Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.21
One of the major problems of lithium polymer electrolyte systems is the development of
high interfacial resistance at the lithium/ polymer electrolyte interphase.
20
This resistance
grows with time and could be as high as 10 k
⍀ cm
⫺
2
.
21
This resistance layer is due to the
reactions of lithium with water, other impurities, and the salt anions. Similar to nonaqueous
electrolytes, the solid electrolyte interphase (SEI) also exists in the lithium/ polymer electro-
lyte systems.
22
In this case, the solid electrolyte interface (SEI) consists of the inorganic
reduction products of the polymer electrolyte and its impurities.
The capacity of a lithium polymer electrolyte battery decreases gradually on cycling. This
capacity loss is most likely related to degradation at the electrode interfaces due to repeated
plating-stripping cycles at the lithium metal anode and the repeated intercalation-
deintercalation cycles at the composite cathode. The cycling performance of lithium polymer
electrolyte cells can be improved by reducing the lithium passivation phenomena and by
improving the cathode morphology and homogeneity (particle size).
The energy density of the SPE batteries is projected to be close to that of the liquid-
electrolyte lithium rechargeable batteries. The thin cell design, however, usually requires a
larger percentage of materials of construction and nonreactive components, which tend to
lower the energy density. Larger electrode areas are also required for a given cell capacity
than with conventional battery design. The thin separator and larger electrode area could
adversely affect the cycle life, safety, and reliability of the battery by increasing the chance
for internal short circuits, lithium dendrite penetration, and other deleterious effects.
Another potential advantage of the solid polymer battery is that the design lends itself to
manufacture by automatic processes and the capability to be easily fabricated in a variety
of shapes and forms. Very thin batteries for cell phones, PDAs and similar applications can
be manufactured. At the other extreme, large thin plates can be manufactured and assembled
in multiplate prismatic or bipolar batteries.
Solid polymer batteries using lithium metal have not yet been introduced in the market-
place, and most of the performance characteristics are based on laboratory prototypes. The
technology offers several potential advantages compared to the other rechargeable lithium
batteries, including high energy density, low self-discharge rate, improved safety, and thin
cell design. The success of this approach will depend on the ability to achieve these advan-
tages, first in small cells and batteries and then to be able to scale up the technology to the
larger-size cells and multicell batteries required, for example, for electric vehicles. Lithium
ion batteries using polymer electrolytes have been introduced to the market in 2000.
Inorganic Electrolyte Batteries. The rechargeable lithium batteries with SO
2
-based inor-
ganic electrolytes are attractive because they can operate at high charge and discharge rates
due to the high ionic conductivity of the electrolyte. Other advantages are a high energy
density, a low self-discharge rate, the ability to accept limited overcharge and overdischarge,
and a wide electrochemical voltage window (5.0 V versus lithium).
Their drawback is the toxicity of the electrolyte and concern with safety problems. High-
capacity fade and relatively low capacity at low temperatures are other disadvantages.
Several types of batteries have been investigated using the SO
2
-based electrolyte cells
(with LiAlCl
4
as a solute) and metallic lithium for the negative electrode. These use either
carbon
11
or CuCl
2
26
for the positive electrode material.
With a high-surface-area carbon black such as Ketjen black, the SO
2
-based LiAlCl
4
elec-
trolyte forms a complex which is believed to take part in the discharge-charge process as

34.22 CHAPTER THIRTY-FOUR
During overcharge, more lithium is deposited from the electrolyte solution at the anode, and
the decomposition of AlCl
4
⫺
occurs at the cathode with the generation of free chlorine. This
free chlorine combines with metallic lithium to form LiCl, which subsequently recombines
with AlCl
3
and regenerates the electrolyte,
⫹
Li ⫹ e → Li
⫺
1
–
AlCl → AlCl ⫹ Cl ⫹ e
4322
1
–
Li ⫹ Cl → LiCl
22
LiCl ⫹ AlCl → LiAlCl
34
While this scheme provides an overcharge mechanism, the chloride generated will attack
the separator and compromise safety.
With CuCl
2
as the positive electrode material, the discharge-charge process involves a
simple redox reaction. The high reduction potential of SO
2
(2.9 V versus lithium on carbon
surfaces) limits the selection of positive electrode materials to only those compounds that
reduce at potentials above the reduction potentials of SO
2
. Thus CuCl
2
, which has an open-
circuit potential of 3.45 V and discharges at about 3.35 V, corresponding to the reduction
process of
2
⫹⫹
Cu ⫹ e → Cu
meets the criteria. The second reduction process,
⫹
Cu ⫹ e → Cu
occurs at about 2.5 V. In principle, the discharge of this Li/CuCl
2
cell in SO
2
-based elec-
trolytes should not be permitted beyond 2.9 V, below which the CuCl
2
electrode would be
passivated by the lithium dithionite produced by the reduction of SO
2
.
Although the SO
2
-based inorganic electrolyte system has been investigated for some time,
the toxicity and the corrosivity of the electrolyte present a potential safety hazard, restricting
commercial development. This system is also known to have severe explosive tendencies for
the reason cited above.
34.3.2 Summary of Design and Performance Characteristics
The different types of rechargeable lithium metal batteries operating at ambient temperature
are classified in four categories (see Table 34.10).
The cell components and the reaction mechanisms of typical cells for each of these
categories are presented in Table 34.11, and their characteristics are summarized in Table
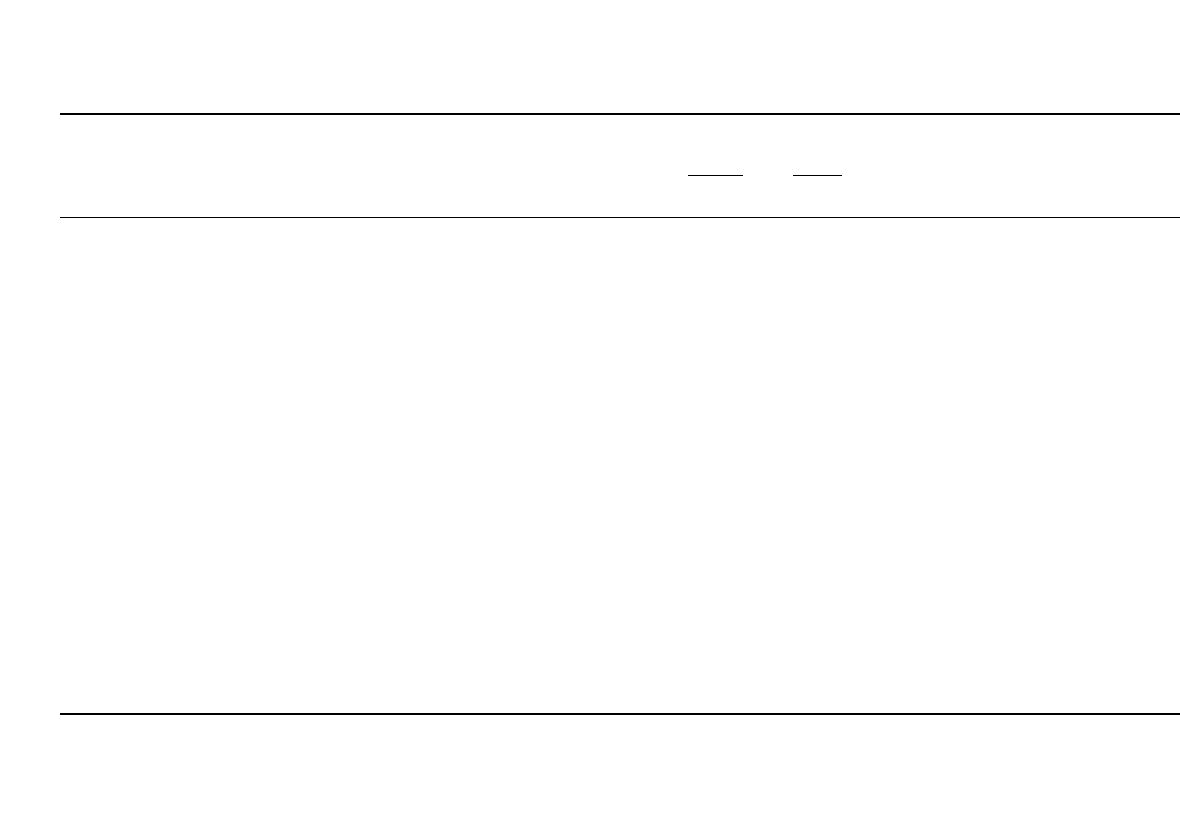
34.12. Typical discharge curves are shown in Fig. 34.11. Detailed electrical and performance
data are presented in Sec. 34.4.

34.23
TABLE 34.11 Chemistry of Rechargeable Lithium Systems
System Anodes Cathodes Electrolytes Separator
Cell reactions
discharge
—— —
———
冉冊
charge
1. Liquid organic electrolyte, solid
cathode cells:
Lithium molybdenum disulfide
(Li/ MoS
2
)
Lithium manganese dioxide
(Li/Li
0.3
MnO
2
)
Lithium titanium diosulfide
(Li/ TiS
2
)
Lithium niobium selenide
(Li/ NbSe
3
)
Lithium/ lithiated cobalt oxide
(Li/Li
x
CoO
2
)
Lithium/ lithiated nickel oxide
(Li/Li
x
NiO
2
)
Li
Li
Li
Li
Li
Li
MoS
2
Li
0.3
MnO
2
TiS
2
NbSe
3
Li
x
CoO
2
Li
x
NiO
2
LiAsF
6
, PC/EC
LiAsF
6
, DN,
Bu
3
N,
LiAsF
6
, 2-MeTHF,
THF
LiAsF
6
, PC/EC
LiAsF
6
/LiBF
4
,
MF/MA
LiAsF
6
, MF/MA
Polypropylene
Polypropylene
Polypropylene
Polypropylene
Polypropylene
Polypropylene
xLi
⫹ MoS
2
Li MoS
x 2
xLi ⫹ MnO
2
Li MnO
x 2
xLi ⫹ TiS
2
Li TiS
x 2
xLi ⫹ NbSe
3
Li NbSe
x 3
xLi ⫹ Li
1
⫺
x
CoO
2
LiCoO
2
xLi ⫹ Li
1
⫺
x
NiO
2
LiNiO
2
2. Polymer electrolyte cells:
Lithium/ vanadium oxide
(Li/PEO/VO
x
)
Lithium/ titanium disulfide
Li
Li
VO
x
TiS
2
SPE
SPE
—
—
yLi
⫹ VO
x
Li
y
VO
x
xLi ⫹ TiS
2
Li
x
TiS
2
3. Inorganic electrolyte cells:
Lithium/ sulfur dioxide
(Li/SO
2
)
Li Carbon (Ketjen
black)
LiAlCl
4
䡠 xSO
2
Tefzel
LiAlCl
4
䡠 3SO
2
⫹ xC ⫹ 3Li
Lithium/ copper chloride
(Li/ CuCl
2
)
Li CuCl
2
LiAlCl
4
䡠 xSO
2
Tefzel
Li
⫹ CuCl
2
LiCl ⫹ CuCl
4. Lithium alloy and other coin
cells:
Lithium-aluminum/ manganese
dioxide
(LiAl/ MnO
2
)
Lithium-aluminum/ vanadium
pentoxide
(LiAl/ V
2
O
5
)
Carbon-lithium cells
Lithium carbon/ vanadium
pentoxide
Lithium-aluminum/ polymer
LiAl
LiAl
Sn
x
Bi
y
Li/C
LiAl
MnO
2
V
2
O
5
Activated carbon
V
2
O
5
Polypyrrole (PP)
or
Polyaniline (PA)
LiAsF
6
,
EC/ BC/ DME
—
LiClO
4
,
PC
—
—
Polypropylene
Polypropylene
Polypropylene
Polypropylene
Polypropylene
LiAl
⫹ MnO
2
Li
x
MnO
2
⫹ Li
1
⫺
x
Al
LiAl ⫹ V
2
O
5
Li
x
V
2
O
5
⫹ Li
1
⫺
x
Al
Sn
x
Bi
y
⫹ nC ⫹ LiClO
4
LiSn
x
Bi
y
⫹ C
n
ClO
4
Li
y
C ⫹ V
2
O
5
Li
x
V
2
O
5
⫹ Li
y
⫺
x
C
LiAl ⫹ PP Li
x
PP ⫹ Li
1
⫺
x
Al
34.24
TABLE 34.12 Performance Characteristics of Rechargeable Lithium Metal Systems*
System
Midpoint
voltage,
V Size
Weight,
g
Capacity,
mAh
Specific
energy
Wh/kg
Energy
density
Wh/L
Self-
discharge† Cycle life‡
Liquid organic
batteries:
Li/ MoS
2
Li/Li
0.3
MnO
2
Li/TiS
2
Li/ NbSe
3
Li/ LiCoO
2
Li/ LiNiO
2
1.75
3.0
2.1
1.95
3.8
3.6
AA
AA
AA
AA
AA
D
21
17
20
21
21
105
600
800
900
1100
500
4500
50
140
95
100
95
155
135
270
235
270
235
325
1–2
1.25
1–2
1
See Table 34.14
—
200
200
250
250
50
See Fig. 34.20 (b)
Polymer electrolyte
batteries:
Li/SPE/VO
x
— — — — 97 110 — —
Li/ SPE/ S-based
polymer
950 310 350 10–15 200
Inorganic electrolyte
batteries:
Li/SO
2
3.0 AA 20 500 75 200 0.1 ⬎50
Li/ CuCl
2
3.2 AA 21 500 75 220 0.1 ⬎100
Lithium alloy batteries:
LiAl/ MnO
2
LiAl/ V
2
O
5
LiAl/ C
LiTiO
2
/LiMn
2
O
4
2.5
1.8
2.4
1.5
2430
2320
2320
1620
4.0
2.8
2.8
1.3
70
30
1.5
14
45
30
1.6
16
120
100
5.4
52
0.4
0.2
—
—
See Tables 34.19,
34.20
See Table 34.21
See Fig. 34.45,
Table 34.22
See Table 34.23
* Some data on experimental cells; see text.
† Self-discharge—% capacity loss per month at 20
⬚C (value-dependent on charge/ discharge conditions and cycling).
‡ Cycle life—to 80% capacity, 100% depth of discharge.
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.25
FIGURE 34.11 Typical discharge curves for rechargeable
lithium batteries.
34.4 CHARACTERISTICS OF SPECIFIC RECHARGEABLE LITHIUM
METAL BATTERIES
34.4.1 Liquid Organic Electrolyte, Solid-Cathode Batteries
These rechargeable batteries were attractive because they can deliver the highest energy
densities of all of the ambient-temperature lithium rechargeable batteries. They use a metallic
lithium anode, a liquid aprotic organic electrolyte, and one of several different cathode ma-
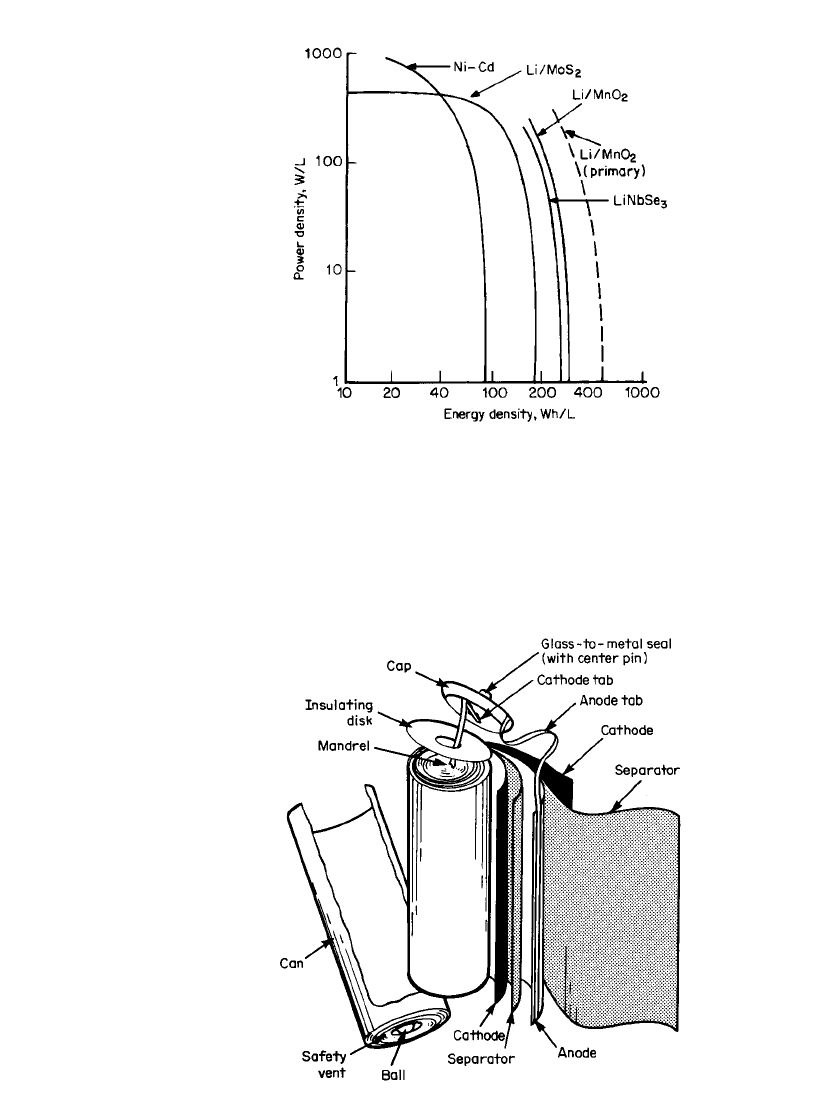
terials. Most of the development of these batteries has focused on the cylindrical spirally
wound construction and flat-plate prismatic designs in order to optimize rate capability. The
properties of these batteries, in cylindrical AA or comparable sizes, are compared in Table
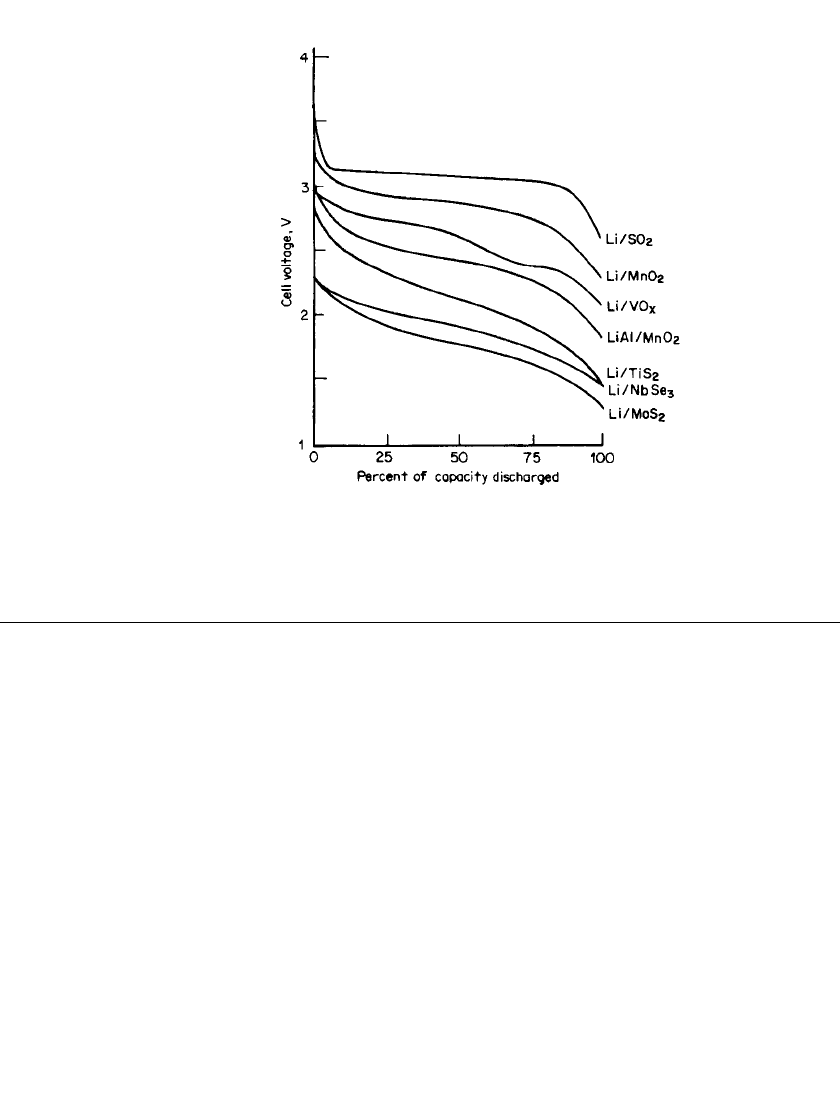
34.12. A plot comparing the energy density and power density of the different rechargeable
batteries and lithium/manganese dioxide primary batteries is given in Fig. 34.12. Although
several of these batteries were introduced commercially, they have not been viable in the
battery market.
Lithium/ Molybdenum Disulfide (Li/ MoS
2
) Batteries. The Li/ MoS
2
system was the first
rechargeable lithium battery to be manufactured when it was introduced in the mid-1980s
in a cylindrical AA-size.
27
The cell used thin lithium metal anodes (125
m), with a stoi-
chiometric excess of about three times, and MoS
2
on a thin aluminum foil (150
m) for the
cathode. A spirally wound construction, as illustrated in Fig. 34.13, was used. The electrolyte
was 1M LiAsF
6
dissolved in a 50:50 mixture of propylene carbonate and ethylene carbonate.
Two types of batteries were manufactured. Table 34.12 describes the more advanced of the
two.
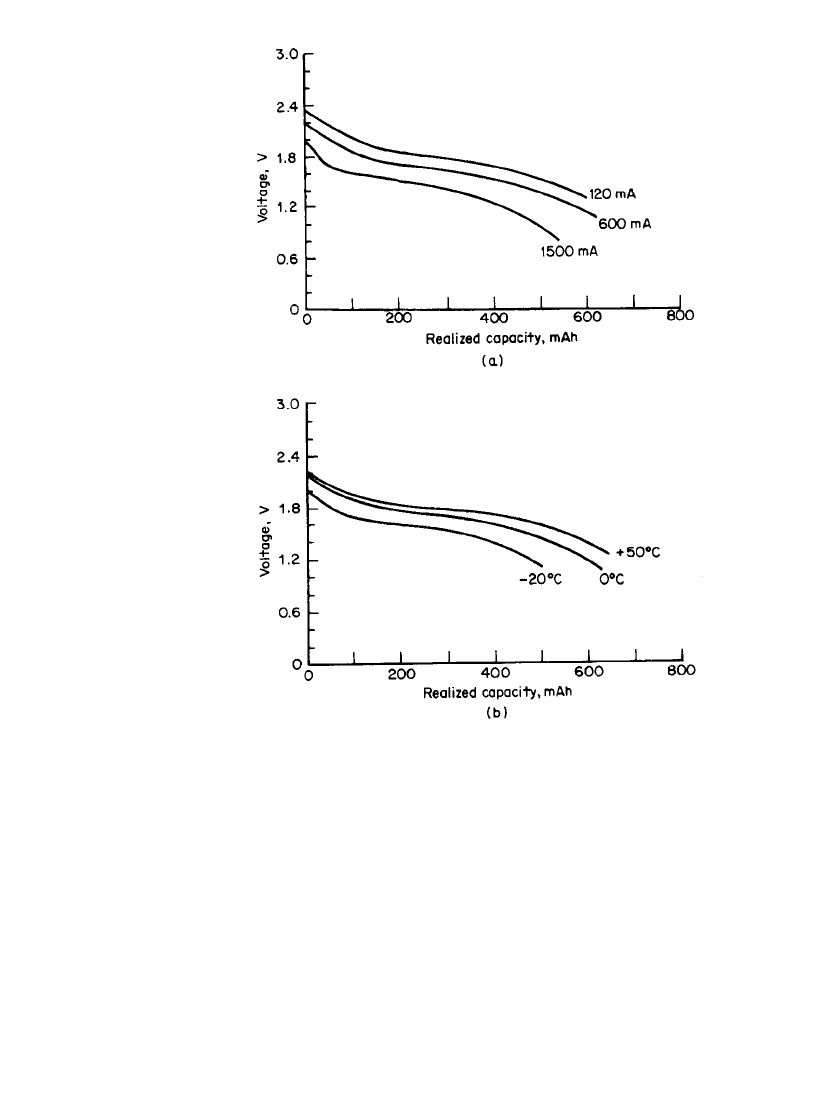
The discharge characteristics of the battery at various discharge loads temperatures are
shown in Fig. 34.14. The sloping voltage profile makes it necessary to discharge the battery
over a wide voltage range to obtain full capacity. However, because of this characteristic,
34.26 CHAPTER THIRTY-FOUR
FIGURE 34.12 Comparison of liquid organic electro-
lytes, solid cathode batteries.
FIGURE 34.13 Construction of the Li / MoS
2
cell. (Cour-
tesy Moli Energy, Ltd.)
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.27
FIGURE 34.14 Discharge characteristics of Li / MoS
2
battery. Bat-
tery charged at 20⬚C, 60 mA to 2.4 V. (a) Discharge characteristics
at 20⬚C. (b) Discharge characteristics at 120 mA. (From Moli Energy
Ltd.)
the voltage can be used as a state-of-charge indicator. The performance of the battery and
its cycle life depend on the manner in which it is discharged as shown in Table 34.13.
Discharging the cells to 0 V could result in irreversible damage due to a phase change of
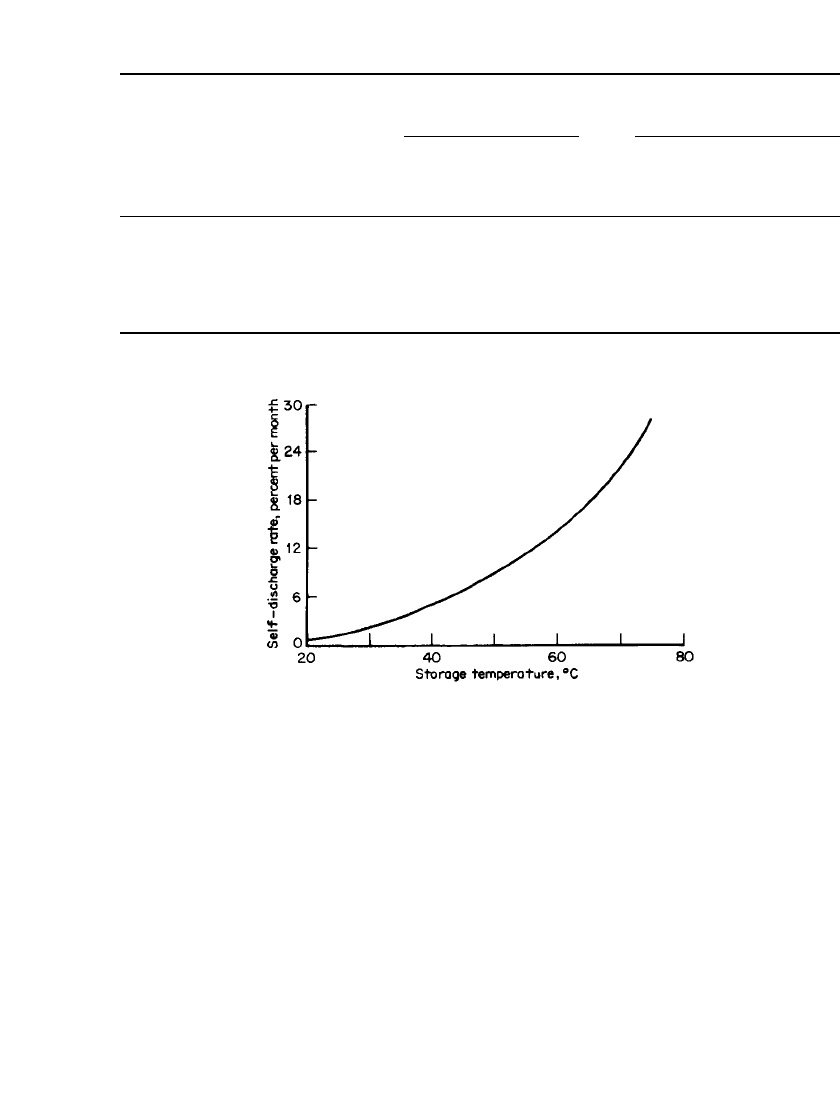
the cathode material, and low-voltage cutoff circuitry is recommended. The self-discharge
characteristics of the cell as a function of temperature are shown in Fig. 34.15. A constant-
current charge at the C/ 10 rate to a voltage limit of 2.2 or 2.3 V is the recommended charging
procedure. These batteries were withdrawn from the market after several safety incidents
occurred.
28
Lithium/ Manganese Dioxide (Li/Li
0.3
MnO
2
) Batteries. Rechargeable spirally wound cy-
lindrical AA-size cells using the Li/MnO
2
chemistry have also been developed.
29–35
The
same construction was used as with the Li/MoS
2
cells. A specially heat-treated Li
x
MnO
2
was used as the positive electrode material with carbon black. Commercial cells used an
electrolyte of 1M LiAsF
6
in dioxolane with added tributylamine as a stabilizer.
34.28 CHAPTER THIRTY-FOUR
TABLE 34.13 Performance Characteristics of Li / MoS
2
AA-Size Batteries
User
mode
Voltage
limits
10th cycle
Capacity,
Ah
Average
energy,
Wh
Cycle life,
number of cycles
To 80% of
10th-cycle
capacity
To 50% of
10th-cycle
capacity
High capacity 2.6–1.1 0.82 1.52 100 110
2.4–1.1 0.70 1.23 180 200
Normal 2.4–1.3 0.60 1.10 400 500
High cycle life 2.1–1.6 0.40 0.74 800 1000
1.95–1.75 0.15 0.27 1800 3000
Source: Moli Energy Ltd.
FIGURE 34.15 Self-discharge characteristics of Li/ MoS
2
AA-
size battery. (From Moli Energy, Ltd.)
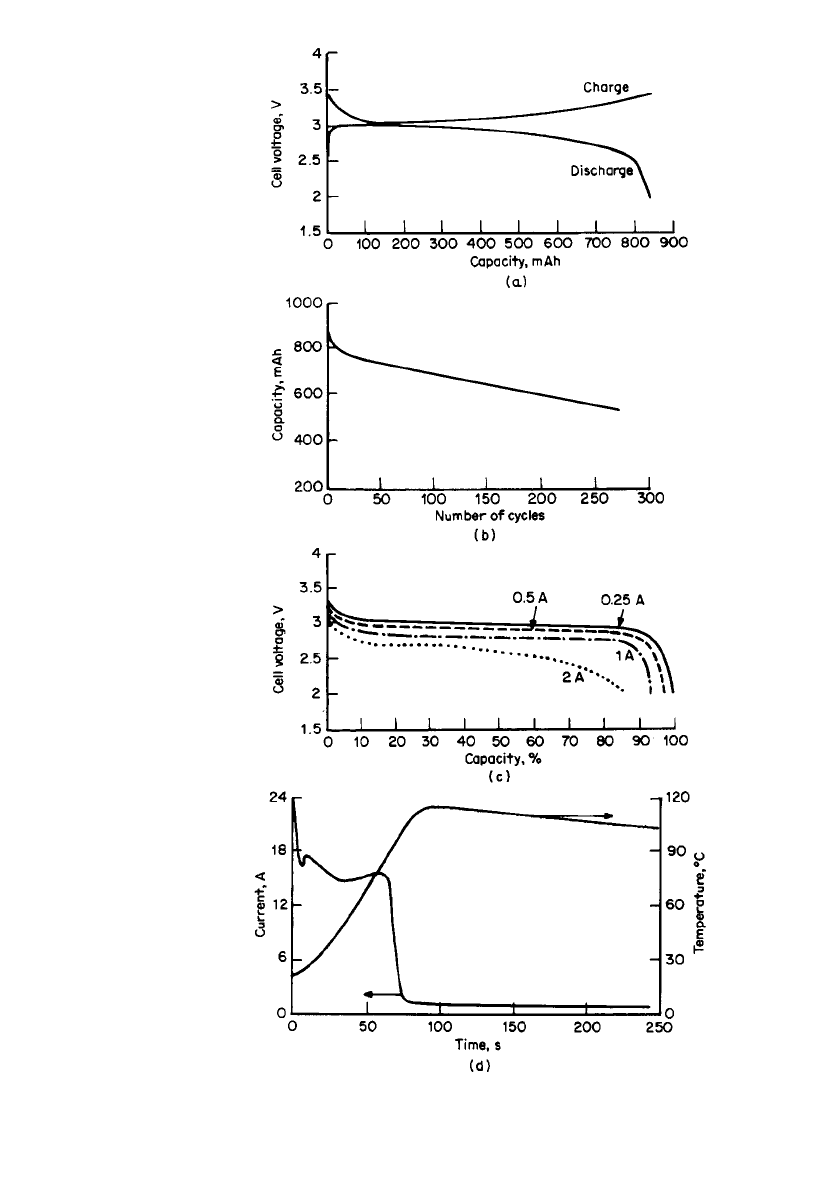
Some of the performance characteristics of this AA-size battery are listed in Table 34.12
and are plotted in Fig. 34.16. The discharge profile is fairly flat with a midpoint voltage, at
moderate discharge rates, close to 3 V. The cell can be discharged at current levels of up to
about 2A, to a 2.0 V cut-off. The self-discharge rate is low, and is typical of the self-discharge
rate of many of the lithium metal /organic electrolyte rechargeable cells. Two hundred cycles
were obtained with a C /3 discharge and a C /12.5 charge rate when cycled to 100% depth
of discharge (DOD). Three hundred to 350 cycles can be obtained to 65% of initial capacity
at 100% DOD when discharged at 250 mA and changed at 80 mA to 3.40 V.
These AA-size batteries with lithium metal anodes were introduced into the consumer
market, with claims of safe performance under abusive electrical conditions due to an in-
trinsic shut-down mechanism involving polymerization of the electrolyte which protects the
cell under short circuit and overcharge (shutdown at 4.0 V).
33,34
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.29
FIGURE 34.16 Performance characteristics of Li / LiO
0.3
MnO
2
re-
chargeable AA-size battery. (a) Charge and discharge curves. I
c
⫽
250 mA; I
d
⫽ 250 mA (cycle no. 5). (b) Capacity vs. cycle number.
I
c
⫽ 60 mA; I
d
⫽ 250 mA (100% DOD). (c) Capacity vs. discharge
rate. (d ) Short-circuit behavior after 50 cycles. (From Tadiran Elec-
tronic Industries, Inc.).
34.30 CHAPTER THIRTY-FOUR
These AA-size batteries have been withdrawn from the commercial market because it has
been found that, at high charging rates, lithium deposition produces small grains which react
with the electrolyte in spite of the passivating tendency of the LiAsF
6
-dioxolane-tributylam-
ine electrolyte. The amount of electrolyte is limited in spiral-wound construction and is
spread in a thin layer within the electrode structure. During cycling, the lithium-electrolyte
reaction depletes the amount of electrolyte so that only a part of the active material continues
to function, leading to a pronounced increase in internal resistance and ultimate failure as a
result of the high internal impedance and reduced amount of active material. Another failure
mechanism is the high current densities which develop as the area of active material de-
creases, leading to dendritic short circuits.
Lithium/ Titanium Disulfide (Li /TiS
2
) Cells. This rechargeable battery system was first
developed as a button cell for use in watches with a lithium-aluminum alloy anode. This
product never achieved commercial success due to fragmentation of the alloy anode on
cycling which limited cycle life. Later the battery was investigated in cylindrical and pris-
matic designs of up to about 5-Ah capacity.
36–39
Its advantageous characteristics are a rela-
tively flat discharge and high energy density.
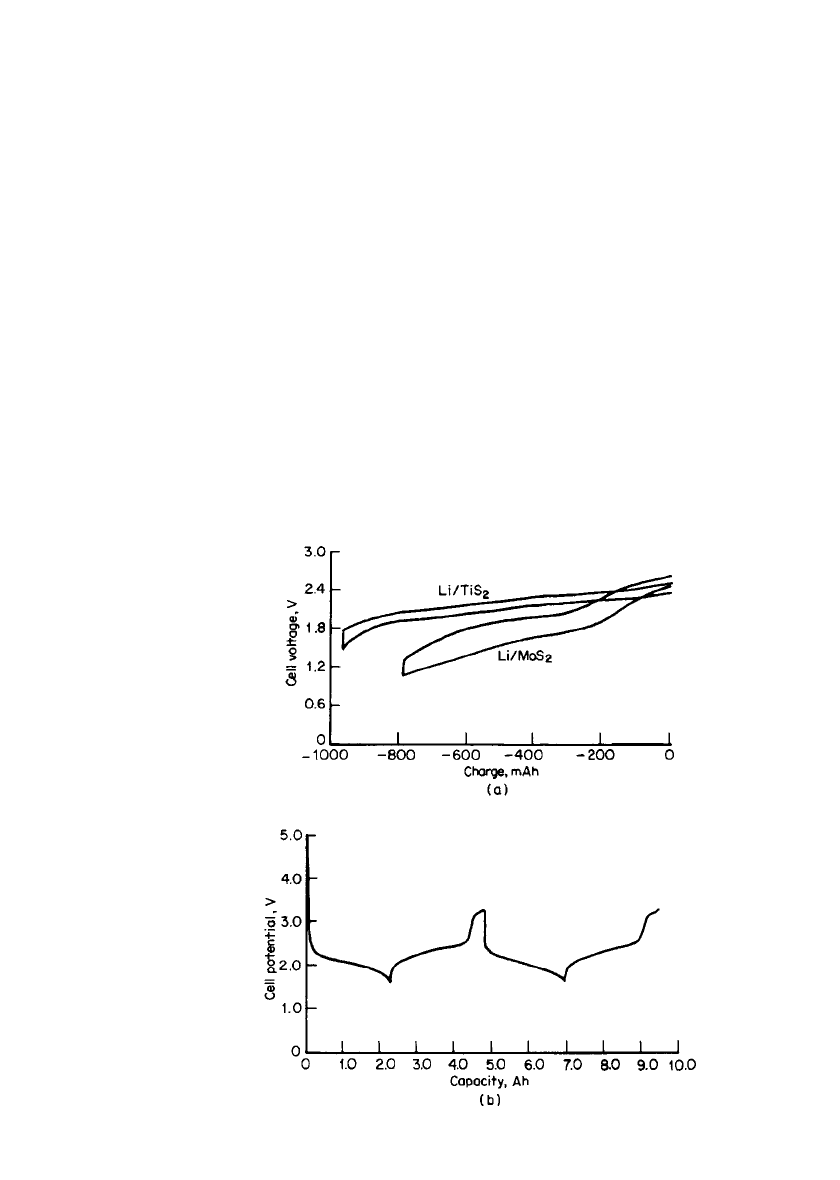
Figure 34.17a compares the discharge characteristics of the Li/TiS
2
battery, which used
an electrolyte composed of 1.5M LiAsF
6
in a mixture of 2-methyl tetrahydrofuran and te-
trahydrofuran, with that of the Li/MoS
2
cell. Figure 34.17b shows the discharge-charge
characteristics of a 2-Ah battery.
36
More than 250 cycles were achieved with a specific energy
of about 100 Wh/kg.
FIGURE 34.17 (a) Comparative performance of AA-size batter-
ies. Discharge rate ⫽ 200 mA; 20⬚C. (b) Successive cycles of a 2-
Ah Li/ TiS
2
battery. The battery is overcharged at the end of each
cycle, with the overcharge plateau time-limited to be at ⬃0.4 Ah.
