Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

9.4 CHAPTER NINE
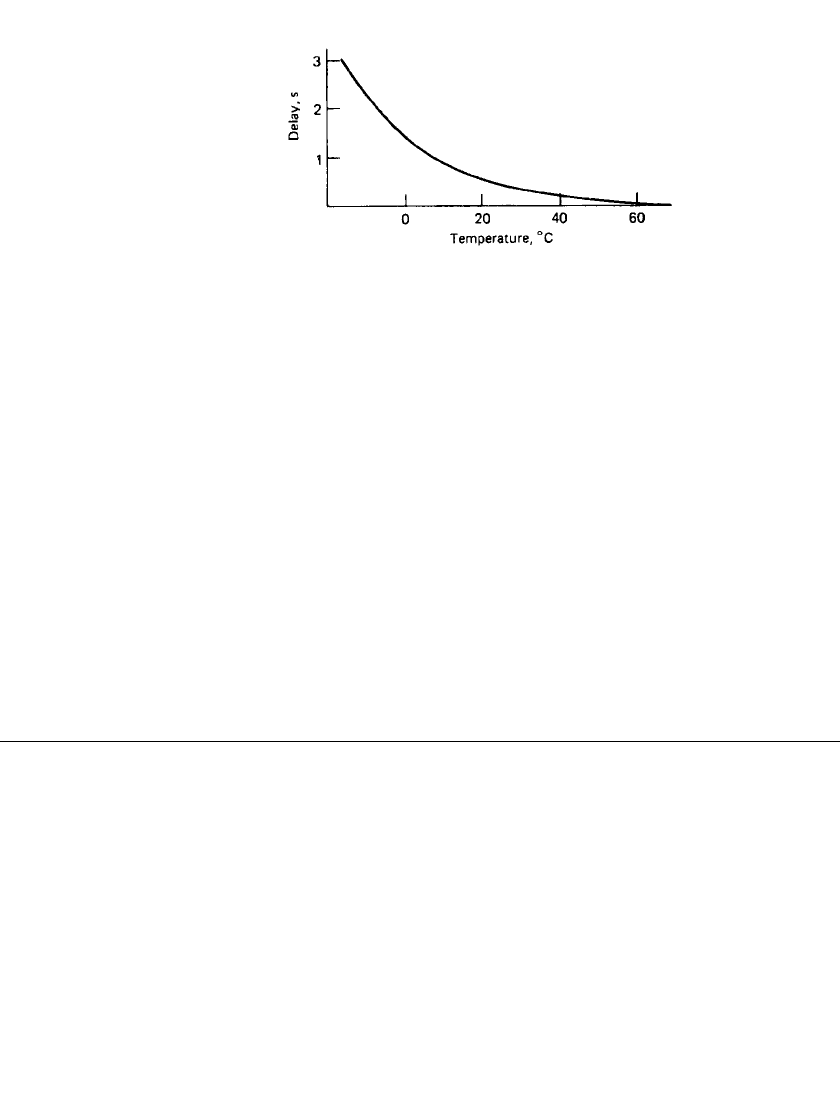
FIGURE 9.2 Voltage delay vs. temperature, Mg/ MnO
2
battery.
9.2.1 Aluminum
The standard potential for aluminum in the anode reaction,
3
⫹
Al → Al ⫹ 3e
is reported as
⫺1.7 V. A battery with an aluminum anode should have a potential about
0.9 V higher than the corresponding zinc battery. However, this potential is not attained, and
the potential of an Al /MnO
2
battery is only about 0.1 to 0.2 V higher than that of a zinc
battery. The Al/MnO
2
battery never progressed beyond the experimental stage because of
the problems with the oxide film, excessive corrosion when the film was broken, voltage
delay, and the tendency for aluminum to corrode unevenly. The experimental batteries that
were fabricated used a two-layer aluminum anode (to minimize premature failure due to can
perforation), an electrolyte of aluminum or chromium chloride, and a manganese dioxide-
acetylene black cathode similar to the conventional zinc/manganese dioxide battery. The
reaction mechanism is
Al
⫹ 3MnO ⫹ 3H O → 3MnO 䡠 OH ⫹ Al(OH)
22 3
9.3 CONSTRUCTION OF Mg /MnO
2
BATTERIES
Magnesium/ manganese dioxide (nonreserve) primary batteries are generally constructed in
a cylindrical configuration.
9.3.1 Standard Construction
The construction of the magnesium battery is similar to the cylindrical zinc-carbon battery.
A cross section of a typical battery is shown in Fig. 9.3. A magnesium alloy can, containing
small amounts of aluminum and zinc, is used in place of the zinc can. The cathode consists
of an extruded mix of manganese dioxide, acetylene black for conductivity and moisture
retention, barium chromate (an inhibitor), and magnesium hydroxide (a pH buffer). The
electrolyte is an aqueous solution of magnesium perchlorate with lithium chromate. A carbon
rod serves as the cathode current collector. The separator is an absorbent kraft paper as in
the paper-lined zinc battery structure. Sealing of the magnesium battery is critical, as it must
be tight to retain battery moisture during storage but provide a means for the escape of
hydrogen gas which forms as the result of the corrosion reaction during the discharge. This
is accomplished by a mechanical vent—a small hole in the plastic top seal washer under the
retainer ring which is deformed under pressure, releasing the excess gas.
8
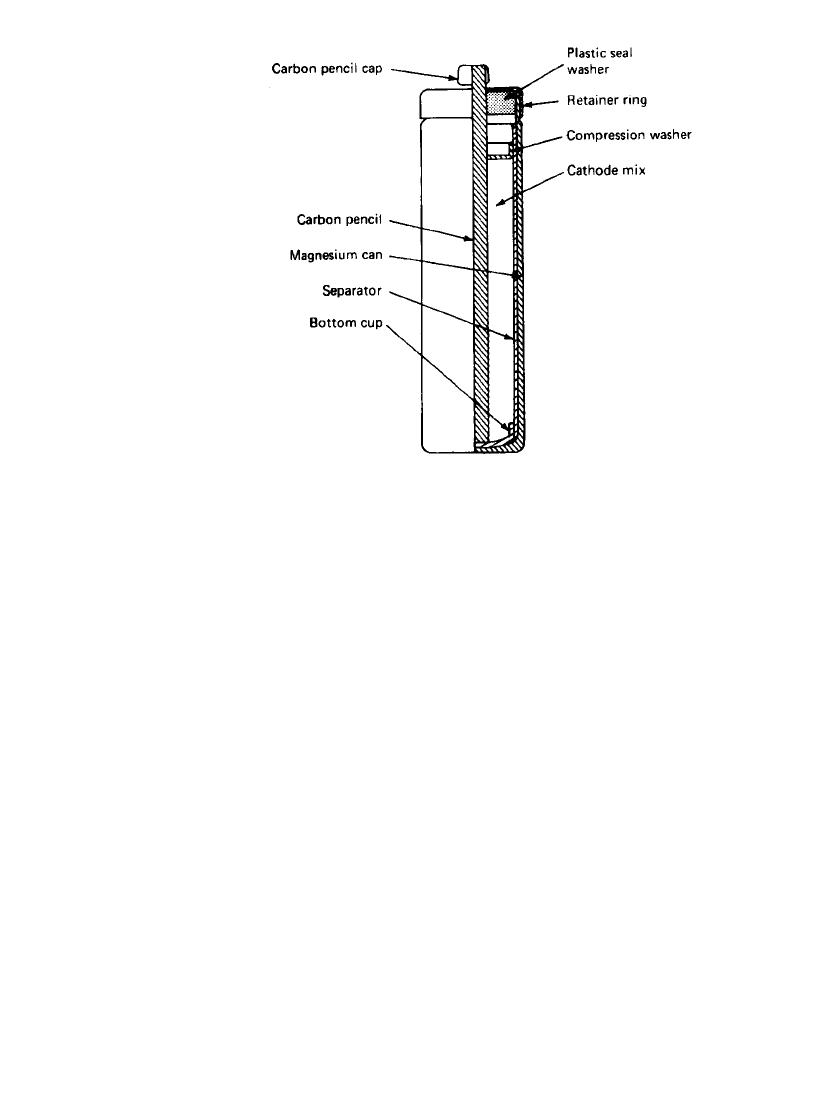
MAGNESIUM AND ALUMINUM BATTERIES 9.5
FIGURE 9.3 Cylindrical construction of magnesium primary
battery.
9.3.2 Inside-Out Construction
The basis of the inside-out design (Fig. 9.4) is a highly conductive carbon structure which
can be molded readily into complex shapes. The carbon structure is formed in the shape of
a cup which serves as the battery container; an integral center rod is incorporated to reduce
current paths. The cups are structurally strong, homogeneous, and impervious to the passage
of liquids and gases and the corrosive effects of the electrolyte. The battery consists of the
carbon cup, a cylindrical magnesium anode, a paper separator, and a cathode mix consisting
of manganese dioxide, carbon black, and inhibitors with aqueous magnesium bromide or
perchlorate as the electrolyte. The cathode mix is packed into the spaces on both sides of
the anode and is in intimate contact with the inside and outside surfaces of the anode, the
center rod, and the inside surfaces of the cup. This configuration provides larger electrode
surface areas. External contacts are made by two metallic end pieces. The positive terminal
is bonded during the forming process to the closed end of the carbon cup. The negative
terminal, to which the anode is attached, together with a plastic ring forms the insulated
closure and seal for the open end of the cup. The entire battery assembly is enclosed in a
crimped tin-plated steel jacket.
9–11
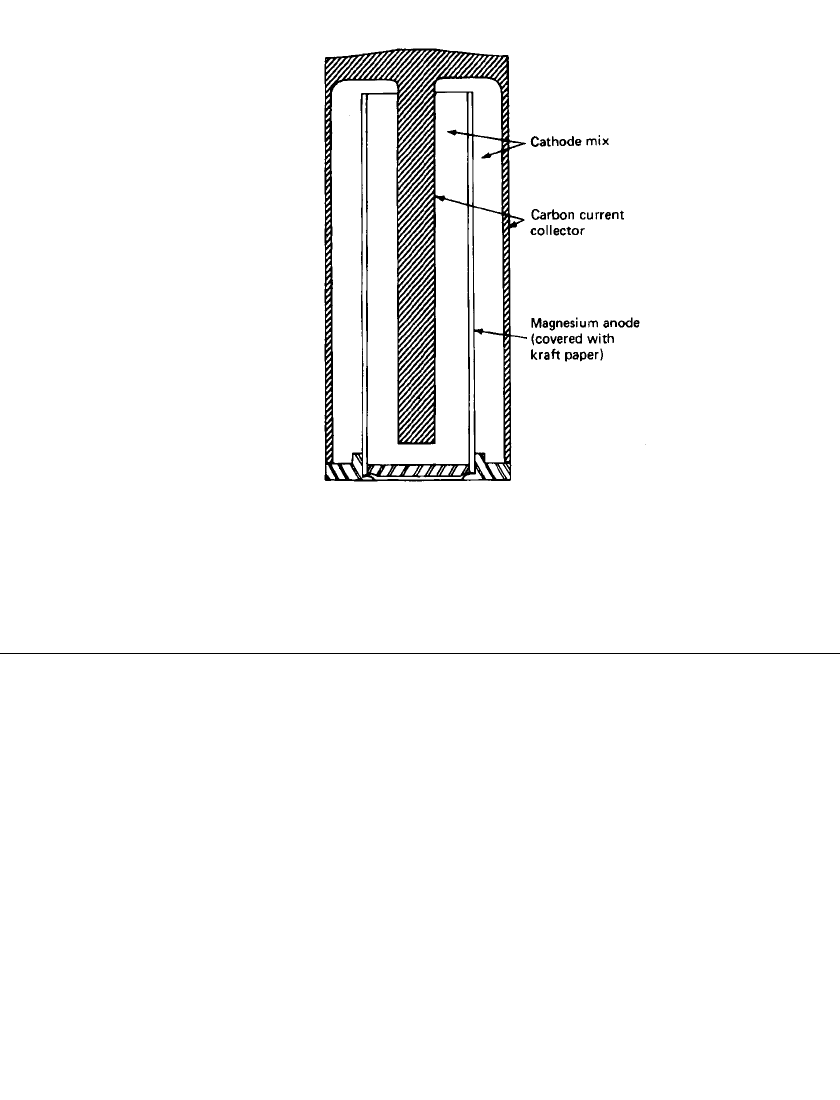
9.6 CHAPTER NINE
FIGURE 9.4 Inside-out construction of mag-
nesium primary battery. (Courtesy of ACR Elec-
tronics, Inc.)
9.4 PERFORMANCE CHARACTERISTICS OF Mg /MnO
2
BATTERIES
9.4.1 Discharge Performance
Typical discharge curves for the cylindrical magnesium /manganese dioxide primary battery
are shown in Fig. 9.5. The discharge profile is generally flatter than for the zinc-carbon
batteries; the magnesium battery also is less sensitive to changes in the discharge rate. The
average discharge voltage is on the order of 1.6 to 1.8 V, about 0.4 to 0.5 V above that of
the zinc-carbon battery; the typical end voltage is 1.2 V. The performance characteristics of
the cylindrical magnesium battery, type 1LM, on continuous and intermittent discharge are
summarized in Figs. 9.6 to 9.8 and Table 9.2. The batteries were discharged under a constant-
resistance load at 20⬚C.
Figure 9.6 provides a summary of the battery’s performance under continuous load to
1.1-V end voltage.
Figure 9.7 shows the relationship of discharge current to delivered ampere-hour capacity
of the battery on continuous constant-current discharge to several end voltages. The inter-
mittent discharge characteristics are illustrated in Table 9.2. The sizable reduction in per-
formance under low-rate or long-term discharge is attributed to the corrosion reaction be-
tween the discharging magnesium anode and the cell electrolyte. The reaction, which results
in the evolution of hydrogen and the concomitant reduction of water, causes a loss of total
cell efficiency. This phenomenon is illustrated in Fig. 9.8, which summarizes the ampere-
hour output under continuous discharge of the 1LM cell to an 0.8-V end voltage. This loss
of capacity on the low-rate (high-resistance) discharges is evident.
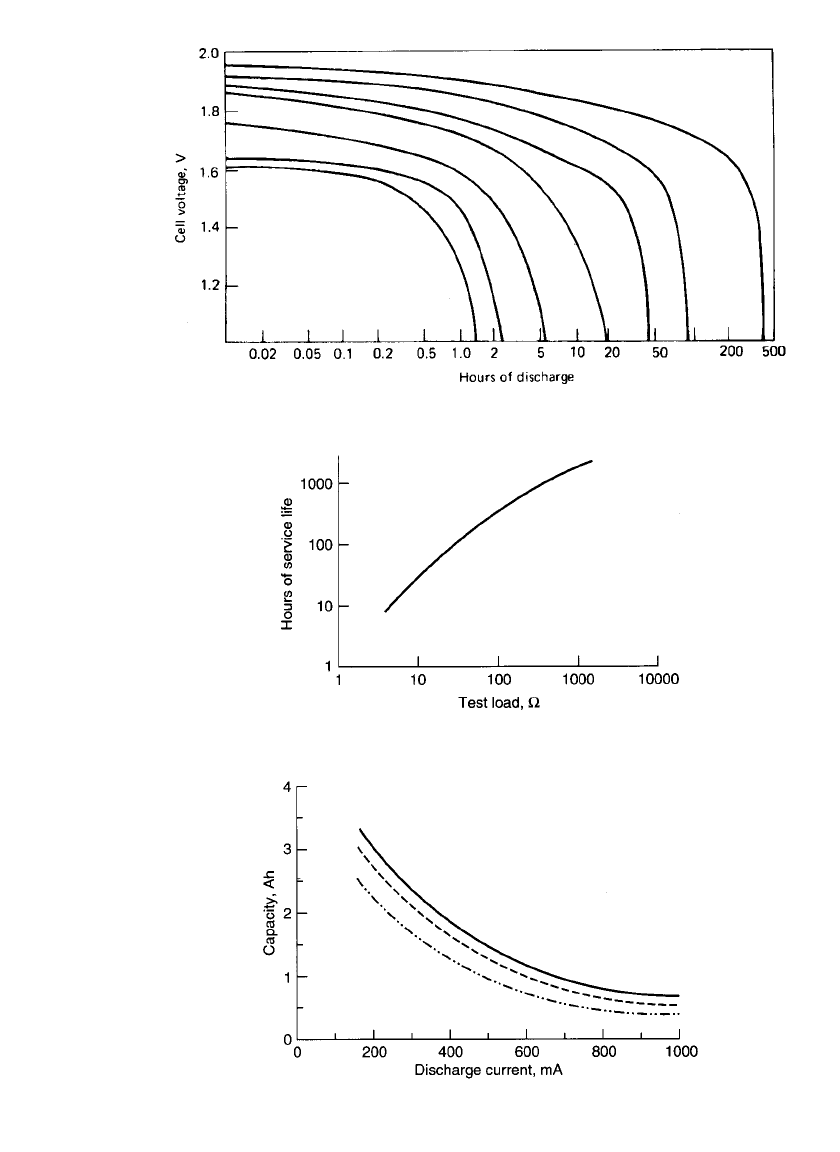
MAGNESIUM AND ALUMINUM BATTERIES 9.7
FIGURE 9.5 Typical discharge curves of magnesium / manganese dioxide cylindrical battery
at 20⬚C.
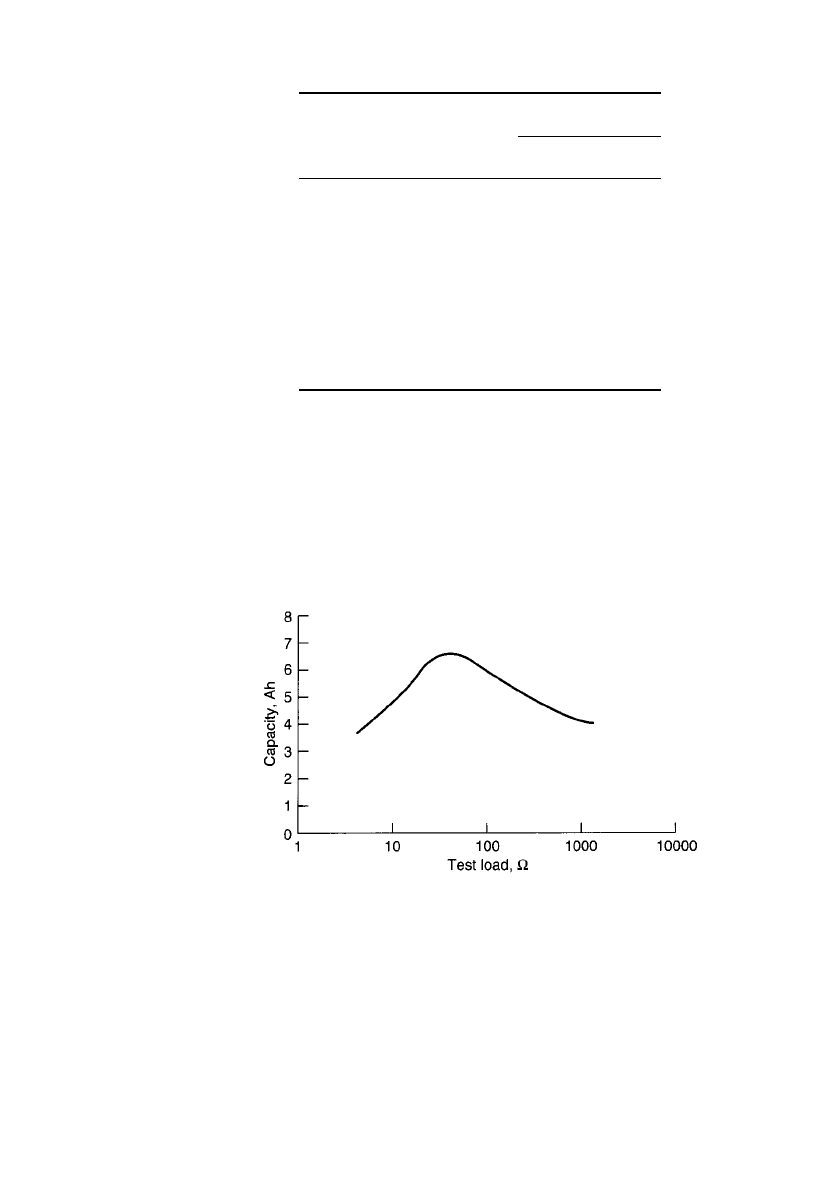
FIGURE 9.6 1LM service life (hours to 1.1 V) vs. test load
at room temperature. (Courtesy of Rayovac Corporation.)
FIGURE 9.7 1LM service life (ampere-hours) vs. constant-
current discharge. Dotted line—1.4-V end voltage; dashed line—
1.2-V end voltage; solid line—1.0-V end voltage. (Courtesy of
Rayovac Corporation.)
9.8 CHAPTER NINE
TABLE 9.2 Performance, in Hours, of 1LM
Batteries on Continuous and Intermittent Discharge
Type of Discharge
End voltage
1.1 V 0.8 V
4 ⍀, continuous 8.9 9.9
4
⍀, LIFT* 10.7 11.6
4
⍀, HIFT† 11 12
4
⍀, 30 min /h, 8 h / d 9.72 10.60
25-
⍀ constant resistance
Continuous 100 104
4 h /d 84.2 88.4
500-
⍀ constant resistance
Continuous 1265 1312
4 h /d 752 776
* Light industrial flashlight test, 4 min / h, 8 h / day.
† Heavy industrial flashlight test, 4 min / 15 mm, 8 h /
day.
Source: Rayovac Corporation.
FIGURE 9.8 1LM service life (ampere-hours to 0.8 V) vs. test
load. (Courtesy of Rayovac Corporation.)
MAGNESIUM AND ALUMINUM BATTERIES 9.9
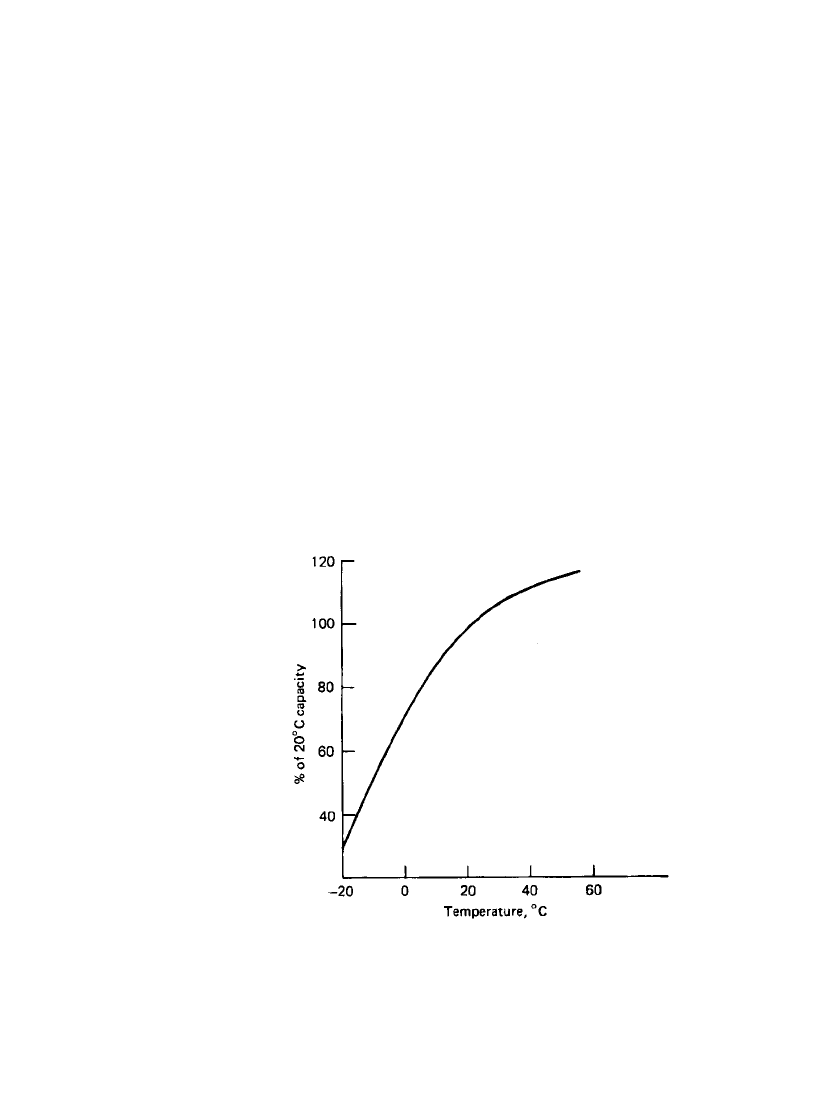
The performance of the magnesium primary battery at low temperatures is also superior
to that of the zinc-carbon battery, operating to temperatures of
⫺20⬚C and below. Figure 9.9
shows the performance of the magnesium battery at different temperatures based on the 20-h
discharge rate. The low temperature performance is influenced by the heat generated during
discharge and is dependent on the discharge rate, battery size, battery configuration, and
other such factors. Actual discharge tests should be performed if precise performance data
are needed.
On extended low-rate discharges, the magnesium battery may split open. This rupture is
due to the formation of magnesium hydroxide which occupies about one and one-half times
the volume of the magnesium. It expands and presses against the cathode mix which has
hardened appreciably from the loss of water during the discharge. This opening of the can
cause the voltage to rise about 0.1 V, also increasing capacity due to the air that can enter
into the reaction.
The service life of the magnesium/ manganese dioxide primary battery, normalized to
unit weight (kilogram) and volume (liter), at various discharge rates and temperatures is
summarized in Fig. 9.10. The data are based on a rated performance of 60 Ah/kg and
120 Ah /L.
FIGURE 9.9 Performance vs. temperature of
magnesium / manganese dioxide cylindrical battery.
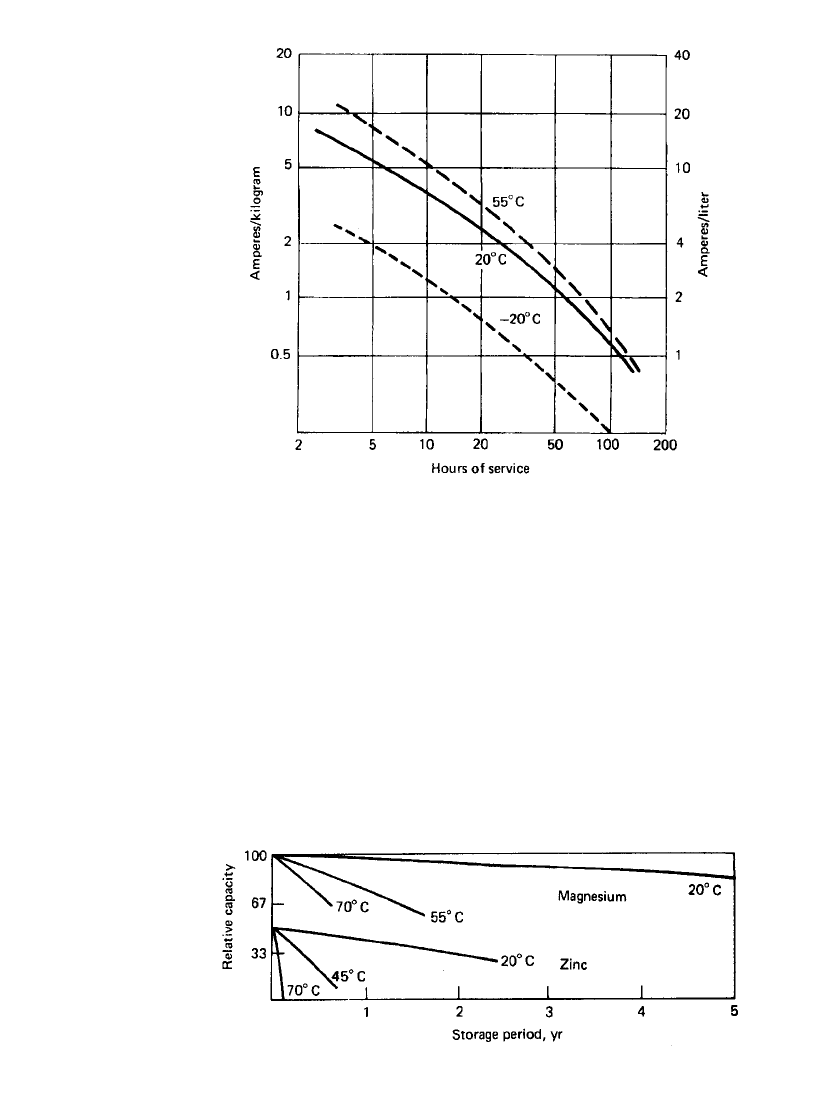
9.10 CHAPTER NINE
FIGURE 9.10 Service life of magnesium/ manganese dioxide pri-
mary battery at various discharge rates and temperatures (to 1.2-V / cell
end voltage).
9.4.2 Shelf Life
The shelf life of the magnesium /manganese dioxide primary battery at various storage tem-
peratures is compared with the shelf life of the zinc-carbon battery in Fig. 9.11. The mag-
nesium battery is noted for its excellent shelf life. The battery can be stored for periods of
5 years or longer at 20
⬚C with a total capacity loss of 10 to 20% and at temperatures as
high as 55
⬚C with losses of about 20% /year.
FIGURE 9.11 Comparison of service vs. storage of magnesium / manganese
dioxide and zinc-carbon batteries.
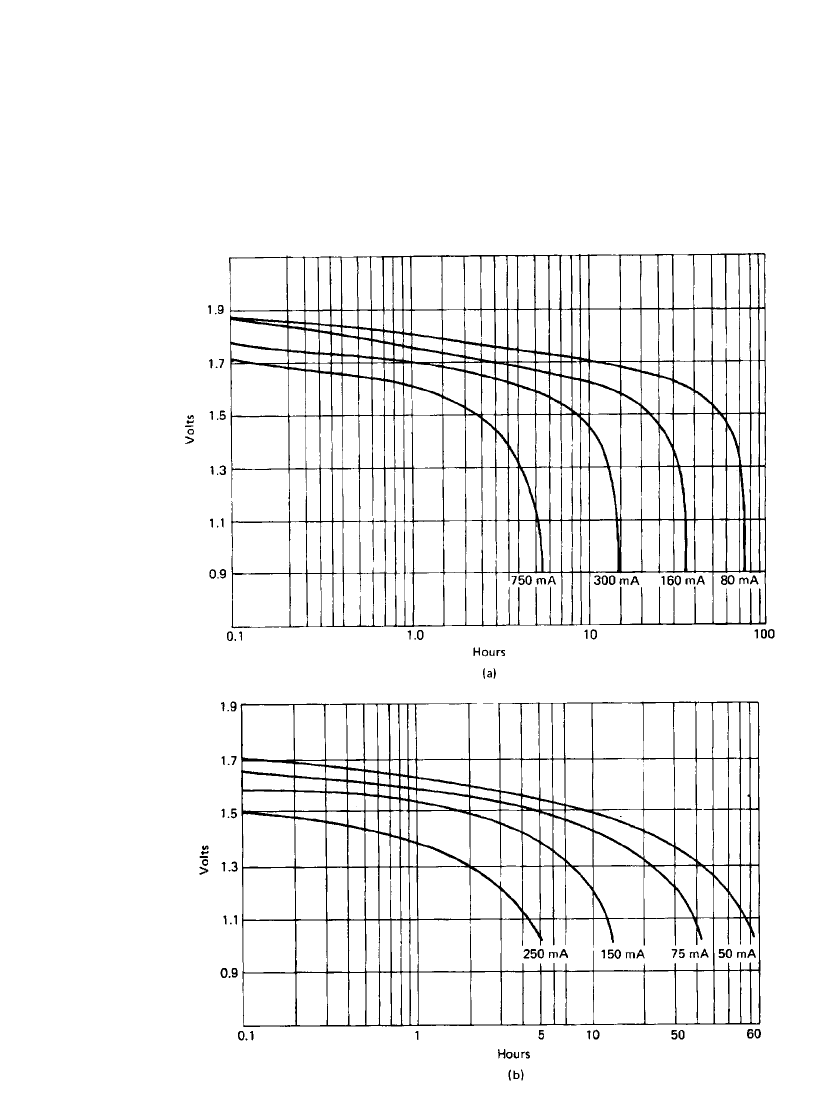
MAGNESIUM AND ALUMINUM BATTERIES 9.11
FIGURE 9.12 Typical discharge curves of magnesium inside-out primary battery, D
size. (a)20⬚C. (b) ⫺20⬚C. (Courtesy of ACR Electronics, Inc.)
9.4.3 Inside-Out Cells
The discharge characteristics of the cylindrical inside-out magnesium primary batteries are
shown in Fig. 9.12 for various discharge rates and at 20
⬚C and ⫺20⬚C. This structure has
better high-rate and low temperature performance than the conventional structure. These
batteries can be discharged at temperatures as low as
⫺40⬚C, although at lighter discharge
loads at the lower temperatures. Discharge curves are characteristically flat. They also have
good and reproducible low-drain, long-term discharge characteristics as they do not split
under these discharge conditions. Discharges for a 2
1
⁄
2
-year duration are realized with a
D-size battery at a 270-
A drain at 20⬚C.

9.12 CHAPTER NINE
9.4.4 Battery Design
Battery configuration has an important influence on the performance of the magnesium/
manganese dioxide battery because of the heat generated during the discharge. As discussed
in Sec. 9.2, proper battery design must allow for the dissipation of this heat to prevent
overheating, premature dry-out, and shortened performance—or for using this heat to im-
prove performance at low ambient temperatures. In some low-temperature applications it is
advantageous to insulate the battery against heat loss. Actual discharge tests will be required
to obtain precise performance data under a variety of possible conditions and battery designs.
The battery and equipment design must also consider the hydrogen that is generated
during discharge. The hydrogen must be vented and kept from accumulating because
hydrogen-air mixtures are flammable above 4.1% hydrogen and explosive above 18%
hydrogen.
9.5 SIZES AND TYPES OF Mg /MnO
2
BATTERIES
The cylindrical magnesium/manganese dioxide batteries were manufactured in several of
the popular standard ANSI sizes, as summarized in Table 9.3. Most of the production of the
conventional battery is used for military radio transceiver applications, and mainly in the
1LM size. The batteries are no longer available commercially. Inside-out batteries are no
longer manufactured.
TABLE 9.3 Cylindrical Magnesium Prmary Batteries
Battery type
Diameter,
mm
Height,
mm
Weight,
g
Capacity, Ah*
Conventional
structure†
Inside-out
cell‡
N
B
C
1LM§
D
FD
No. 6
11.0
19.2
25.4
22.8
33.6
41.7
63.5
31.0
53.0
49.7
84.2
60.5
49.1
159.0
5
26.5
45
59
105
125
1000
0.5
2.0
—
4.5
—
—
—
3.0
7.0
8.0
65
* 50-h discharge rate.
† Manufacturer: Rayovac Corp.
‡ Manufacturer: ACR Electronics, Inc., Hollywood, Fla. (no longer manufactured).
§ Only size now being manufactured.

MAGNESIUM AND ALUMINUM BATTERIES 9.13
9.6 OTHER TYPES OF MAGNESIUM PRIMARY BATTERIES
Magnesium primary batteries have been developed in other structures and with other cathode
materials, but these designs have not achieved commercial success. Flat cells, using a plastic-
film envelope, were designed but were never produced commercially.
The use of organic depolarizers, such as meta-dinitrobenzene (m-DNB), in place of man-
ganese dioxide was of interest because of the high capacity that could be realized with the
complete reduction of m-DNB to n-phenylenediamine (2 Ah/ g). The discharge of actual
batteries, while having a flat voltage profile and a higher ampere-hour capacity than the
manganese dioxide battery, had a low operating voltage of 1.1 to 1.2 V per cell. Watthour
capacities were not significantly higher than for the magnesium/manganese dioxide batteries.
The m-DNB battery also was inferior at low temperatures and high current drains. Com-
mercial development of these batteries never materialized.
Magnesium/ air batteries were studied, again because of the higher operating voltage than
with zinc (see Chap. 38). These batteries, too, were never commercialized. Magnesium,
however, is a very useful anode in reserve batteries. Its application in these types of batteries
is covered in Chap. 17.
9.7 ALUMINUM PRIMARY BATTERIES
Experimental work on Al /MnO
2
primary or dry batteries was concentrated on the D-size
cylindrical battery using a construction similar to the one used for the Mg/MnO
2
battery
(Fig. 9.3). The most successful anodes were made of a duplex metal sheet consisting of two
different aluminum alloys. The inner, thicker layer was more electrochemically active, leav-
ing the outer layer intact in the event of pitting of the inner layer. The cathode bobbin
consisted of manganese dioxide and acetylene black, wetted with the electrolyte. Aqueous
solutions of aluminum or chromium chloride, containing a chromate inhibitor, were the most
satisfactory electrolytes.
Aluminum active primary batteries were never produced commercially. While the exper-
imental aluminum batteries delivered a higher energy output than conventional zinc batteries,
anode corrosion, causing problems on intermittent and long-term discharges and irregularities
in shelf life, and the voltage-delay problem restrained commercial acceptance. Aluminum /
air batteries are covered in Chap. 38.
REFERENCES
1. J. L. Robinson, ‘‘Magnesium Cells,’’ in N. C. Cahoon and G. W. Heise (eds.), The Primary Battery,
vol. 2, Wiley-Interscience, New York, 1976, chap. 2.
2. G. R. Hoey and M. Cohen, ‘‘Corrosion of Anodically and Cathodically Polarized Magnesium in
Aqueous Media,’’ J. Electrochem. Soc. 105:245 (1958).
3. J. E. Oxley, R. J. Ekern, K. L. Dittberner, P. J. Spellman, and D. M. Larsen, in Proc. 35th Power
Sources Symp., IEEE, New York, 1992, p. 18.
4. B. V. Ratnakumar and S. Sathyanarayana, ‘‘The Delayed Action of Magnesium Anodes in Primary
Batteries. Part I: Experimental Studies,’’ J. Power Sources 10:219 (1983).
5. S. Sathyanarayana and B. V. Ratnakumar, ‘‘The Delayed Action of Magnesium Anodes in Primary
Batteries. Part II: Theoretical Studies,’’ J. Power Sources 10:243 (1983).
6. S. R. Narayanan and S. Sathyanarayana, ‘‘Electrochemical Determination of the Anode Film Resis-
tance and Double Layer Capacitance in Magnesium-Manganese Dioxide Cells,’’ J. Power Sources
15:27 (1985).
