Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

10.30 CHAPTER TEN
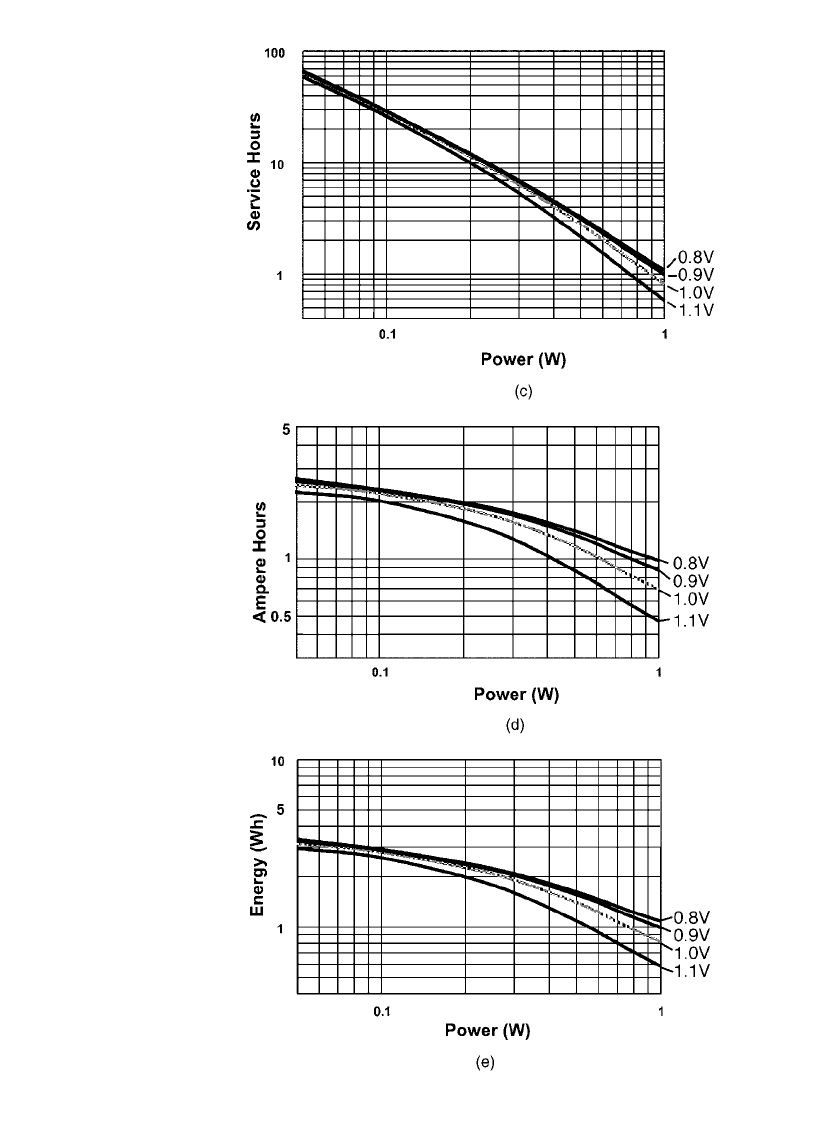
FIGURE 10.22 (c) Service hours vs. power drain to specified end
voltage. (d ) Ampere-hours vs. power drain to specified end voltage.
(e) Energy (Wh) vs. power drain to specified end voltage. (Courtesy
of Duracell, Inc.)(Continued ).
ALKALINE-MANGANESE DIOXIDE BATTERIES 10.31
Service Hours
Discharge Current, mA
(a)
10000
1000
100
10
1
0
1 10 100 1000 10000
1.1
1.2
1.3
0.8
0.9
1.0
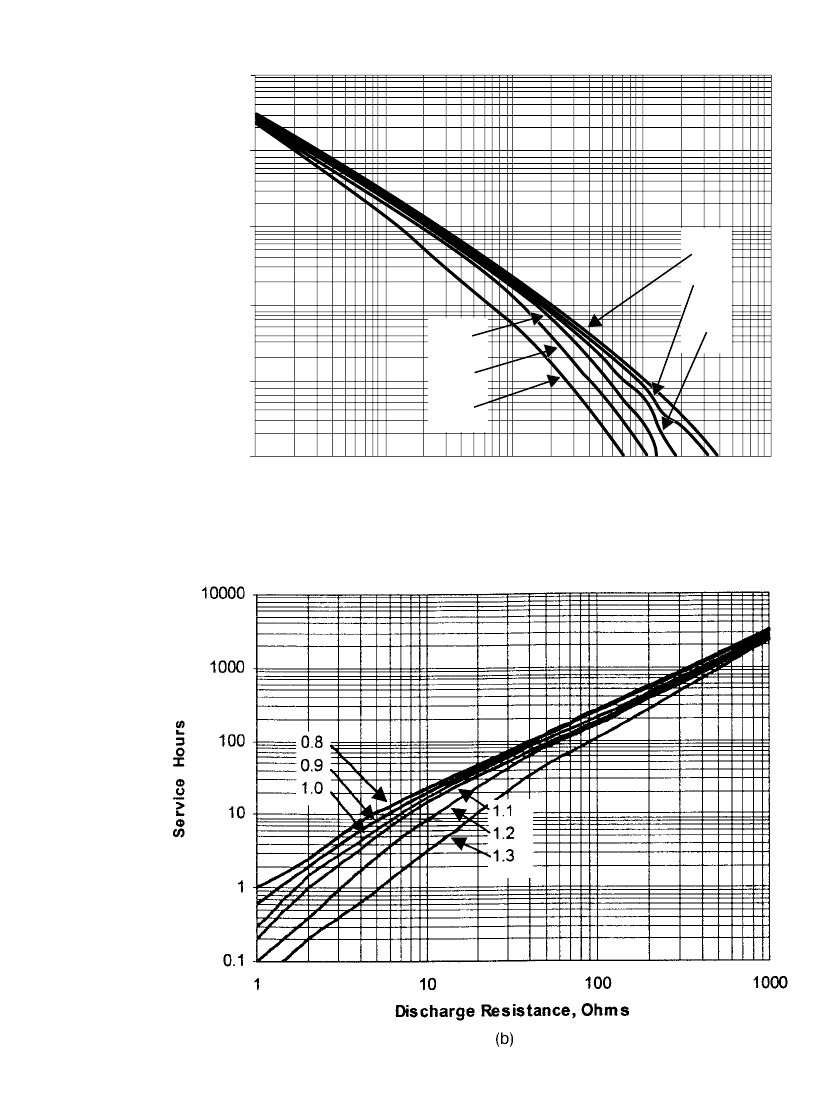
FIGURE 10.23 Performance characteristics of premium zinc / alkaline / manganese dioxide
primary batteries AA-size (a) Discharge characteristics at various current drains to specified
end voltages. (b) Discharge characteristics at various resistance drains to specified end voltages.
10.32 CHAPTER TEN
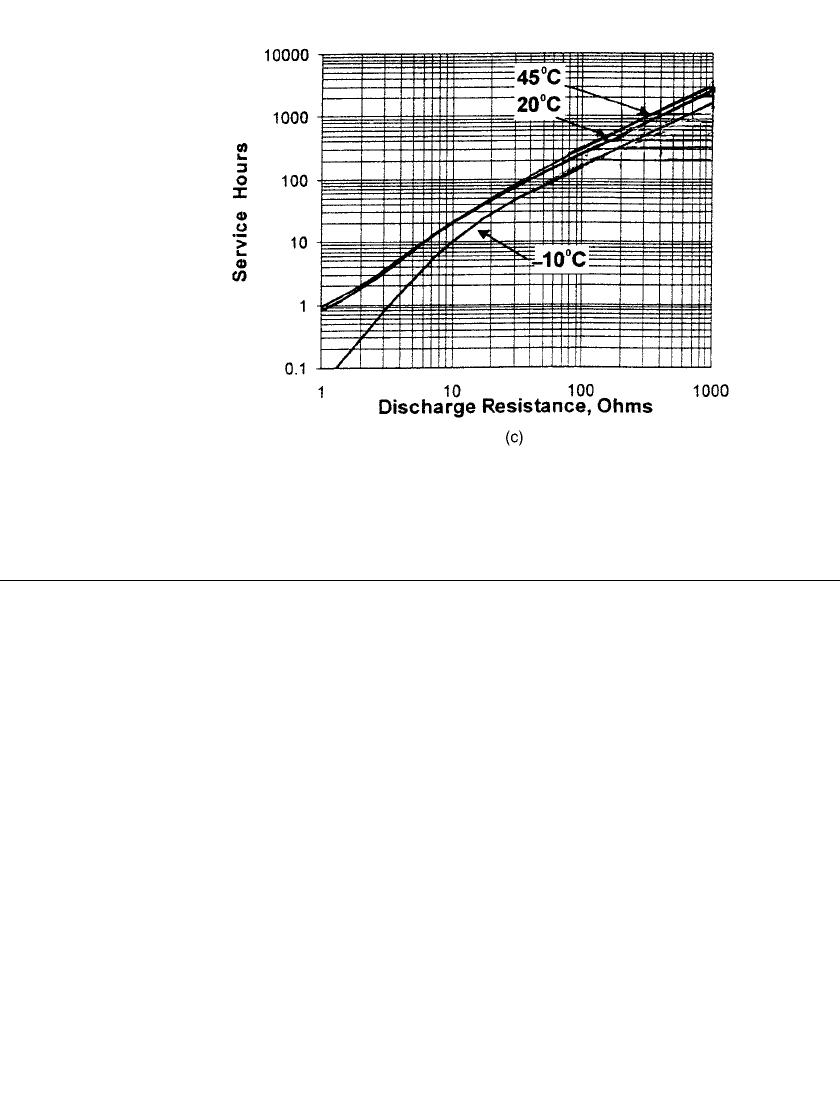
FIGURE 10.23 (c) Constant resistance discharge performance at various
temperatures at 0.8 volts. (Courtesy of Eveready Battery Company.)
(Continued ).
REFERENCES
1. A. Kozawa and R. A. Powers, J. Chem. Educ. 49:587 (1972).
2. D. M. Holton, W. C. Maskell, and F. L. Tye, in Proc. 14th International Power Sources Symp.
(Brighton), Pergamon, New York, 1984.
3. Eveready Battery Engineering Data, vol. 2a, Eveready Battery Co., St. Louis, Mo.
4. Duracell Tech. Bull., Alkaline Cells, Duracell, Inc., Bethel, Conn.
5. Eveready Battery Engineering Data, vol. la, Eveready Battery Co., St. Louis, Mo.

11.1
CHAPTER 11
MERCURIC OXIDE BATTERIES
Denis Naylor*
11.1 GENERAL CHARACTERISTICS
The alkaline zinc/mercuric oxide battery is noted for its high capacity per unit volume,
constant voltage output, and good storage characteristics. The system has been known for
over a century, but it was not until World War II that a practical battery was developed by
Samuel Ruben in response to a requirement for a battery with a high capacity-to-volume
ratio which would withstand storage under tropical conditions.
1,2
Since that time, the zinc/mercuric oxide battery has been used in many applications where
stable voltage, long storage life or high energy-to-volume ratios were required. The char-
acteristics of this battery system were particularly advantageous in applications such as hear-
ing aids, watches, cameras, some early pacemakers and small electronic equipment where it
was widely used. The battery has also been used as a voltage reference source and in
electrical instruments and electronic equipment, such as sonobuoys, emergency beacons,
rescue transceivers, radio and surveillance sets, small scatterable mines and early satellites.
These applications, however, did not become widespread, except for military and special
uses, because of the relatively higher cost of the mercuric oxide system.
The use of cadmium in place of zinc results in a very stable battery with excellent storage
and performance at extreme temperatures due to the low solubility of cadmium in caustic
alkali over a wide range of temperatures. However, the cost of the material is high and the
cell voltage is low, less than 1.0 V. Hence, the cadmium/mercuric batteries were used, but
to a lesser degree, in special applications requiring the particular performance capabilities of
the system. These include gas and oil well logging, telemetry from engines and other heat
sources, alarm systems, and for operation of remote equipment such as data-monitoring,
surveillance buoys, weather stations and emergency equipment.
3
During the last several years, the market for mercuric oxide batteries has almost com-
pletely evaporated, due mainly to environmental problems associated with mercury and cad-
mium and few are manufactured. They have been removed from the International Electro-
technical Commission (IEC) and the American National Standards Institute (ANSI)
standards. In applications, they have been replaced by alkaline-manganese dioxide, zinc/ air,
silver oxide and lithium batteries.
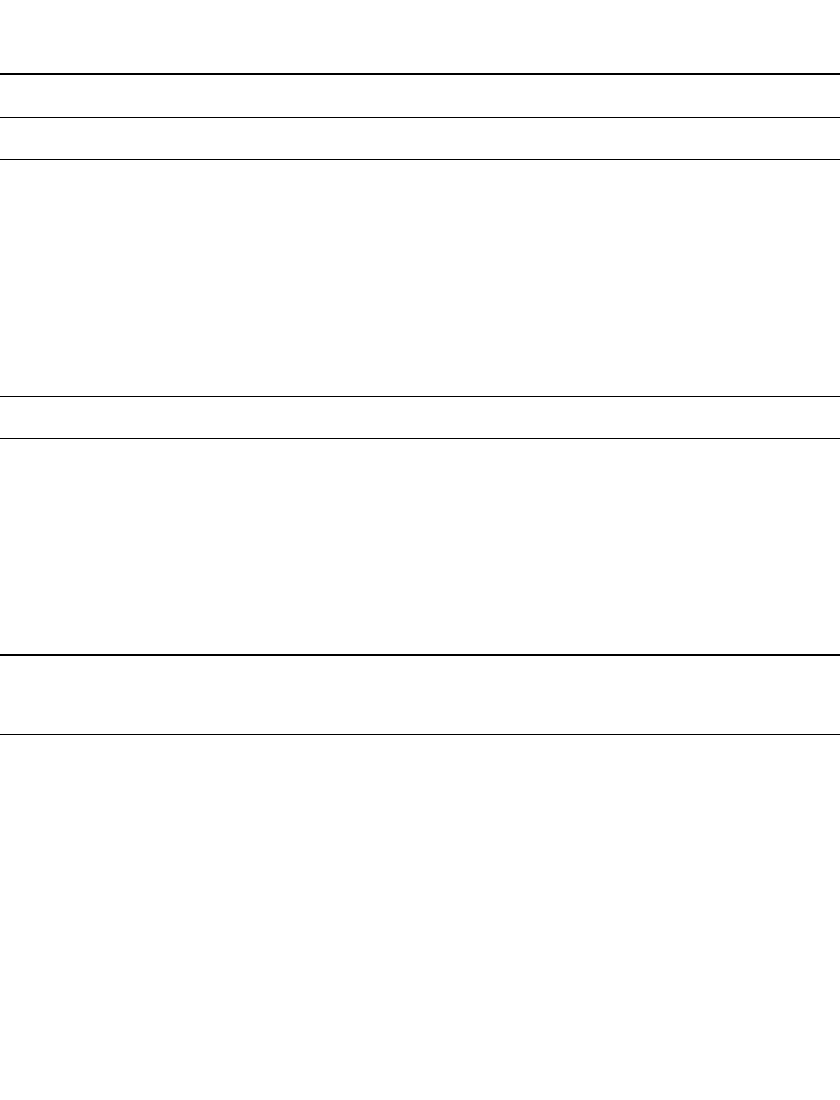
The major characteristics of these two battery systems are summarized in Table 11.1.
*The chapter on Mercuric Oxide Batteries was written by Denis Naylor, now deceased,
for the 1
st
and 2
nd
Editions. His work was updated and modified for the 3
rd
Edition by
David Linden.
11.2 CHAPTER ELEVEN
TABLE 11.1 Characteristics of the Zinc/ Mercuric Oxide and Cadmium/ Mercuric Oxide Batteries
Advantages Disadvantages
Zinc/ mercuric oxide battery
High energy-to-volume ratio, 450 Wh / L
Long shelf life under adverse storage conditions
Over a wide range of current drains recuperative
periods are not necessary to obtain a high capacity
from the battery
High electrochemical efficiency
High resistance to impact, acceleration, and vibration
Very stable open-circuit voltage, 1.35 V
Flat discharge curve over wide range of current drains
Batteries were expensive; although widely used in
miniature sizes, but only for special applications in
the larger sizes
After long periods of storage, cell electrolyte tends to
seep out of seal which is evidenced by white
carbonate deposit at seal insulation
Moderate energy-to-weight ratio
Poor low-temperature performance
Disposal of quantities of spent batteries creates
environmental problems
Cadmium/ mercuric oxide battery
Long shelf life under adverse storage conditions
Flat discharge curve over wide range of current drains
Ability to operate efficiently over wide temperature
range, even at extreme high and low temperatures
Can be hermetically sealed because of inherently low
gas evolution level
Batteries are more expensive than zinc/ mercuric oxide
batteries due to high cost of cadmium
System has low output voltage (open-circuit voltage
⫽
0.90 V)
Moderate energy-to-volume ratio
Low energy-to-weight ratio
Disposal of spent batteries creates environmental
problem, with both cadmium and mercury being
toxic
11.2 CHEMISTRY
It is generally accepted that the basic cell reaction for the zinc /mercuric oxide cell is
Zn
⫹ HgO → ZnO ⫹ Hg
For the overall reaction,
⌬G
0
⫽ 259.7 kJ. This gives a thermodynamic value for E
0
at 25⬚C
of 1.35 V, which is in good agreement with the observed values of 1.34 to 1.36 V for the
open-circuit voltage of commercial cells.
4
From the basic reaction equation it can be cal-
culated that 1 g of zinc provides 819 mAh and1gofmercuric oxide provides 247 mAh.
Some types of zinc/ mercuric oxide cells exhibit open-circuit voltages between 1.40 and
1.55 V. These cells contain a small percentage of manganese dioxide in the cathode and are
used where voltage stability is not of major importance for the application.
The basic cell reaction for the cadmium/mercuric oxide cell is
Cd
⫹ HgO ⫹ HO→ Cd(OH) ⫹ Hg
22
For the overall reaction, ⌬G
0
⫽⫺174.8 kJ. This gives a thermodynamic value for E
0
at
25
⬚C of 0.91 V, which is in good agreement with the observed values of 0.89 to 0.93 V.
From the basic reaction it can be calculated that 1 g of cadmium should provide 477 mAh.
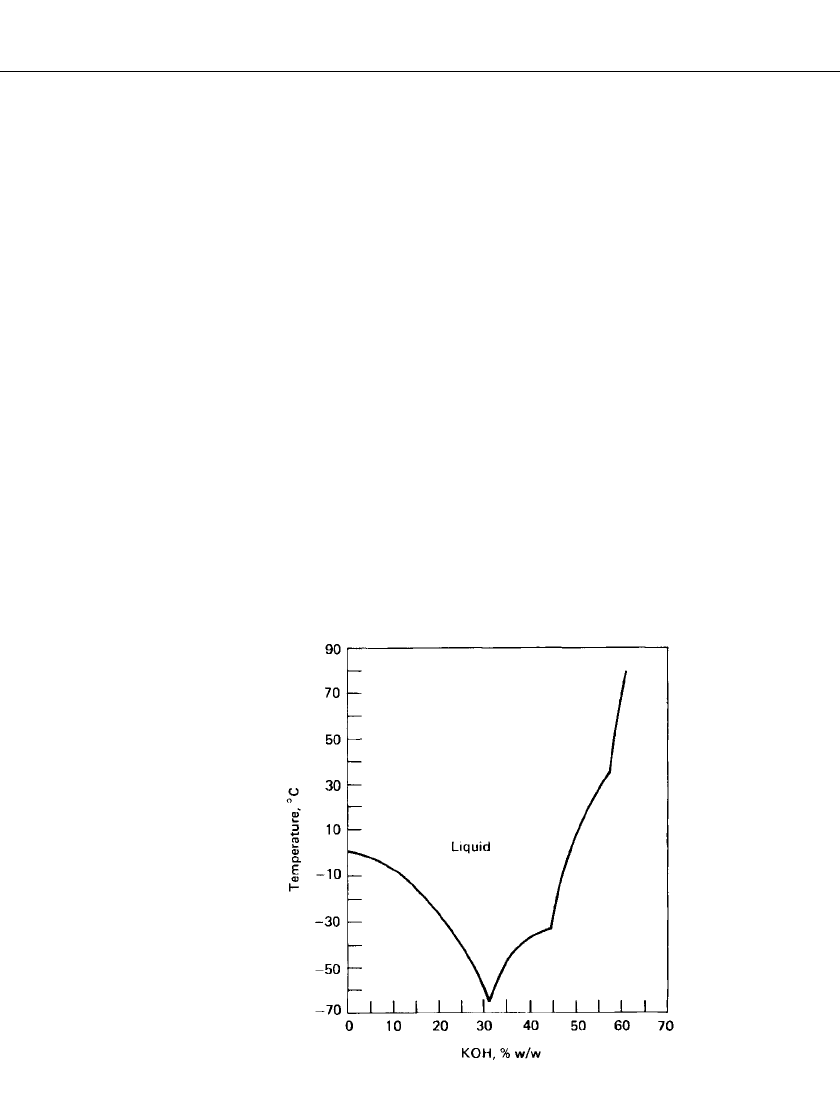
MERCURIC OXIDE BATTERIES 11.3
FIGURE 11.1 Freezing-point curve for aqueous caustic
potash solutions.
11.3 CELL COMPONENTS
11.3.1 Electrolyte
Two types of alkaline electrolyte are used in the zinc/mercuric oxide cell, one based on
potassium hydroxide and one on sodium hydroxide. Both of these bases are very soluble in
water and highly concentrated solutions are used; zinc oxide is also dissolved in varying
amounts in the solution to suppress hydrogen generation.
Potassium hydroxide electrolytes generally contain between 30 and 45% w/w KOH and
up to 7% w/ w zinc oxide. They are more widely used than the sodium hydroxide electrolytes
because of their greater operating temperature range and ability to support heavier current
drains. For low temperature operation, both the potassium hydroxide and the zinc oxide
contents are reduced, and this introduces some instability at higher temperatures with respect
to hydrogen generation in the cell.
Sodium hydroxide electrolytes are prepared in similar concentration ranges and are used
in cells where low temperature operations or high current drains are not required. These
electrolytes are suitable for long-term discharge applications because of the reduced tendency
of the electrolyte to seep out of the cell seal after long periods of storage.
Generally only potassium-based alkaline electrolytes are used in the cadmium /mercuric
oxide cell. As cadmium is practically insoluble in all concentrations of aqueous potassium
hydroxide solutions, the electrolyte can be optimized for low-temperature operation.
The freezing-point curve for caustic potash solutions is shown in Fig. 11.1. It shows that
the eutectic with a freezing point below
⫺60⬚C is 31% w/ w KOH, which is the electrolyte
most frequently used. Improvements in low-temperature performance have been made in
some cases by the addition of a small percentage of cesium hydroxide to the electrolyte.

11.4 CHAPTER ELEVEN
11.3.2 Zinc Anode
Alkaline electrolytes act as ionic carriers in the cell reactions. The reaction at the zinc
negative electrode may be written
⫺
2
⫺
Zn ⫹ 4OH → Zn(OH) ⫹ 2e
4
2
⫺⫺
Zn(OH) → ZnO ⫹ 2OH ⫹ HO
42
These reactions imply the dissolution of the zinc electrode, with the crystallization of zinc
oxide from the electrolyte. The reaction at the anode can be simplified to
⫺
Zn ⫹ 2OH → ZnO ⫹ HO⫹ 2e
2
Direct solution of the zinc electrode in the alkaline solution on open circuit is minimized by
dissolving zinc oxide in the electrolyte and amalgamating the zinc in the electrode. Mercury
levels used in zinc electrodes are usually in the range of 5 to 15% w/w. Great attention is
also paid to the impurity levels in the zinc since minor cathodic inclusions in the electrode
can drive the hydrogen generation reaction despite the precautions indicated.
5,6
11.3.3 Cadmium Anode
The reaction at the anode is
⫺
Cd ⫹ 2OH → Cd(OH) ⫹ 2e
2
This implies the removal of water from the electrolyte during discharge, necessitating an
adequate quantity of electrolyte in the cell and the desirability of a high percentage of water
in the electrolyte. Cadmium has a high hydrogen overvoltage in the electrolyte, and so
amalgamation is neither necessary nor desirable, since the electrode potential is some 400
mV less electropositive than zinc.
Cadmium metal powders as produced conventionally are unsuitable for use as electrode
materials. Activated cadmium anodes are produced by (1) electroforming the anode, (2)
electroforming powder by a special process followed by pelleting, or (3) precipitating by a
special process as a low-nickel alloy and pelleting. All of these processes have been used
by different manufacturers to give cells with various performance parameters.
7
11.3.4 Mercuric Oxide Cathode
At the cathode, the overall reaction may be written
⫺
HgO ⫹ HO⫹ 2e → Hg ⫹ 2(OH)
2
Mercuric oxide is stable in alkaline electrolytes and has a very low solubility. It is also a
nonconductor, and adding graphite is necessary to provide a conductive matrix. As the dis-
charge proceeds, the ohmic resistance of the cathode falls and the graphite assists in the
prevention of mass agglomeration of mercury droplets. Other additives which have been used
to prevent agglomeration of the mercury are manganese dioxide, which increases the cell
voltage to 1.4–1.55 V, lower manganese oxides, and silver powder, which forms a solid-
phase amalgam with the cathode product.
Graphite levels usually range from 3 to 10% and manganese dioxide from 2 to 30%.
Silver powder is used only in special-purpose cells because of cost considerations, but may
be up to 20% of the cathode weight. Again, great care is taken to obtain high-purity materials
for use in the cathode. Trace impurities soluble in the electrolyte are liable to migrate to the
anode and initiate hydrogen evolution. An excess of mercuric oxide capacity of 5 to 10% is

MERCURIC OXIDE BATTERIES 11.5
FIGURE 11.2 Zinc / mercuric oxide battery—button con-
figuration. (Courtesy of Duracell, Inc.)
usually maintained in the cathode to ‘‘balance’’ the cell and prevent hydrogen generation in
the cathode at the end of discharge.
11.3.5 Materials of Construction
Materials of construction for the zinc/mercuric oxide cells are limited not only by their
ability to survive continuous contact with strong caustic alkali, but also by their electro-
chemical compatibility with the electrode materials. As far as the external contacts are con-
cerned, these are decided by corrosion resistance, compatibility with the equipment interface
with respect to galvanic corrosion, and, to some degree, cosmetic appearance. Metal parts
may be homogeneous, plated metal, or clad metal. Insulating parts may be injection-, com-
pression-, or transfer-molded polymers or rubbers.
With the exception of the anode contact (where slight modification of the top /anode
interface is necessary), materials for the cadmium/mercuric oxide cell are generally the same
as for the zinc /mercuric oxide cell. However, because of the wide range of storage and
operating conditions of most applications, cellulose and its derivatives are not used, and low-
melting-point polymers are also avoided. Nickel is usually used on the anode side of the
cell and also, conveniently, at the cathode.
11.4 CONSTRUCTION
The mercuric oxide batteries were manufactured in three basic structures—button, flat, and
cylindrical configurations. There are several design variations within each configuration.
11.4.1 Button Configuration
The button configuration of the zinc /mercuric oxide battery is shown in Fig. 11.2. The top
is copper or copper alloy on the inner face and nickel or stainless steel on the outer face.
This part may also be gold plated, depending on the application. Within the top is a dispersed
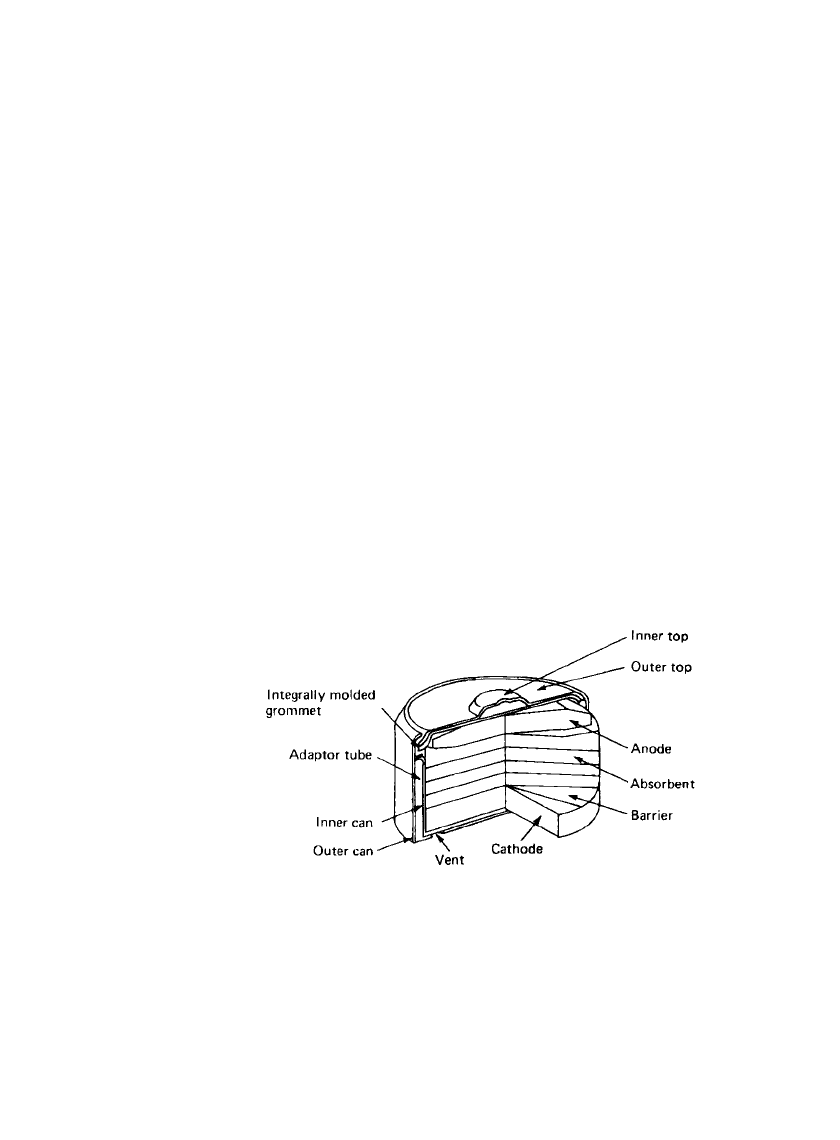
11.6 CHAPTER ELEVEN
mass of amalgamated zinc powder (‘‘gelled anode’’), and the top is insulated from the can
by a nylon grommet. The whole top-grommet-anode assembly presses down onto an ab-
sorbent which contains most of the electrolyte, the remainder being dispersed in the anode
and cathode. Below the absorbent is a permeable barrier, which prevents any cathode material
from migrating to the anode. The cathode of mercuric oxide and graphite is consolidated
into the can, and a sleeve support of nickel-plated steel prevents collapse of the cathode
mass as the battery discharges. The can is made of nickel-plated steel, and the whole cell is
tightly held together by crimping the top edge of the can as shown.
The cadmium /mercuric oxide button battery uses a similar configuration.
11.4.2 Flat-Pellet Configuration
A form of a larger-sized zinc/ mercuric oxide battery is shown in Fig. 11.3. In these cells
the zinc powder is amalgamated and pressed into a pellet with sufficient porosity to allow
electrolyte impregnation. A double top is used, with an integrally molded polymer grommet,
as a safeguard to relieve excessive gas pressures and maintain a leak-resistant structure. The
outer top is of nickel-plated steel, and the inner top is nickel-plated steel but tin plated on
its inner face. This cell also uses two nickel-plated steel cans with an adaptor tube between
the two, the seal being effected by pressing the top-grommet assembly against the inner can
and crimping over the outer can. A vent hole is pierced into the outer can so that if gas is
generated within the cell, it can escape between the inner and outer cans, any entrained
electrolyte being absorbed by the paper adaptor tube.
FIGURE 11.3 Zinc / mercuric oxide battery—flat-pellet config-
uration. (Courtesy of Duracell, Inc.)
11.4.3 Cylindrical Configuration
The larger cylindrical zinc/mercuric oxide battery is constructed from annular pressings, as
shown in Fig. 11.4. The anode pellets are rigid and pressed against the cell top by the
neoprene insulator slug. A number of variations of the cylindrical cell were used with dis-
persed anodes, where contact with the anode is made either by a nail welded to the inner
top or a spring extending from the base insulator to the top.
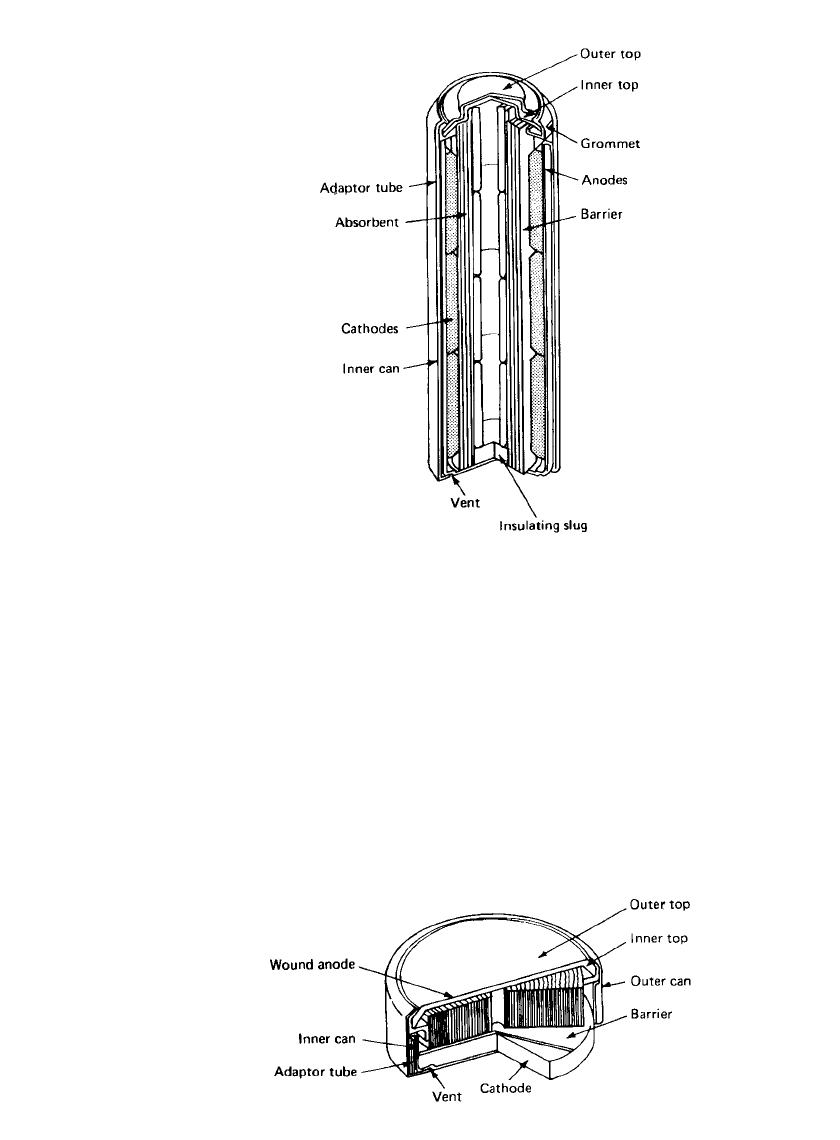
MERCURIC OXIDE BATTERIES 11.7
FIGURE 11.4 Zinc / mercuric oxide battery cy-
lindrical configuration. (Courtesy of Duracell,
Inc.)
11.4.4 Wound-Anode Configuration
Another design of the zinc/mercuric oxide battery which operates better at low temperatures
is the wound-anode or jelly-roll structure shown in Fig. 11.5. Structurally the cell is similar
to the flat cell shown in Fig. 11.3, but the anode and absorbent have been replaced by a
wound anode which consists of a long strip of corrugated zinc interleaved with a strip of
absorbent paper. The paper edge protrudes at one side and the zinc strip at the other. This
provides a large surface area anode. The roll is held in a plastic sleeve and the zinc is
amalgamated in situ. The paper swells in the electrolyte and forms a tight structure, which
is compressed in the cell at the assembly stage with the zinc edge in contact with the top.
Electrolyte formulations can be adjusted for low-temperature operation, long storage life
at elevated temperature, or a compromise between the two. The performance is optimized
by careful adjustment of the anode geometry.
FIGURE 11.5 Zinc/ mercuric oxide battery—wound-anode
configuration. (Courtesy of Duracell, Inc.)
