Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


10.10 CHAPTER TEN
conductive coatings containing carbon may be placed on the surface. Nickel plating may
also be present on the outside surface of the container, either for contact purposes or for
appearance.
The seal is typically a plastic material, such as nylon or polypropylene, combined with
some metal parts, including the anode collector, to make a seat assembly. It closes off the
open end of the cylindrical can, preventing leakage of electrolyte from the cell, and providing
electrical insulation between the cathode collector (can) and the anode collector contact.
The cylindrical alkaline cell has some additional parts, collectively referred to as finish.
There usually are metal pieces at each end for positive and negative contact. These may be
nickel- or tin-plated for appearance and corrosion resistance. There may be a metal jacket
around the cell, with a printed label on it. In many recent designs the finish is just a thin
plastic jacket or printed label. In the latter type of cell, the use of the thin plastic allows the
cell container to be made slightly larger in diameter, which results in a significant increase
in cell capacity.
Miniature Cell. The container, seal, and finish materials for the miniature alkaline-
manganese dioxide button cell are essentially the same as those for other miniature cells.
The can (container and cathode collector) is made of mild steel plated on both sides with
nickel. The seat is a thin plastic gasket. The anode cup makes up the rest of the exterior of
the cell. The outer surfaces of the can and anode cup are highly finished, with manufacturer
identification and cell number inscribed on the can. No additional finish is needed.
10.4 CONSTRUCTION
10.4.1 Cylindrical Configuration
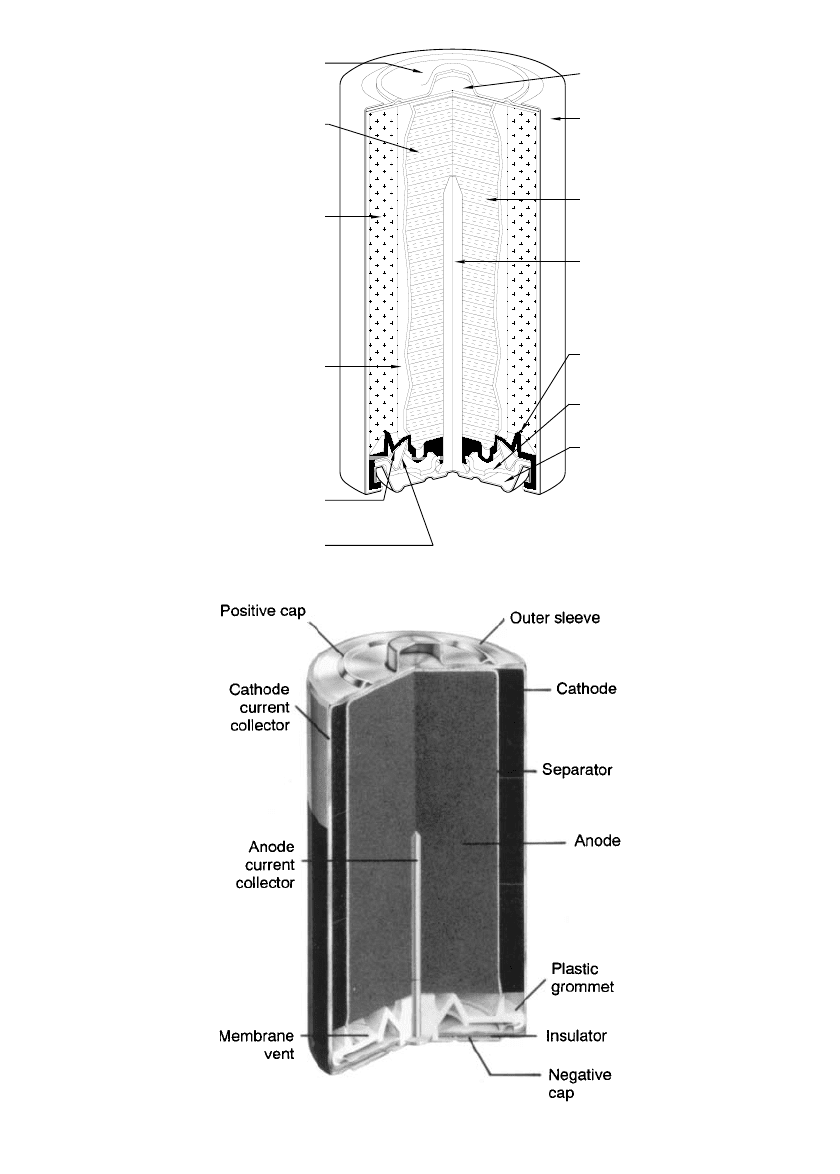
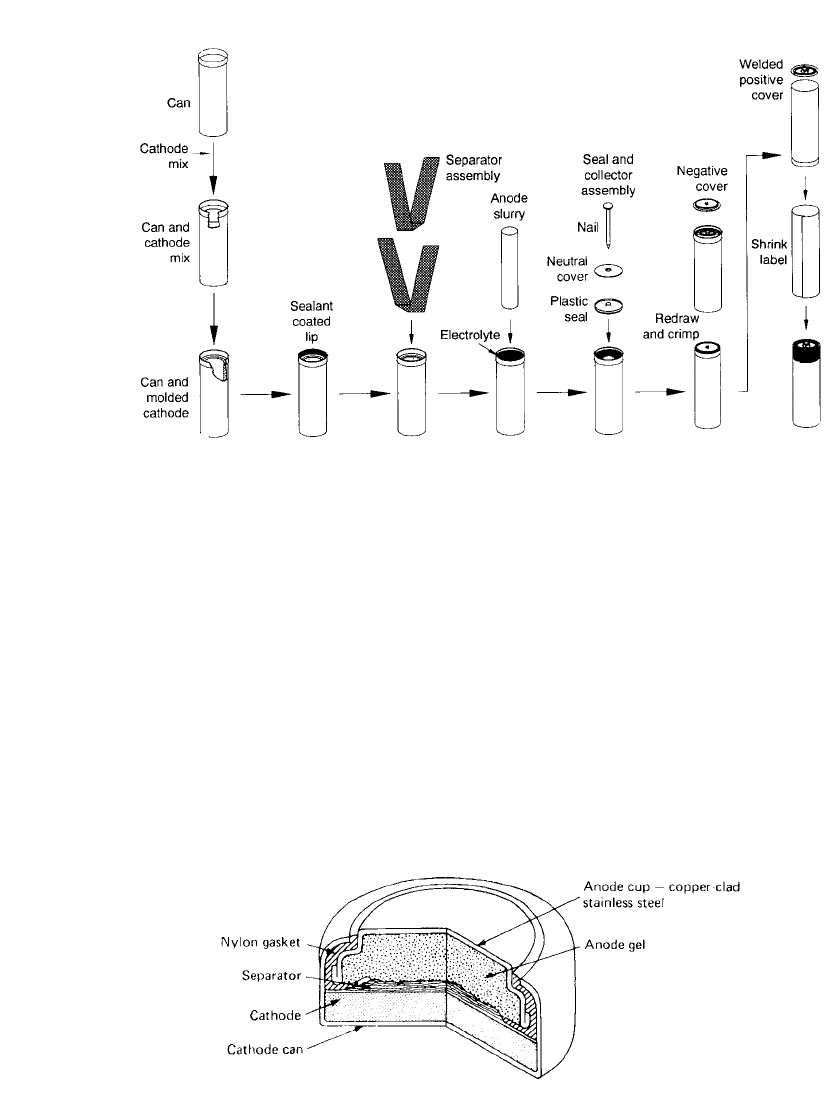
Figure 10.2 shows the construction of typical cylindrical alkaline-manganese dioxide batter-
ies from two manufacturers. Figure 10.3 illustrates the process for assembling the battery. A
cylindrical steel can is the container for the cell. It also serves as the cathode current collector.
The cathode, a compressed mixture of manganese dioxide, carbon, and possibly other ad-
ditives, is positioned inside the can in the form of a hollow cylinder in close contact with
the can inner surface. The cathode can be formed by directly molding it in the can. Alter-
natively, rings of cathode material can be formed outside the cell and then pushed into the
can. Inside the hollow center of the cathode are placed layers of separator material. Inside
of that is the anode, with a metal collector contacting it, and making connection through a
plastic seal to the negative terminal of the cell. The cell has top and bottom covers and a
metal or plastic jacket applied. The covers serve a dual purpose. Besides providing a dec-
orative and corrosion-resistant finish, they also provide for the proper polarity of the battery.
This is necessary because the cylindrical alkaline manganese battery is used as a direct
replacement for Leclanche´ batteries. Leclanche´ batteries have a flat contact on the negative
(zinc can) end, and a button contact on the positive end to accommodate the carbon rod
used as current collector. The cylindrical alkaline-manganese dioxide cell is built ‘‘inside-
out’’ in relation to the Leclanche´ cell, with the cell container as the positive current collector
and the end of the negative collector protruding from the center of the seal. Therefore to
give it an external form similar to the Leclanche´ battery, the cylindrical alkaline battery must
use a flat cover to contact the terminus of the negative collector, and a bottom cover con-
taining the Leclanche´ positive protrusion in contact with the bottom of the can. (Some
manufacturers mold the protrusion into the can itself, and thus do not need the bottom cover.)
ALKALINE-MANGANESE DIOXIDE BATTERIES 10.11
METALIZED
CAN-STEEL
PLASTIC FILM LABEL
PLATED STEEL
WATER
POTASSIUM
HYDROXIDE /
ELECTROLYTE-
POSITIVE COVER-
BRASS PIN
STEEL
INNER CELL COVER-
SEAL - NYLON
NEGATIVE COVER-
CURRENT COLLECTOR
POWDERED ZINC
PLATED STEEL
CATHODE-
MANGANESE
CARBON
DIOXIDE,
NON-WOVEN
SEPARATOR-
FABRIC
METAL
WASHER
METAL SPUR
ANODE-
(a)
(b)
FIGURE 10.2 Cross section of cylindrical alkaline-
manganese dioxide batteries. [(a) From Eveready Battery En-
gineering Data.
3
(b) From Duracell Technical Bulletin.
4
]
10.12 CHAPTER TEN
FIGURE 10.3 Assembly process for AA-size cylindrical alkaline-manganese dioxide battery. (Courtesy
of Eveready Battery Company.)
10.4.2 Button Configuration
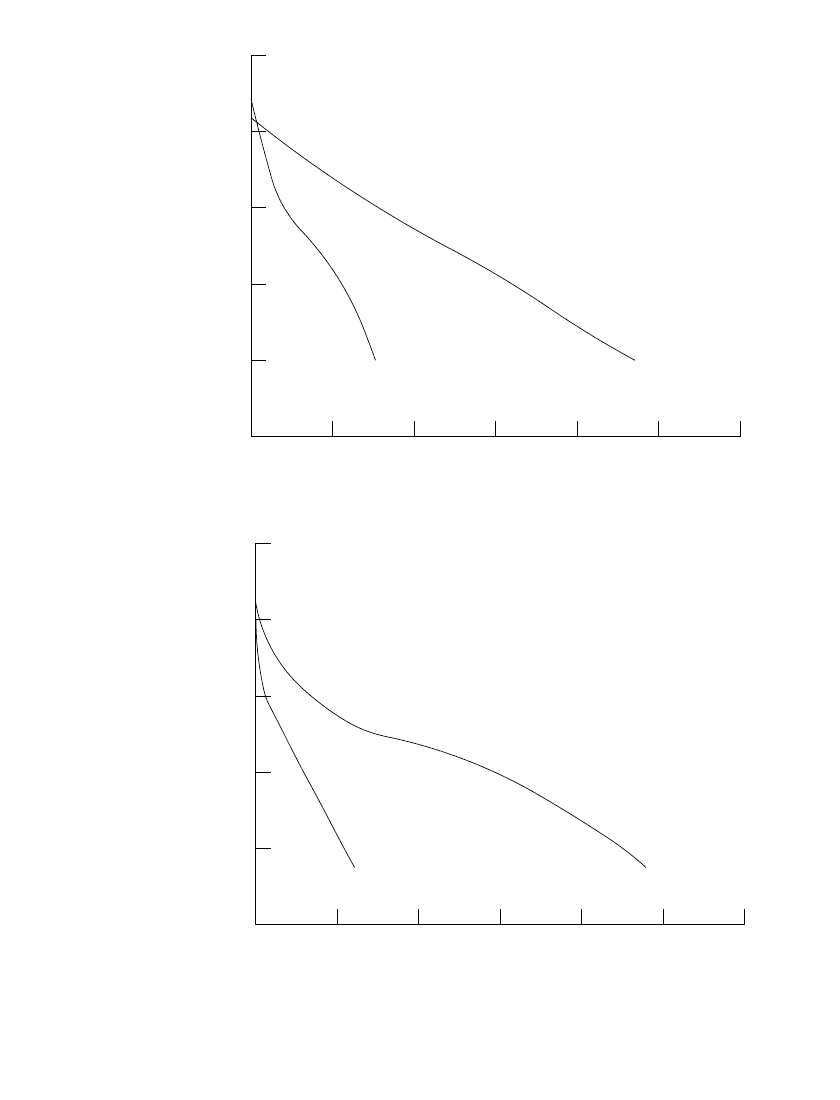
The construction of the miniature alkaline-manganese dioxide cell is shown in Fig. 10.4. It
is essentially the same as the construction of other miniature alkaline cells. There are a
bottom cup with a cathode pellet in it, an anode cup containing the anode mix, one or more
round disks of separator material between them, and a plastic seal that is compressed between
the bottom cup and the anode cup to prevent leakage.
FIGURE 10.4 Cross section of miniature alkaline-manganese dioxide battery.
(From Eveready Battery Engineering Data.
5
)

ALKALINE-MANGANESE DIOXIDE BATTERIES 10.13
10.5 PERFORMANCE CHARACTERISTICS
10.5.1 General Characteristics, Comparison with Leclanche´ Batteries
Alkaline-manganese dioxide batteries have a relatively high theoretical capacity, considerably
higher than Leclanche´ batteries of the same size. There are several reasons for this. The
alkaline-manganese dioxide cell uses manganese dioxide of much higher purity and activity
than is used in most Leclanche´ cells. Moreover, the alkaline-manganese dioxide cell can
function with a very dense cathode, which contains only a small amount of electrolyte.
Furthermore the space taken up by other cell components (separator, current collectors, and
so on) is minimized.
In addition to having a high input capacity, these batteries use that capacity very effi-
ciently. The KOH electrolyte has a very high conductivity, and the zinc anode is in the form
of a high-area powder (compared to the low-area zinc can used in the Leclanche´ cell).
Consequently the internal resistance of the battery is very low at the beginning of discharge
and remains quite low up to the end of its service life (see Sec. 10.5.4).
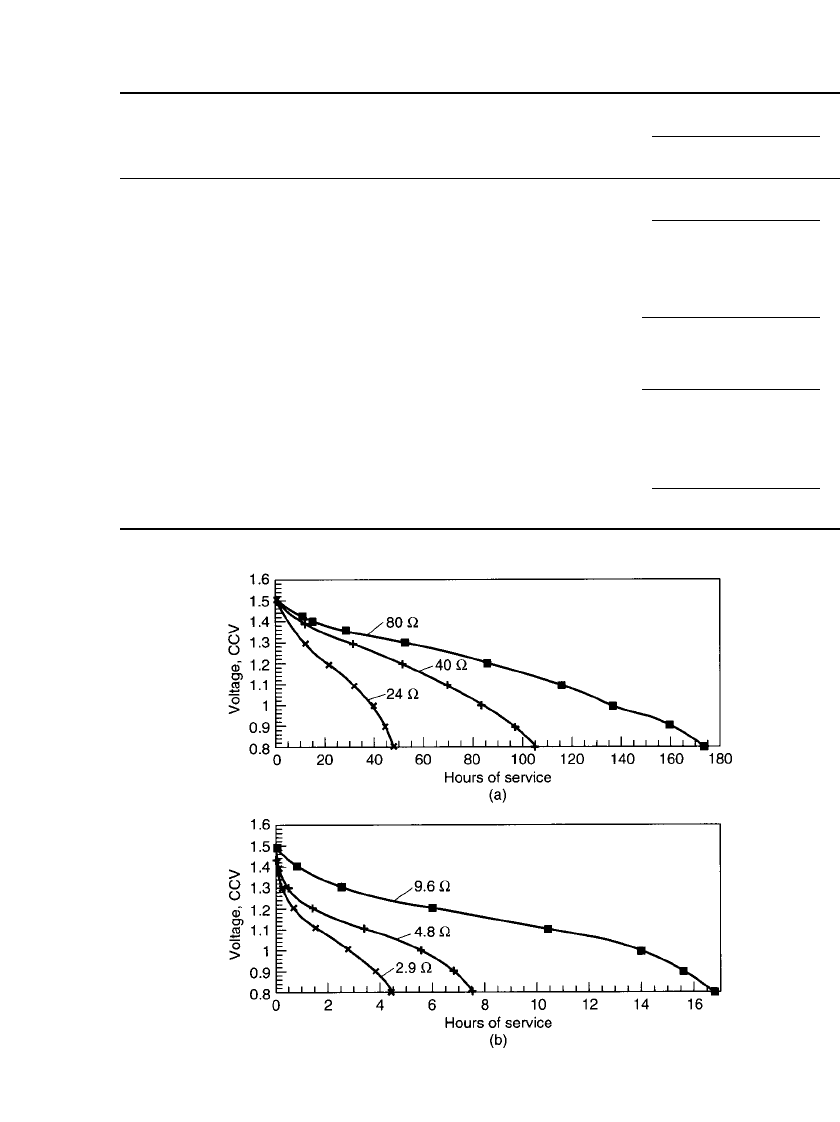
Alkaline-manganese dioxide batteries perform better than Leclanche´ batteries under a
wide range of conditions. Figure 10.5a compares the discharge curve for an alkaline-
manganese dioxide battery with that for a Leclanche´ battery under a single light-drain con-
tinuous discharge condition. In this case both types of batteries function efficiently, delivering
a significant part of their theoretical capacity. The alkaline-manganese battery outperforms
the Leclanche´ battery simply because of its higher theoretical capacity. Figure 10.5b shows
a similar comparison of an alkaline-manganese dioxide battery and a Leclanche´ battery under
heavy-drain continuous discharge conditions. Again, the alkaline-manganese dioxide battery
outperforms the Leclanche´ battery, but this time the difference is much larger. The alkaline-
manganese dioxide battery still functions quite efficiently due to its superior high-rate ca-
pability, but the Leclanche´ battery is unable to deliver more than a fraction of its theoretical
capacity. Other comparisons of these two types of cells are covered in Chap. 7.
10.14 CHAPTER TEN
Alkaline
Carbon Zinc
2400
2000
1600
800
Service (Hours)
(a)
1200
1.7
1.5
1.1
0.9
Closed Circuit Voltage
1.3
400
0.7
0
Alkaline
25
30
1.6
10 15
20
Closed Circuit Voltage
0.6
0
0.8
5
1.0
1.2
1.4
Carbon Zinc
Service (Hours)
(b)
FIGURE 10.5 Performance comparison of D-size alkaline-manganese dioxide and
zinc-carbon batteries. (a) Typical light drain test (30 mA continuous test at 20⬚C).
(b) Typical heavy drain test (500 mA continuous test at 20⬚C). (Courtesy of Eveready
Battery Company.)

ALKALINE-MANGANESE DIOXIDE BATTERIES 10.15
TABLE 10.7 Estimated Average Service at 21⬚C for an AA-Size Alkaline-Manganese Dioxide
Battery
Schedule
Typical
drains at
1.2 V,
mA
Load,
⍀
Cutoff voltage, V
0.75 0.8 0.9 1.0 1.1 1.2 1.3
Hours
24 h /d 0.8 1500 3300 3200 3100 2400 2100 1800 1200
24 h /d 8 150 325 320 310 240 210 180 138
8 h /d 8 150 325 320 310 240 210 180 138
2 h /d 8 150 330 325 310 240 210 180 138
24 h /d 80 15 33 32 28 24 20 13 5
8 h /d 80 15 33 32 28 24 21 14 5
2 h /d 80 15 34 32 28 24 21 15 7
Minutes
24 h /d 800 1.5 120 115 86 54 25 12 3
10.5.2 Discharge Profile
The voltage profiles for the cylindrical and button alkaline-manganese dioxide batteries are
similar. The voltage starts above 1.5 V and decreases gradually during discharge. This occurs
because of the nature of the homogeneous-phase discharge of manganese dioxide, discussed
in Sec. 10.2. Figure 10.5 illustrates the sloping discharge characteristic of the alkaline-
manganese dioxide battery but that it is flatter than that of the zinc-carbon cell. While not
necessarily an advantageous characteristic, most devices that use cylindrical alkaline-
manganese dioxide batteries at low to moderate drains (radios, flashlights, toys, etc.) are
generally tolerant of this discharge characteristic. The sloping discharge can also be advan-
tageous because the gradual decrease in voltage gives the user advance warning of when the
battery is nearing the end of its useful life.
Other types of applications and devices that use miniature alkaline batteries are often less
tolerant of a sloping discharge profile. Many devices are designed for a particular type of
battery, such as zinc/silver oxide. These devices may not function properly when used with
miniature alkaline-manganese dioxide batteries which have a sloping discharge profile. But
for those devices that will tolerate the voltage profile of the alkaline-manganese dioxide
battery, it can provide an economical source of power.
Tables 10.7 and 10.8 present typical service for a particular size, the AA alkaline-
manganese dioxide battery, under various resistive loads and discharge schedules. Discharge
curves for the AA-size alkaline-manganese dioxide battery under continuous resistive dis-
charge conditions at 20
⬚C are shown in Fig. 10.6. The service to several cutoff voltages as
a function of the resistive load is summarized in Fig. 10.7.
Similar curves for constant-current discharges are presented in Fig. 10.8, which shows
the discharge curves at rates ranging from the C/3 to C /250. Figure 10.9 shows the hours
of service delivered to various cutoff voltages over a range of discharge currents. Figure
10.10 shows the capacity service hours of the AA-size and D-size batteries as a function of
the constant-current load to various cutoff voltages. The midpoint voltages for these dis-
charges are plotted in Fig. 10.11.
10.16 CHAPTER TEN
TABLE 10.8 Estimated Average Service at 21⬚C for Simulated Application Tests for an AA-Size
Alkaline-Manganese Dioxide Battery
Schedule
Typical drains at
1.2 V, mA
Load,
⍀
Cutoff voltage, V
0.75 0.8 0.9 1.0
Hours
Radio, 4 h /d 16 75 169 167 155 125
Calculator, 1 h / d 80 15 33 29 26 21
Cassette, 1 h / d 120 10 21 19 17 14
Minutes
Camera, 4 min / 15 min, 8 h/ d, 16 h rest 250 mA constant
current
368
Minutes
Portable light, 4 min /h, 8 h/ d, 16 h rest 308 3.9 404 372 338 292
Toy, continuous 308 3.9 425 399 339 273
Compact disk / TV, 1 h /d 308 3.9 446 404 348 282
Pulses
Pulse 15 s /min, 24 h / d 667 1.8 584
FIGURE 10.6 Typical discharge performance characteristics for AA-size
alkaline-manganese dioxide battery. (a) continuous moderate-drain discharge
at 20⬚C. (b) Continuous heavy-drain discharge at 21⬚C. (Courtesy of Eveready
Battery Company.)
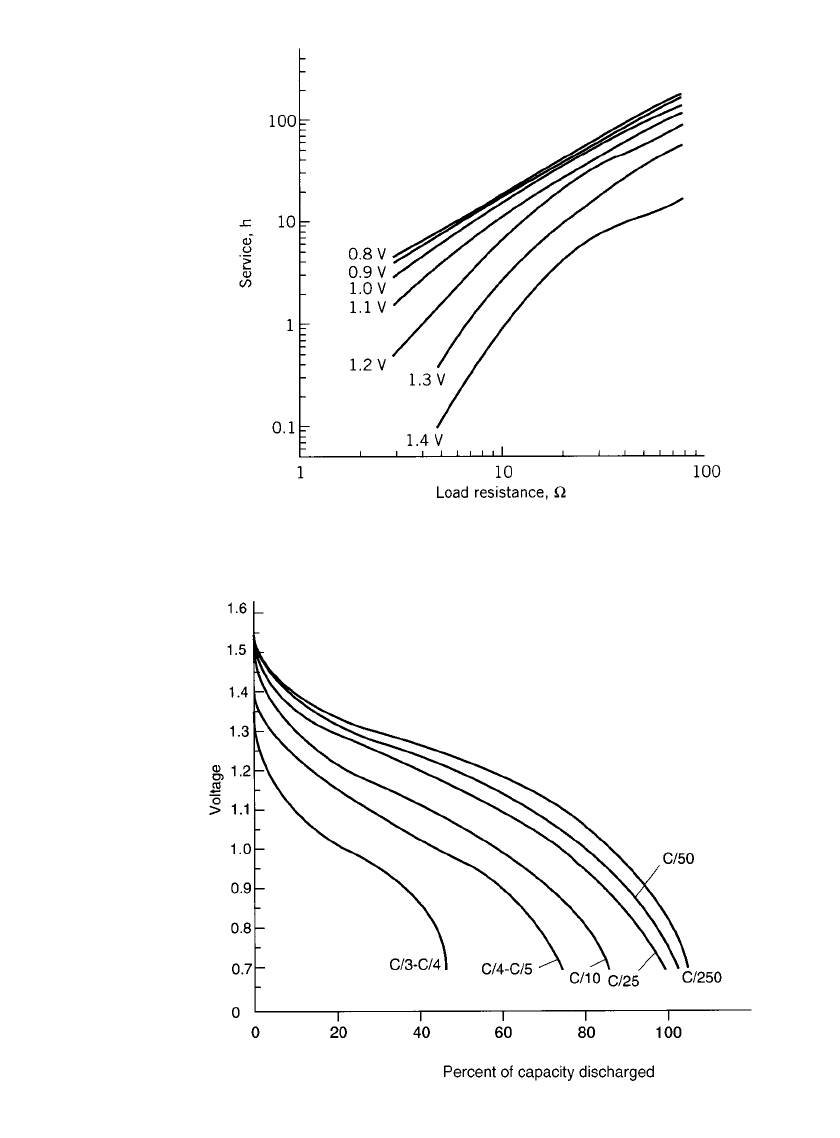
ALKALINE-MANGANESE DIOXIDE BATTERIES 10.17
FIGURE 10.7 Typical continuous discharge service to various cutoff
voltages at various loads for AA-size alkaline-manganese dioxide battery
at 21⬚C. (Courtesy of Eveready Battery Company.)
FIGURE 10.8 Typical constant-current discharge curves at 20⬚C for an AA-size
alkaline-manganese dioxide battery; voltage vs. percent of capacity at various dis-
charge rates. (Courtesy of Duracell, Inc.)
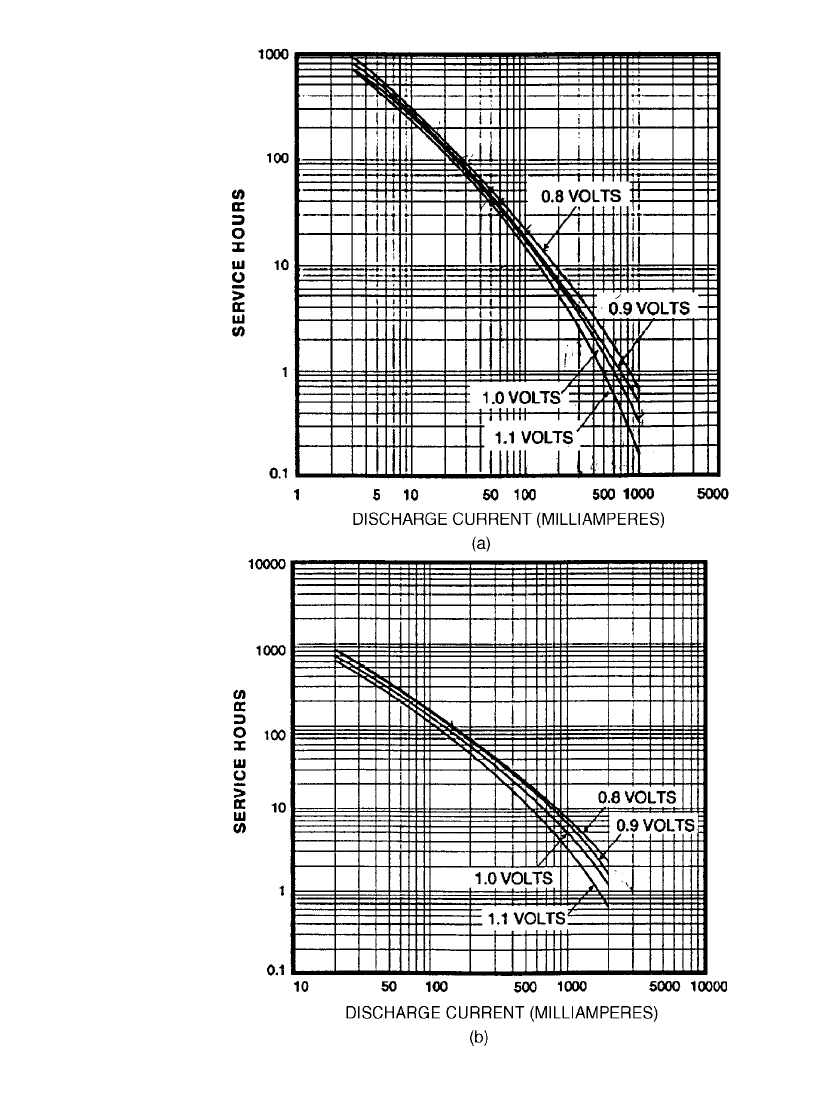
10.18 CHAPTER TEN
FIGURE 10.9 Typical constant-current discharge performance at 20⬚Cof
typical zinc-alkaline batteries; (a) AA-size battery. (b) D-size battery; service
to various cutoff voltages vs. discharge current. (Courtesy of Duracell, Inc.)
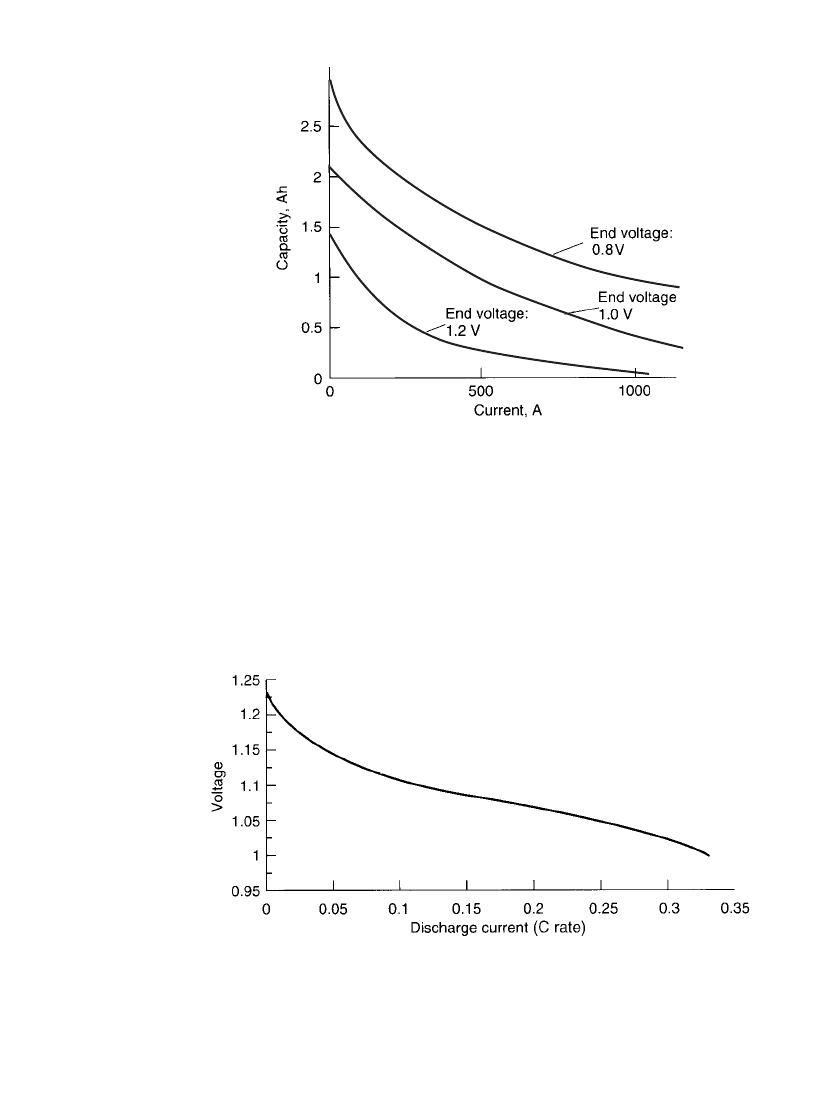
ALKALINE-MANGANESE DIOXIDE BATTERIES 10.19
3
:
FIGURE 10.10 Capacity of typical AA-size alkaline battery
on constant-current discharge at 20⬚C vs. current drain for var-
ious end-point voltages.
FIGURE 10.11 Typical midpoint voltages during discharge of a typical AA-size
alkaline battery at various discharge currents at 20⬚C, 0.8-V end voltage.
