Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.11
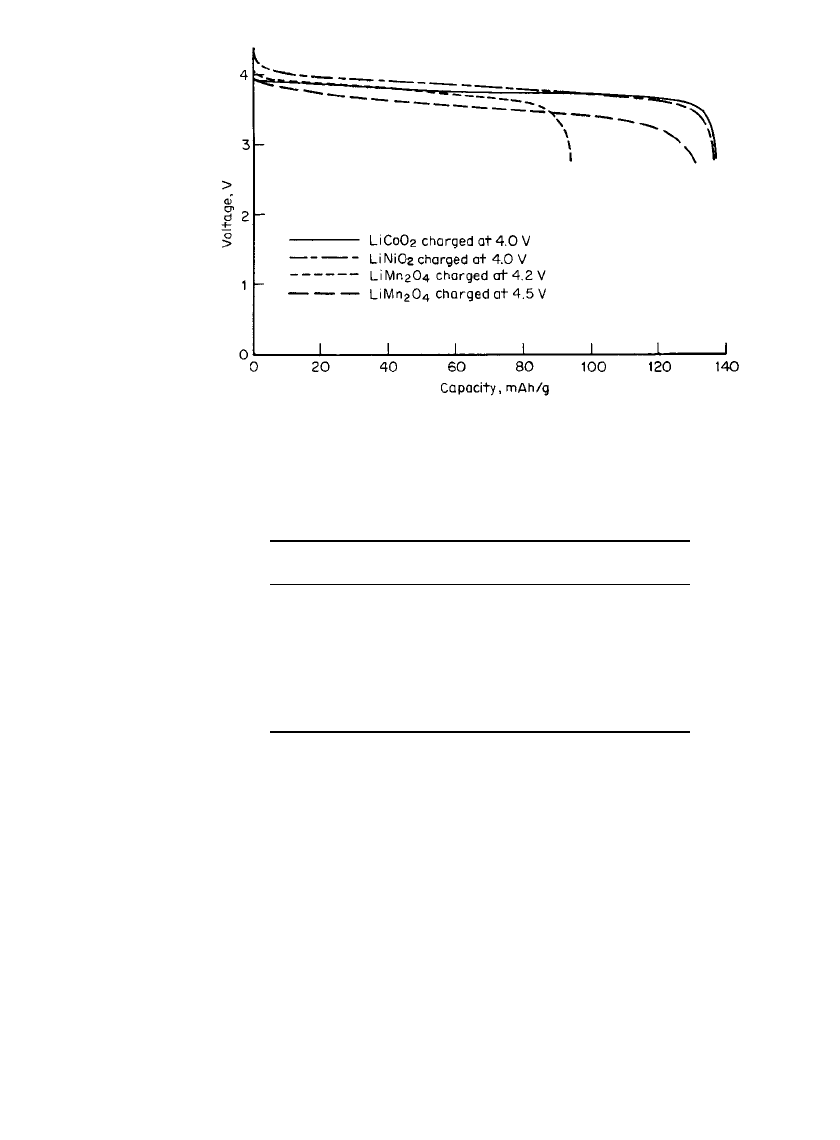
FIGURE 34.5 Reversible discharge capacity of cathode materials for lithium-
ion cells (cells fabricated with graphite anode). (Courtesy Yardney Technical
Products, Inc.)
TABLE 34.7 Diffusion Coefficients of Li-Ion in Lithiated
Transition Metal Oxides
Metal oxides Techniques D
Li
⫹
,cm
2
/s References
LiCoO
2
Transient
Transient
Impedance
5
⫻ 10
⫺
9
5 ⫻ 10
⫺
9
5 ⫻ 10
⫺
8
a
b
c
LiNiO
2
Impedance 2 ⫻ 10
⫺
7
d
LiMn
2
O
4
Transient
Transient
10
⫺
11
10
⫺
9
e
ƒ
a
K. Mizushima, P. C. Jones, P. J. Wieseman, and J. B. Good-
enough, Solid State Ionics 3/4:171, 1981.
b
S. Kikkawa, S. Miyazaki, and M. Koizumi, J. Power Sources 14:
231, 1985.
c
M. G. S. R. Thomas, P. Bruce, and J. Goodenough, Solid State
Ionics 18 / 19:794, 1986.
d
P. G. Bruce, A. Lisowska-Oleksiak, M. Y. Saidi, and C. A. Vin-
cent, Solid State Ionics 57:353, 1992.
e
P. G. Dickens and G. F. Reynolds, Solid State Ionics 5:53, 1981.
ƒ
D. Guyomard and J. M. Tarascon, J. Electrochem. Soc. 139:937,
1992.
Inorganic Electrolytes. Another choice of active materials for the cathode is the inorganic
electrolytes such as liquid SO
2
-based LiAlCl
4
or LiGaCl
4
. Reduction and oxidation occur
during the discharge-charge process on carbon surfaces. The cell reaction depends on the
type of carbon and electrolytes used. On relatively low-surface-area carbon such as acetylene
black, a simple SO
2
/S
2
couple is involved in the charge-discharge process,
⫽
O
4
discharge
⫽
—— —
2SO ⫹ 2e SO
———
2
24
charge
The reduction of SO
2
occurs at about 2.9 V at 1 mA/cm
2
.

34.12 CHAPTER THIRTY-FOUR
When high-surface-area carbons such as Ketjen black and SO
2
-based LiAlCl
4
electrolytes
are used,
11
a carbon-electrolyte complex is believed to take part in the discharge-charge
process.
Solid Cathodes. Solid-state couples can also be used as cathode materials in SO
2
-based
inorganic electrolytes. The high reduction potential of SO
2
limits the selection of the positive
electrode materials that can be used in SO
2
electrolytes to those compounds that reduce at
potential above 2.90 V. Thus CuCl
2
, which has an open-circuit voltage of 3.43 V and dis-
charges at about 3.35 V at 1 mA /cm
2
, corresponding to the reduction process of
discharge
2
⫹
—— —
Cu ⫹ e Cu
———
charge
meets these criteria. The second reduction process (Cu
⫹
⫹ e → Cu) occurs at 2.5 V. In
principle, the discharge of Li/CuCl
2
cells should not occur below 2.9 V, since the cathode
will be passivated by lithium dithionite (LiS
2
O
4
) produced from the reduction of SO
2
.
LiCoO
2
is being investigated in SO
2
-based inorganic electrolytes.
12,65,66
This cathode ma-
terial has a higher voltage and the SO
2
-based LiAlCl
4
electrolyte is potentially suitable
because of its wide electrochemical voltage window.
34.2.3 Electrolytes
The choice of electrolyte for rechargeable lithium batteries is also critical. The electrolyte
should have the following characteristics:
1. Good ionic conductivity (
⬎10
⫺
3
S/ cm from ⫺40 to 90⬚C) to minimize internal resistance.
2. Lithium ion transference number approaching unity (to limit concentration polarization)
3. Wide electrochemical voltage window (0 to 5 V)
4. Thermal stability (up to 70
⬚C)
5. Compatibility with other cell components
Aprotic Organic Electrolytes. Aprotic liquid organic electrolyte solvents, such as dioxo-
lane, propylene carbonate, ethylene carbonate, diethyl carbonate and ethylmethyl carbonate
are the most common electrolyte solvents because of their low reactivity with lithium. A list
of the electrolyte solvents used in rechargeable lithium batteries with their major character-
istics is given in Table 34.8.
8
Choices for the electrolyte solute and their ionic conductivities
in various solvents at different temperatures are listed in Table 34.9. These organic liquid
electrolytes generally have conductivities that are about two orders of magnitude lower than
aqueous electrolytes.
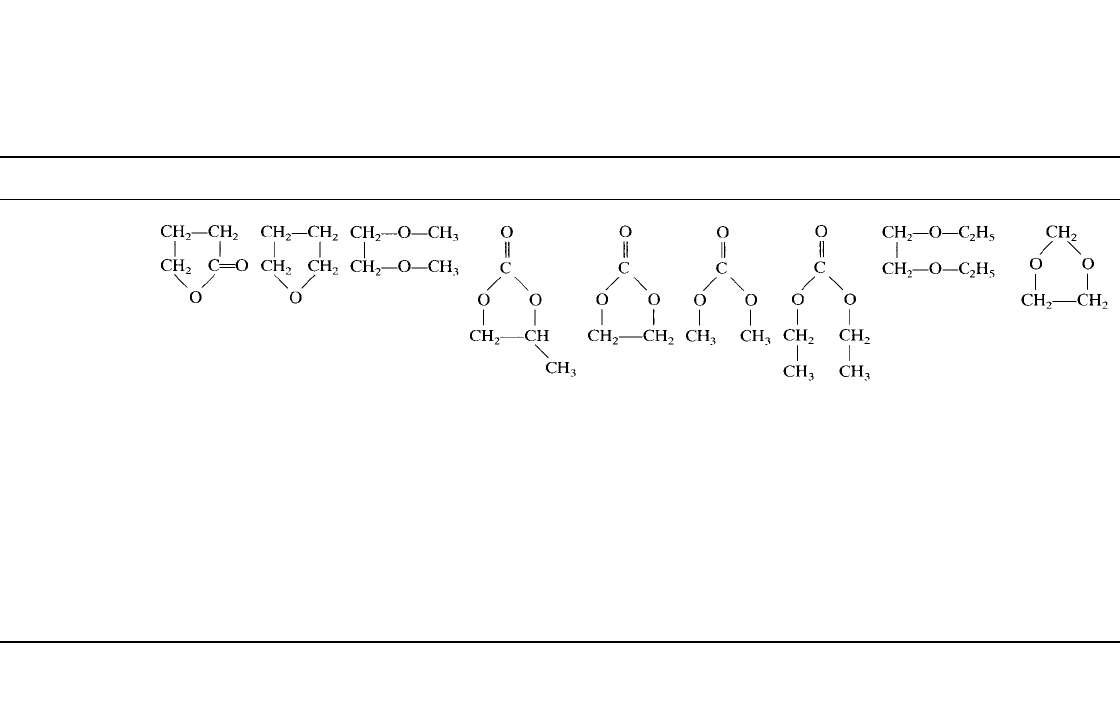
34.13
TABLE 34.8 Characteristics of Organic Solvents*
Characteristic
␥
-BL THF 1,2-DME PC EC DMC DEC DEE Dioxolane (DN)
Structural formula
Boiling point, ⬚C 202–204 65–67 85 240 248 91 126 121 78
Melting point,
⬚C ⫺43 ⫺109 ⫺58 ⫺49 ⫺39–40 4.6 ⫺43 ⫺74 ⫺95
Density, g /cm
3
1.13 0.887 0.866 1.198 1.322 1.071 0.98 0.842 1.060
Viscosity at 25
⬚C, cP 1.75 0.48 0.455 2.5 1.86
(at 40
⬚C)
0.59 0.75 0.65 0.58
Dielectric constant at
20
⬚C
39 7.75 7.20 64.4 89.6
(at 40⬚C)
3.12 2.82 5.1 6.79
Molecular weight 86.09 72.10 90.12 102.0 88.1 90.08 118.13 118.18 74.1
Typical H
2
O content,
ppm
⬍10 ⬍10 ⬍10 ⬍10 ⬍10 ⬍10 ⬍10 ⬍10 ⬍10
Electrolytic
conductivity at 20
⬚C,
1M LiAsF
6
, mS/cm
10.62 12.87 19.40 5.28 6.97 11.00
(1.9 M)
5.00
(1.5 M)
⬃10.00† ⬃11.20†
*
␥
-BL ⫽
␥
-butyrolactone; THF ⫽ tetrahydrofuran; 1,2-DME ⫽ 1,2-dimethoxyethane; PC ⫽ propylene carbonate;
EC
⫽ ethylene carbonate; DMC ⫽ dimethyl carbonate; DEC ⫽ diethyl carbonate; DEE ⫽ diethoxyethane.
† Estimation based on Walden’s rule.
Source: From Ref. 8.
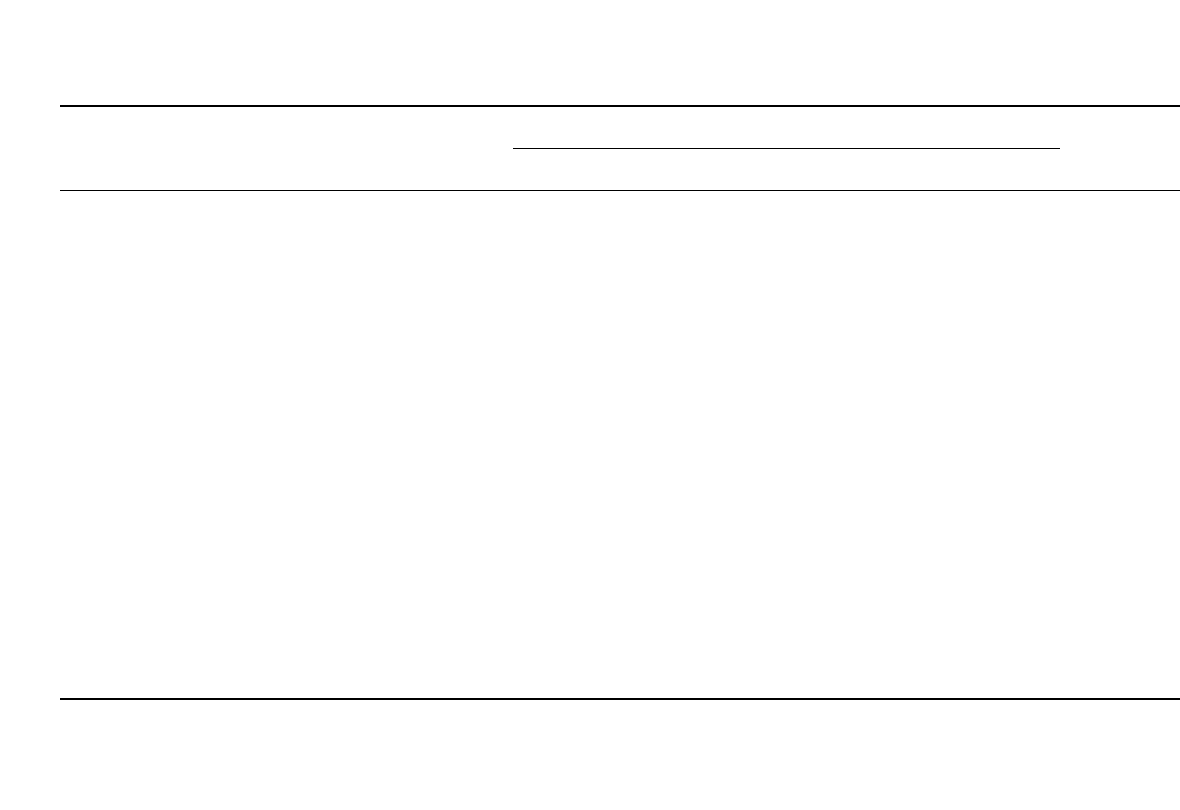
34.14
TABLE 34.9 Ionic Conductivity of Some 1 Molar Organic Liquid Electrolytes Used in Secondary Lithium Battery Systems
Salt Solvents
Solvent,
vol %
Conductivities at
⬚C, mS / cm
⫺40 ⫺20 ⫺0 20 40 60 80 References
LiPF
6
EC/PC
2-MeTHF/ EC / PC
EC/ DMC
EC/ DME
EC/ DEC
50/50
75/ 12.5 / 12.5
33/67
33/67
33/67
0.23
2.43
—
—
—
1.36
4.46
1.2
8.0
2.5
3.45
6.75
5.0
13.6
4.4
6.56
9.24
10.0
18.1
7.0
10.34
11.64
—
25.2
9.7
14.63
14.00
20.0
31.9
12.9
19.35
16.22
—
—
—
*
*
†
‡
‡
LiASF
6
EC/ DME
PC/ DME
2-MeTHF/ EC / PC
50/50
50/50
75/ 12.5 / 12.5
Freeze
Freeze
2.54
5.27
4.43
4.67
9.50
8.37
6.91
14.52
13.15
9.90
20.64
18.46
12.76
26.65
23.92
15.52
32.57
28.18
18.18
*
*
*
LiCF
3
SO
3
EC/PC
DME/PC
DME/PC
2-MeTHF/ EC / PC
50/50
50/50
50/50
75/ 12.5 / 12.5
0.02
—
—
0.50
0.55
2.61
Freeze
0.93
1.24
4.17
5.32
1.34
2.22
5.88
7.41
1.78
3.45
7.46
9.43
2.31
4.88
9.07
11.44
2.81
6.43
10.61
13.20
3.30
*
*
*
*
LiN(CF
3
SO
2
)
2
EC/PC
EC/ DME
PC/ DME
2-MeTHF/ EC / PC
50/50
50/50
50/50
75/ 12.5 / 12.5
0.28
—
—
2.07
1.21
Freeze
3.92
3.40
2.80
7.87
7.19
5.12
5.12
12.08
11.23
7.06
7.69
16.58
15.51
8.71
10.70
21.25
19.88
10.41
13.86
25.97
24.30
12.02
*
*
*
*
LiBF
6
EC/PC
2-MeTHF/ EC / PC
EC/ DMC
EC/ DEC
EC/ DME
50/50
75/ 12.5 / 12.5
33/67
33/67
33/67
0.19
—
—
—
—
1.11
0.38
1.3
1.2
6.7
2.41
0.92
3.5
2.0
9.9
4.25
1.64
4.9
3.2
12.7
6.27
2.53
6.4
4.4
15.6
8.51
3.43
7.8
5.5
18.5
10.79
4.29
—
—
—
*
‡
‡
‡
LiClO
4
EC/ DMC
EC/ DEC
EC/ DME
33/67
33/67
33/67
—
—
—
1.0
1.8
8.4
5.7
3.5
12.3
8.4
5.2
16.5
11.0
7.3
20.3
13.9
9.4
23.9
—
—
—
‡
‡
‡
* J. T. Dudley et al., J. Power Sources, 35:59–82, 1991.
† D. Guyomard and J. M. Tarascon, J. Electrochem. Soc. 140:3071–3081, 1993.
‡ S. Sosnowski and S. Hossain, unpublished results, Yardney Technical Products, Inc.
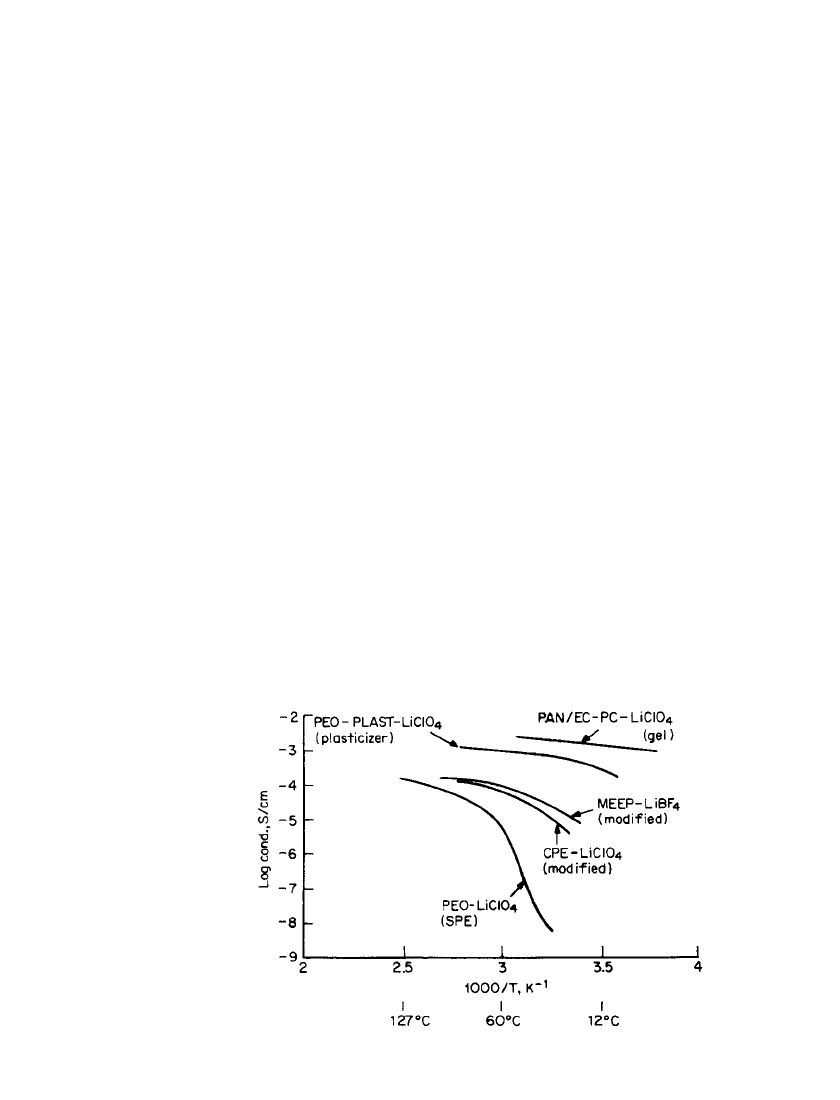
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.15
FIGURE 34.6 Variation of conductivity with temperature of dif-
ferent classes of polymer electrolytes. PEO ⫽ polyethylene oxide.
CPE ⫽ cross-linked polyvinylether. MEEP ⫽ poly[bis(methoxy eth-
oxy ethoxide)]. PAN ⫽ polyacrylonitrile.
Polymer Electrolytes. An alternative to the liquid electrolytes is a solid polymer electrolyte
(SPE) formed by incorporating lithium salts into polymer matrices and casting into thin
films. These can be used as both the electrolyte and separator. These electrolytes have lower
ionic conductivities and low lithium-ion transport numbers compared to the liquid electro-
lytes, but they are less reactive with lithium, which should enhance the safety of the battery.
The use of thin polymer films or operation at higher temperatures (60–100⬚C) compensate
in part for the lower conductivity of the polymer film. The solid polymers also offer the
advantages of a ‘‘nonliquid’’ battery and the flexibility of designing thin batteries in a variety
of configurations.
Initially, high-molecular-weight polymers such as polyethylene oxide (PEO) and lithium
salts such as LiClO
4
and LiN(CF
3
SO
2
)
2
were used.
13
These PEO-lithium salt electrolytes
have good mechanical properties but low conductivities, which are on the order of 10
⫺
8
S/cm at 20⬚C. A significant improvement in conductivity to approximately 10
⫺
5
S/cm has
been achieved with the combination of modified comb-shaped PEO structures with lithium
salts,
14
but these types of solid polymer electrolytes have poor mechanical properties and
their conductivity is still two orders of magnitude lower than that of most organic liquid
electrolytes. Further improvement in conductivity was obtained with the addition of liquid
plasticizers such as polypropylene carbonate.
15,16
The amount of plasticizer may be as high
as 70%, resulting in limited chemical and mechanical stability. Another class of polymer
electrolytes called ‘‘gelled’’ electrolytes has been developed by trapping liquid solutions of
lithium salts in aprotic organic solvents [for example, LiClO
4
in propylene carbonate-ethylene
carbonate (PC/EC) solvent] into a solid polymer matrix such as polyacrylonitrile (PAN).
17,18
The ‘‘gel’’ electrolytes are made by inserting liquid electrolyte solutions into polymer cages
with an immobilization procedure such as cross-linking, gellification, and casting. Cross-
linking may be carried out by ultraviolet, electron-beam, or gamma-ray irradiation. Conduc-
tivities as high as 10
⫺
3
S/cm at 20⬚C and transference number around 0.6 have been obtained.
However, these plasticized and gelled electrolytes are more reactive with lithium than true
solid polymers. The conductivites of the various classes of polymer electrolytes and their
variations with temperature are presented in Fig. 34.6.
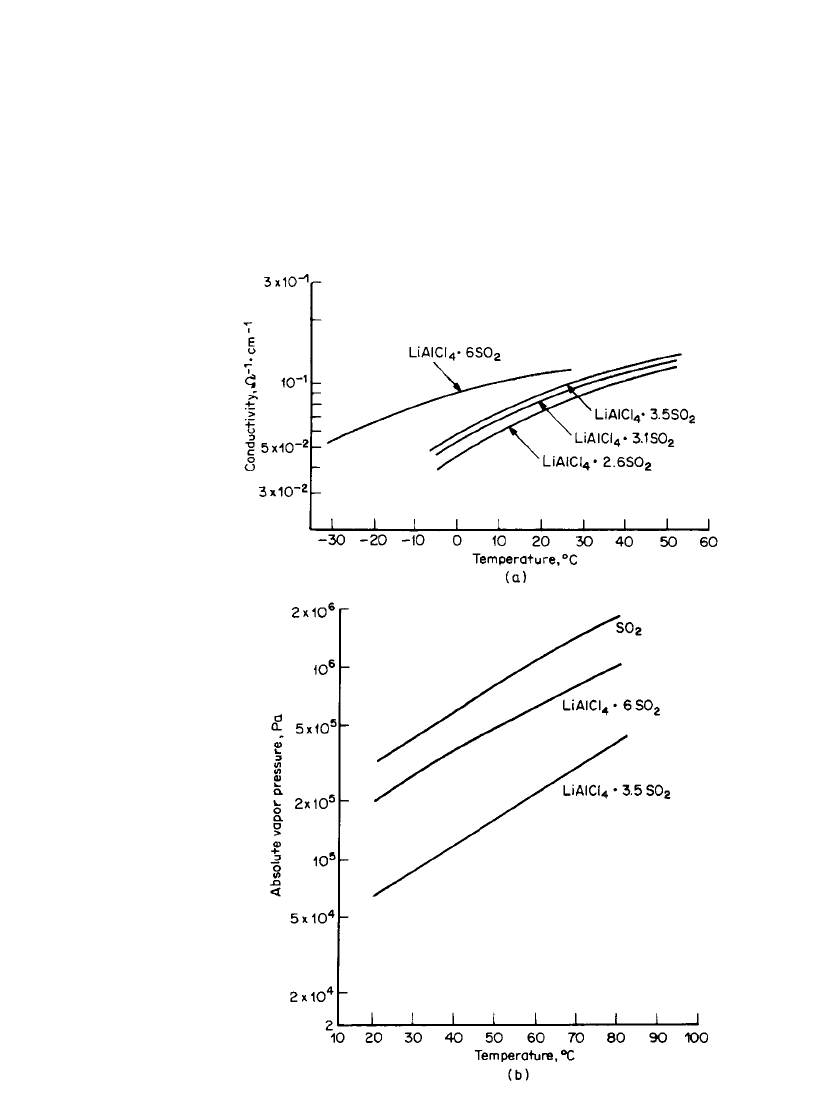
34.16 CHAPTER THIRTY-FOUR
FIGURE 34.7 (a) Conductivity of LiAlCl
4
/SO
2
electrolytes at various
temperatures. (b) Vapor pressure of SO
2
and SO
2
-based electrolytes.
Solid-State Electrolytes. True all solid-state ionic electrolytes such as lithium phosphorus
oxynitride (LiPON) provide adequate conductivity for use in thin film solid-state batteries
(see Sec. 35.8.)
Inorganic Electrolytes. The SO
2
-based inorganic electrolytes are another alternative for
use in rechargeable lithium batteries. These electrolytes are attractive because they offer the
highest ionic conductivity of any electrolyte used in rechargeable lithium batteries. Figure
34.7a shows the ionic conductivity of SO
2
-based LiAlCl
4
electrolytes at various tempera-
tures.
19

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.17
The SO
2
-based electrolytes, however, present potential safety problems. One disadvantage,
the high vapor pressure of SO
2
, can be reduced by adding electrolyte salts such as LiAlCl
4
and LiGaCl
4
. These form complexes exothermically with the SO
2
and reduce the vapor
pressure. For example, as shown in Fig. 34.7b, the vapor pressure of LiAlCl
4
䡠 3SO
2
is below
atmospheric pressure at 20
⬚C.
19
34.3 CHARACTERISTICS OF LITHIUM RECHARGEABLE BATTERIES
A number of different battery systems have been investigated for the development of lithium
rechargeable batteries in order to achieve the high specific energy and charge retention that
lithium batteries can offer without sacrificing other important characteristics, such as specific
power and cycle life, while maintaining safe and reliable operation. These approaches are
summarized in Fig. 34.1.
The rechargeable lithium batteries are generally characterized by a high cell voltage, good
charge retention, higher specific energy but poorer high-rate performance, and poorer cycle
life than conventional aqueous rechargeable batteries.
Rechargeable lithium metal batteries, because of their many potential advantages, have
been considered for use in a wide variety of applications. Because of the reactivity of lithium
and the possibility of safety problems, emphasis also has been placed on achieving safe
operation under normal and abusive conditions.
For these reasons, too, commercialization of rechargeable lithium batteries has been lim-
ited. They have been introduced into the market only on a limited scale and in small cell
sizes. Coin-type batteries have been commercially available for use in low-power portable
applications and as memory backup. Small cylindrical cells, using a lithium metal anode,
have been marketed briefly for consumer electronics applications but were withdrawn when
safety problems arose. Rechargeable lithium metal batteries (including ambient-temperature
as well as high-temperature types), because of their high energy density, have been investi-
gated for applications requiring larger size cells and batteries as, for example, electric ve-
hicles. More recently, the lithium-ion type battery was introduced into the consumer market,
again in small cylindrical and prismatic sizes for camcorders, cell phones and other portable
electronics. The lithium ion battery became the dominant rechargeable lithium system during
the 1990s.
34.3.1 Electrochemical Systems
The different types of ambient-temperature lithium metal rechargeable batteries have been
classified into four design categories. These are identified in Table 34.10, which lists rep-
resentative chemical systems and the key advantages and disadvantages of each class. The
components and chemical reactions and the performance characteristics of typical examples
of the five types are summarize and compared in Sec. 34.3.2.
Liquid Organic Electrolyte Batteries. These cells use lithium metal for the negative elec-
trode, a liquid organic aprotic solution for the electrolyte, and transition metal compounds
(oxides, sulfides, and selenides) for the positive electrode. These transition metal compounds
are insertion or intercalation compounds and possess a structure into which lithium ions can
be inserted or from which they can be removed during discharge and charge, respectively,
discharge
—— —
xLi ⫹ MB LiMB
———
zy
xzy
charge
where M
z
B
y
is the transition metal compound.
34.18 CHAPTER THIRTY-FOUR
TABLE 34.10 Classification of Rechargeable Lithium Metal Batteries (Ambient Temperature)
1. Liquid organic electrolyte cells (solid cathode cells using intercalation compounds for cathode, a
liquid organic electrolyte, and a metallic lithium anode):
High specific energy
Moderate-rate capability
Potential safety problems
Low cycle life
Low self-discharge rate
Examples: Li/ MoS
2
, Li/ MnO
2
, Li/TiS
2
, Li/ NbSe
3
, Li/V
2
O
5
, Li/ LiCoO
2
, Li/ LiNiO
2
2. Polymer electrolyte cells (cells using polymer electrolyte, intercalation compounds for cathode,
and lithium metal for anode):
High energy density
Safer design
Low electrolyte conductivity (poor high-rate capability)
Poor low-temperature performance
Low self-discharge rate
Examples: Li/ PEO-LiClO
4
/VO
x
and others
3. Inorganic electrolyte cells (liquid cathode cells using inorganic cathode materials which also
function as electrolyte solvent):
High energy density
High-rate capability
Excellent shelf life
Ability to sustain overcharge
Safety problems, toxicity
Capacity fade on cycling
Examples: Li/ SO
2
, Li/ CuCl
2
4. Lithium alloy cells (cells with lithium-alloy anodes, liquid organic electrolytes, and a variety of
cathodes):
Coin cell configuration
Lithium alloy considered safer than metallic lithium
Low energy density
Poor cycle life (except on shallow depth of discharge)
Examples: LiAl/ MnO
2
, LiAl/ V
2
O
5
, LiAl/C, LiC/V
2
O
5
, LiAl/ polymer
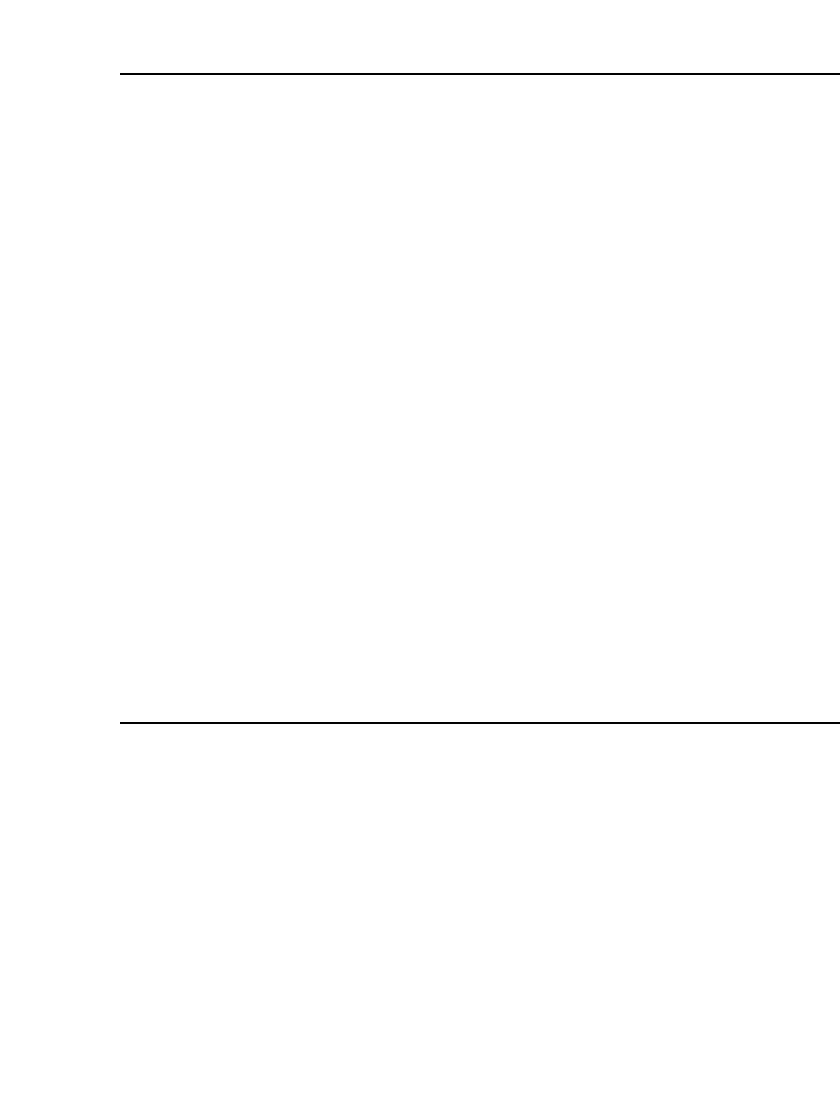
The overall discharge process is shown graphically in Fig. 34.8. Lithium ions formed at
the negative electrode during discharge migrate through the electrolyte, and are inserted into
the crystal structure of the host compound at the positive electrode. The electrons travel in
the external circuit to enter into the electronic band structure of the host compound. The
process is reversed during charge. The electrode reactions that occur during this process are
covered in Sec. 34.2.2.
These batteries have the highest energy density of any of the rechargeable lithium bat-
teries, the highest power density (with the exception of the SO
2
-based batteries), and a low
self-discharge rate, typically less than 1% per month. Safety, however, is a major concern
with this type of lithium battery. Most designs use a three- to five-fold excess of lithium in
order to obtain a reasonable cycle life to make up for the fact that the active lithium is lost
as it is stripped and replated during cycling. Further, the freshly formed porous high-surface-
area lithium that is formed during recharging is highly reactive and susceptible to forming
dendrites, which could cause internal short circuits.
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.19
FIGURE 34.8 Scheme of the discharge process of a
liquid organic electrolyte Li / M
z
B
y
cell. 䡩 ⫽ B; ● ⫽ M.
(From Ref. 8.)
The liquid electrolyte batteries were among the first lithium rechargeable batteries to be
investigated and the first to be introduced commercially. Small coin-type batteries were
marketed for use in small electronic devices and for memory backup. These batteries, how-
ever, use lithium alloys, which are less reactive than metallic lithium, for the negative elec-
trode. The small cylindrical batteries that were introduced briefly in the 1980s, on a limited
basis, were withdrawn as safety problems arose. In the 1990s, cylindrical AA batteries were
reintroduced, but have recently also been withdrawn from the market. Applications will
probably be limited to coin-type and other small-size batteries until a technological break-
through occurs.
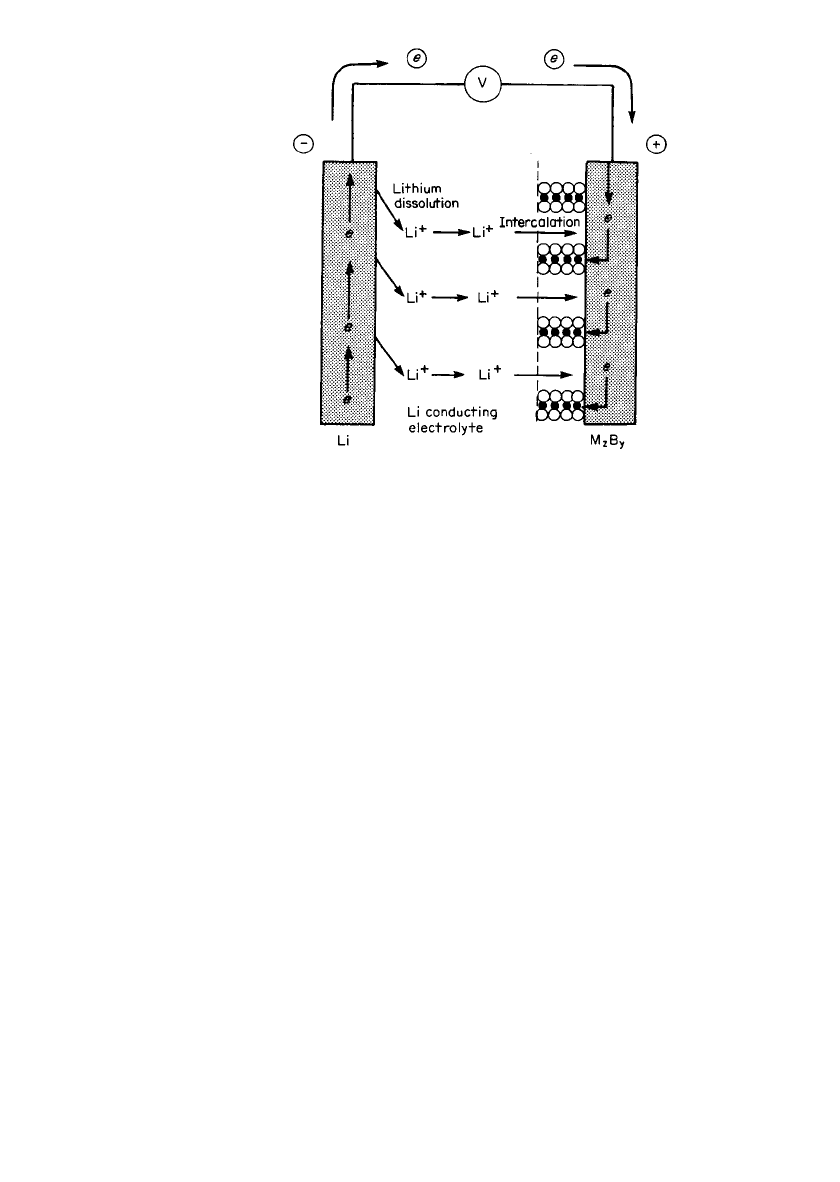
Polymer Electrolyte Batteries. The rechargeable lithium batteries which use solid polymer
electrolytes (SPE) are considered to have a safety advantage over the organic liquid electro-
lytes because of their lower reactivity with lithium and the absence of a volatile, flammable
organic solvent. In their most common form, as shown schematically in Fig. 34.9. these cells
use a lithium-ion conducting polymer membrane which acts both as the electrolyte and as
the separator, a thin lithium metal foil as the negative or anode material and a transition
metal oxide or chalcogenide, such as V
2
O
5
,TiS
2
,orVO
x
, blended with carbon and the
polymer electrolyte and backed by a metal-foil current collector as the positive electrode.
The basic structure can be represented as
Li/ PEO-LiX/ M B , PEO-LiX, C /M*
xy
where M
x
B
y
is the intercalation compound, PEO-LiX the polymer electrolyte, and M* the
current collector.
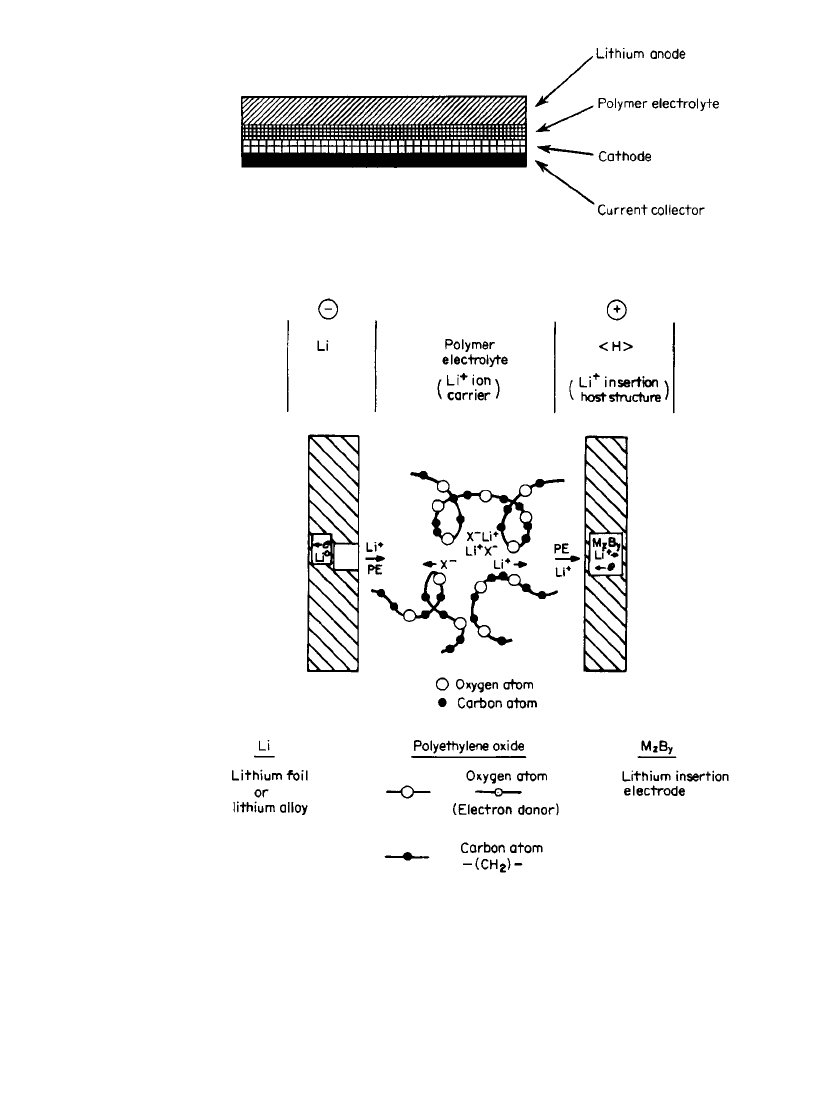
The cell reaction is similar to that in the liquid organic electrolyte cell—intercalation of
lithium into the structure of the cathode during discharge and deintercalation of lithium from
the charged cathode and deposition on the anode during charge,
discharge
—— —
xLi ⫹ MB Li M B
———
zy
xzy
charge
This process is shown schematically in Fig. 34.10.
34.20 CHAPTER THIRTY-FOUR
FIGURE 34.9 Schematic cross section of a solid polymer electrolyte
(SPE) cell. (Courtesy Valence Technology, Inc.)
FIGURE 34.10 Schematic of discharge process of a Li/ SPE / M
z
B
y
cell.
The ionic conductivity of most solid polymer electrolytes is significantly lower than that
of the liquid electrolytes. Cells must be designed with thin electrodes and cell components
to minimize the internal cell resistance. The total thickness of a cell assembly is as low as
200
m or thinner. An alternative is to operate at higher temperatures where the conductivity
is higher. While this may be acceptable for electric-vehicle and stand-by batteries, it will not
be acceptable for many portable consumer applications. Newer polymer electrolytes are being
developed using plasticizers or gel-type polymers. These methods increase the conductivity
of the polymers, but since they contain organic solvents, they will be more reactive with the
lithium anode.
