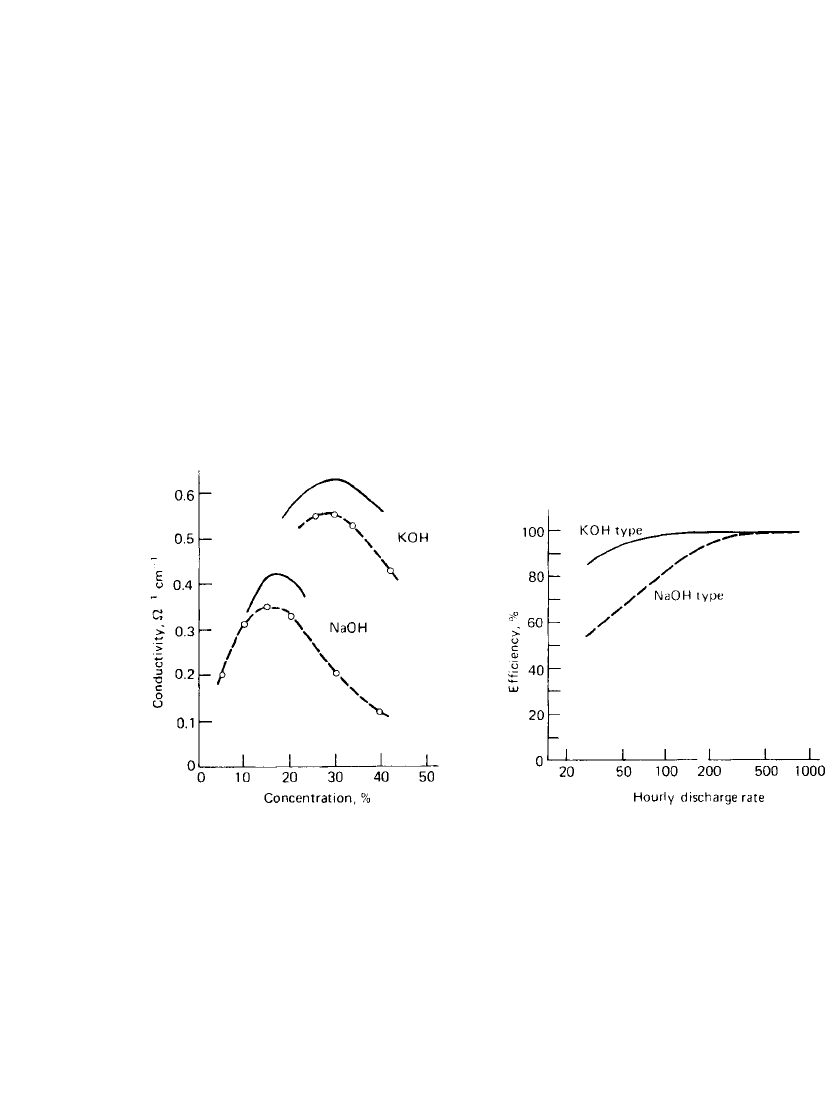
The preferred electrolyte for button cells is potassium hydroxide (KOH). Its higher elec-
trical conductivity
20,21
allows cells to discharge over a wide range of current demands (Fig.
12.6). Sodium hydroxide (NaOH) is used mainly for long life cells not requiring a high-rate
discharge (Fig 12.7). The sodium hydroxide exhibits less creep and such cells are less apt
to leak than the potassium hydroxide cells. Leakage is evidenced as frosting or salting around
the seal. However, this characteristic of potassium hydroxide solutions has been corrected in
recent years due to improvements in seal technology by most manufacturers.
FIGURE 12.6 Specific conductivity of alkaline hy-
droxide solutions. Solid line—25⬚C, Ref. 5; dotted
line—15⬚C, Ref. 6.
FIGURE 12.7 Dependence of discharge efficiency
on discharge rate of zinc / silver oxide button battery
at 20⬚C.
12.2.4 Barriers and Separators
A physical barrier is required to keep the anode and silver cathode apart in the tight volume
constraints of a button cell. Failure of the barrier will result in internal cell shorting and cell
failure. A silver oxide cell requires a barrier with the following properties:
1. Permeable to water and hydroxyl ions
2. Stable in strong alkaline solutions
3. Not oxidized by the solid silver oxide or dissolved silver ions
4. Retards the migration of dissolved silver ions to the anode
12.10 CHAPTER TWELVE
Because of the slight solubility of silver oxides in alkaline electrolyte, little work was done
with zinc /silver oxide cells until 1941 when Andre´
22
suggested the use of a cellophane
barrier. Cellophane prevents migrating silver ions from reaching the anode
23,24
by reducing
them to insoluble silver metal. The cellophane is oxidized and destroyed in the process,
making it less effective for long-life cells.
Many types of laminated membranes are presently available. A commonly used alternate
barrier material is prepared from a radiation graft of methacrylic acid onto a polyethylene
membrane.
23,24
The graft makes the film wettable and permeable to the electrolyte. Studies
have shown that lower resistance polyethylene barrier membrane is suitable for high-rate
KOH cells while higher resistance polyethylene is suitable for low-rate NaOH cells. Cello-
phane is used in conjunction with the grafted membrane as a sacrificial barrier. The lami-
nation of cellophane to either side of the polyethylene membrane results in a synergistic
action for stopping silver migration.
15
A separator is commonly used in conjunction with a barrier membrane layer as added
protection to the barrier. It is located between the barrier and anode cavity and is multi-
functional both during cell manufacture and in performance. Separators in zinc/silver cells
are typically fibrous woven or non-woven polymers such as polyvinyl alcohol (PVA). The
fibrous nature of the separator gives it stability and strength that protects the more fragile
barrier layers from compression failure during cell closure or penetration of zinc particles
through the membranes themselves. The separator also acts in controlling dimensional stress
in the barrier layers which is often derived during original rollup of cellophane or in lami-
nation with polyethylene membranes. As barrier membranes wet out, this stress is relieved.
Differential swelling may then occur which could result in a gap that would allow silver ion
migration to the anode. The separator acts to prevent the formation of these gaps.
12.3 CONSTRUCTION
Figure 12.8 is a cross-sectional view of a typical zinc/ silver oxide button type battery.
Zinc/ silver oxide button cells are designed as anode limited; the cell has 5 to 10% more
cathode capacity than anode capacity. If the cell were cathode limited, a zinc-nickel or zinc-
iron couple could form between the anode and the cathode can resulting in the generation
of hydrogen.
The cathode material for zinc /silver oxide cells is monovalent silver oxide (Ag
2
O) mixed
with 1 to 5% graphite to improve the electrical conductivity. The Ag
2
O cathode material
may also contain manganese dioxide (MnO
2
) or silver nickel oxyhydroxide (AgNiO
2
)as
cathode extenders. The cathode could also be prepared from a controlled blend of divalent
silver oxide and monovalent silver oxide with either silver plumbate (Ag
5
Pb
2
O
6
) or silver
metal to reduce the AgO cathode voltage and cell impedance, but this is no longer a com-
mercial process. A small amount of polytetrafluoroethylene (Teflon
TM
) may be added to the
mix as a binder and to ease pelleting.
The anode is a high surface area, amalgamated, gelled zinc metal powder housed in a
top cup which serves as the external negative terminal for the cell. The top cup is pressed
from a triclad metal sheet: the outer surface is a protective layer of nickel over a core of
steel. The inner surface that is in direct contact with the zinc is high-purity copper or tin.
The cathode pellet is consolidated into the positive can, which is formed from nickel-plated
steel and serves as the positive terminal for the cell. To keep the anode and cathode separated,
a barrier disk of cellophane or a grafted plastic membrane is placed over the consolidated
cathode. The entire system is wetted with potassium or sodium hydroxide electrolyte.
The gasket serves to seal the cell against electrolyte loss and to insulate the top and
bottom cups from contact. The gasket material is made from an elastic, electrolyte-resistant
plastic such as nylon. The seal may be improved by coating the gasket with a sealant such
as polyamide or bitumen to prevent electrolyte leakage at the seal surfaces.
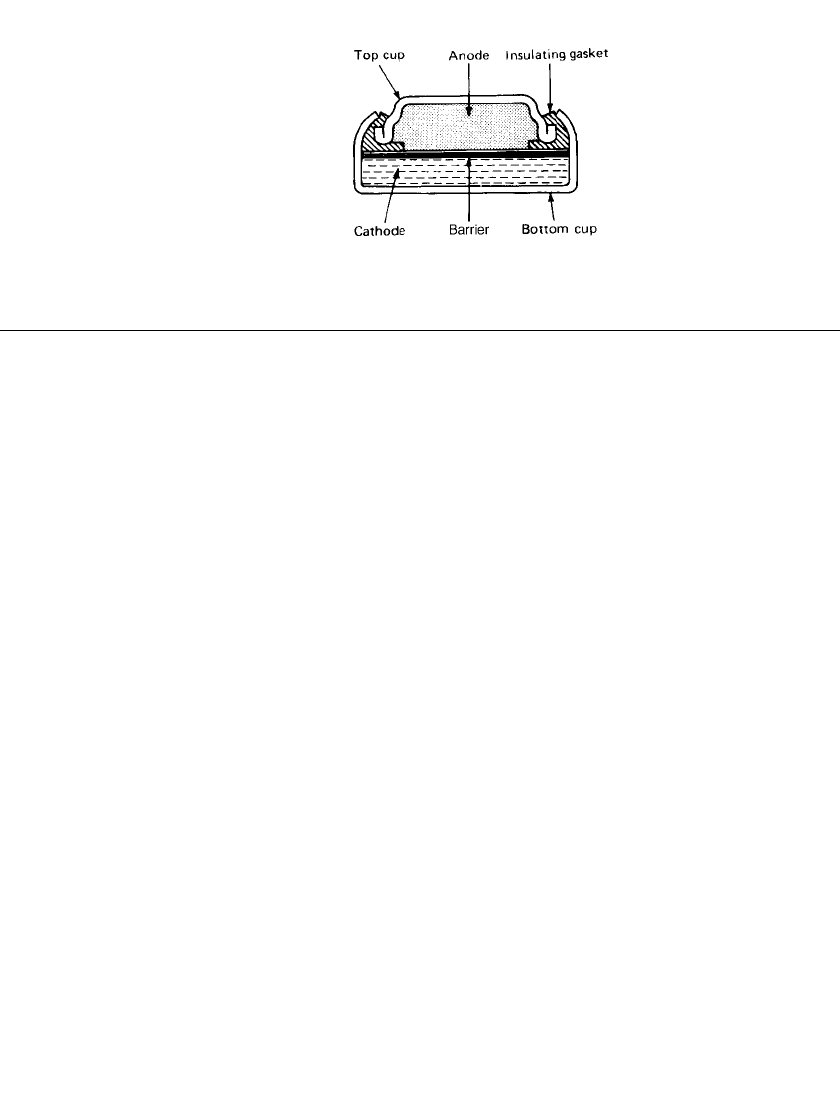
SILVER OXIDE BATTERIES 12.11
FIGURE 12.8 Cutaway view of typical zinc/
silver oxide button type battery.
12.4 PERFORMANCE CHARACTERISTICS
12.4.1 Open-Circuit Voltage
The open-circuit voltage (OCV) of the Zn /Ag
2
O battery is about 1.60 V but will vary slightly
(1.595 to 1.605 V) with electrolyte concentration, concentration of zincate in the electrolyte,
and temperature exposure.
25
The reaction of carbon dioxide with the silver oxide during
battery manufacturing can raise the OCV to 1.65 V due to the formation of silver carbonate.
The increase in voltage, however, is temporary and will drop to the operating voltage level
of 1.58 V in a watch within seconds. The depth of discharge has little effect on the OCV
of a monovalent silver battery; a partially used battery has the same OCV as a new one.
The OCV of the zinc/ divalent silver oxide battery will vary from 1.58 to 1.86 V de-
pending on the ratio of Ag to Ag
2
O to AgO in the cathode. The OCV will decrease with
greater Ag
2
O to AgO ratios and with the presence of silver metal in the cathode. With
divalent silver oxide batteries, the depth of discharge does have an effect on the OCV; a
partially used battery will have more Ag
2
O and silver metal than a new one and will thus
have a lower OCV.
12.4.2 Discharge Characteristics
Figure 12.9 exhibits the typical curves for a 11.6 ⫻ 3.0 mm sized monovalent silver oxide
battery on constant resistance discharge. These are typical voltage curves; the discharge
voltage profiles of other size batteries would be similar. The service life will vary depending
upon the size of the battery and the applied load.
The discharge characteristics of the various types of silver oxide batteries are also covered
in Sec. 12.2.2.
The zinc/silver oxide button battery is capable of operating over a wide temperature
range. The battery can deliver more than 70% of its 20
⬚C capacity at 0⬚C and 35% at ⫺20⬚C,
at moderate loads. At heavier loads, the loss is greater. Higher temperatures tend to accelerate
capacity deterioration, but temperatures as high as 60
⬚C can be tolerated for several days
with no serious effect.
Figures 12.10 and 12.11 show the initial closed-circuit voltage of representative sizes of
zinc/ silver oxide batteries at various loads and temperatures.
Figure 12.12 shows the pulse performance of treated divalent silver oxide batteries using
sodium hydroxide and potassium hydroxide as electrolytes. The low-rate cell is better suited
for analog watches (2000
⍀, 7.8 ms/s) even at ⫺10⬚C, while the high-rate cell is better
suited for LCD watches with backlight (100
⍀, 2 s/h to 8 h/day). Some applications demand
a heavy-current pulse of short duration in addition to the low background current, e.g.,
electronic shutter mechanism of a still camera, LED watch, LCD watch with a backlight,
and some analog watches. In these cases, the minimum voltage at the end of the pulse must
be met to ensure proper device function.
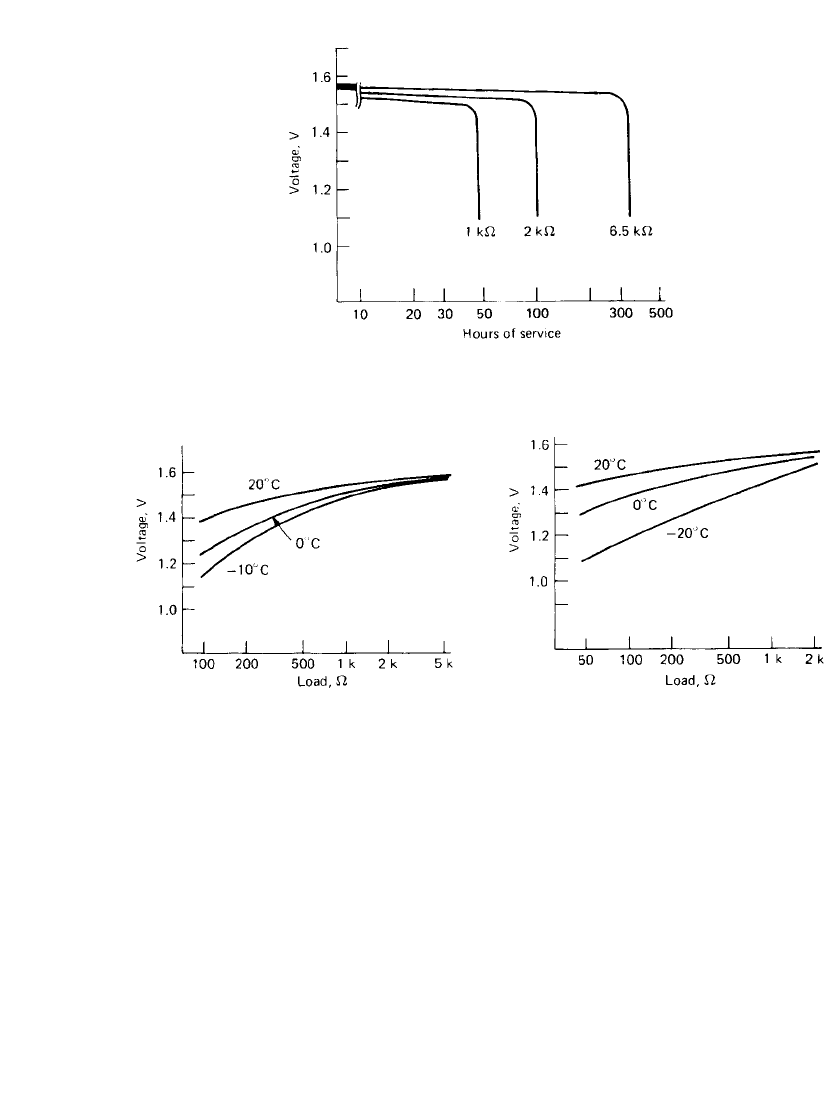
12.12 CHAPTER TWELVE
FIGURE 12.9 Typical discharge curves of zinc / silver
oxide battery at 20⬚C, 11.6 ⫻ 3.0 mm size.
FIGURE 12.10 Closed-circuit voltage of zinc/ sil-
ver oxide battery, 7.9 ⫻ 2.7 mm size, Type 396.
FIGURE 12.11 Closed-circuit voltage of zinc/ sil-
ver oxide battery, 11.6 ⫻ 5.35 mm size.
The manufacturers of these two types of batteries do not distinguish them by service life
tests. In fact, similar mAh output is obtained at loads lighter than the 500 hr rate. The industry
uses pulse CCV tests to differentiate the higher rate version from the low rate version.
Different producers use different CCV loads, durations, and minimum voltages. For example,
at Rayovac, the following tests are used
Battery type 1196 1176
Rayovac number 376 377
CCV load (ohms) 100 2000
Minimum CCV @ 150 mS 1.00V 1.50V
The impedance of a zinc/silver oxide battery is influenced primarily by the conductive
diluents in the cathode, the barrier resistivity and the electrolyte type and concentration.
These factors are balanced by battery manufacturers to obtain the desired values required to
meet the applications. As the cell is discharged, the impedance will decline as the resistive
silver oxide is reduced to conductive metallic silver (Fig. 12.13).
