Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

34.1
CHAPTER 34
RECHARGEABLE LITHIUM
BATTERIES (AMBIENT
TEMPERATURE)
Thomas B. Reddy and Sohrab Hossain
34.1 GENERAL CHARACTERISTICS
Rechargeable lithium batteries operating at room temperature offer several advantages com-
pared to conventional aqueous technologies, including
1. Higher energy density (up to 150 Wh/kg, 400 Wh/L)
2. Higher cell voltage (up to about 4 V per cell)
3. Longer charge retention or shelf life (up to 5 to 10 years)
These advantageous characteristics result in part from the high standard potential and low
electrochemical equivalent weight of lithium metal.
Ambient-temperature lithium rechargeable batteries, on the other hand, do not have the
high-rate capability (because of the lower conductivity of the aprotic organic or inorganic
electrolytes that must be used because of the reactivity of lithium in aqueous electrolytes)
nor, in some instances, the cycle life of conventional rechargeable batteries. In addition,
rechargeable lithium batteries that use lithium metal as the negative electrode present poten-
tial safety problems which are more challenging than those with primary lithium batteries.
This is due to a three- to fivefold excess of lithium, which is required for these types of
cells in order to obtain a reasonable cycle life, and to the reactivity of the high-surface-area
lithium that is formed during cycling.
There is another type of rechargeable ‘‘lithium’’ battery, however, which uses a lithiated
carbon or other intercalation material for the negative electrode in place of lithium. The
absence of metallic lithium in these lithium-ion batteries minimizes these safety concerns.
This type of battery is covered in Chap. 35.
The advantages and disadvantages of rechargeable lithium batteries operating at ambient
temperature are summarized in Table 34.1. (These may not be applicable in all cases because
of the different characteristics of the various rechargeable lithium systems.)

34.2 CHAPTER THIRTY-FOUR
TABLE 34.1 Advantages and Disadvantages of Ambient-Temperature Lithium Rechargeable Batteries
Advantages
High energy density and specific energy
High voltage
Good charge retention, low self-discharge rate
Disadvantages
Low cycle life with metallic lithium systems
Relatively poor high-rate performance (compared to conventional aqueous rechargeable batteries)
Relatively poor low-temperature performance (compared to conventional aqueous rechargeable batteries)
Capacity fading (with some systems)
Potential safety problems with metallic lithium systems
As with primary lithium batteries, a number of different approaches have been taken in
the chemistry and design of rechargeable lithium batteries to obtain the desired performance
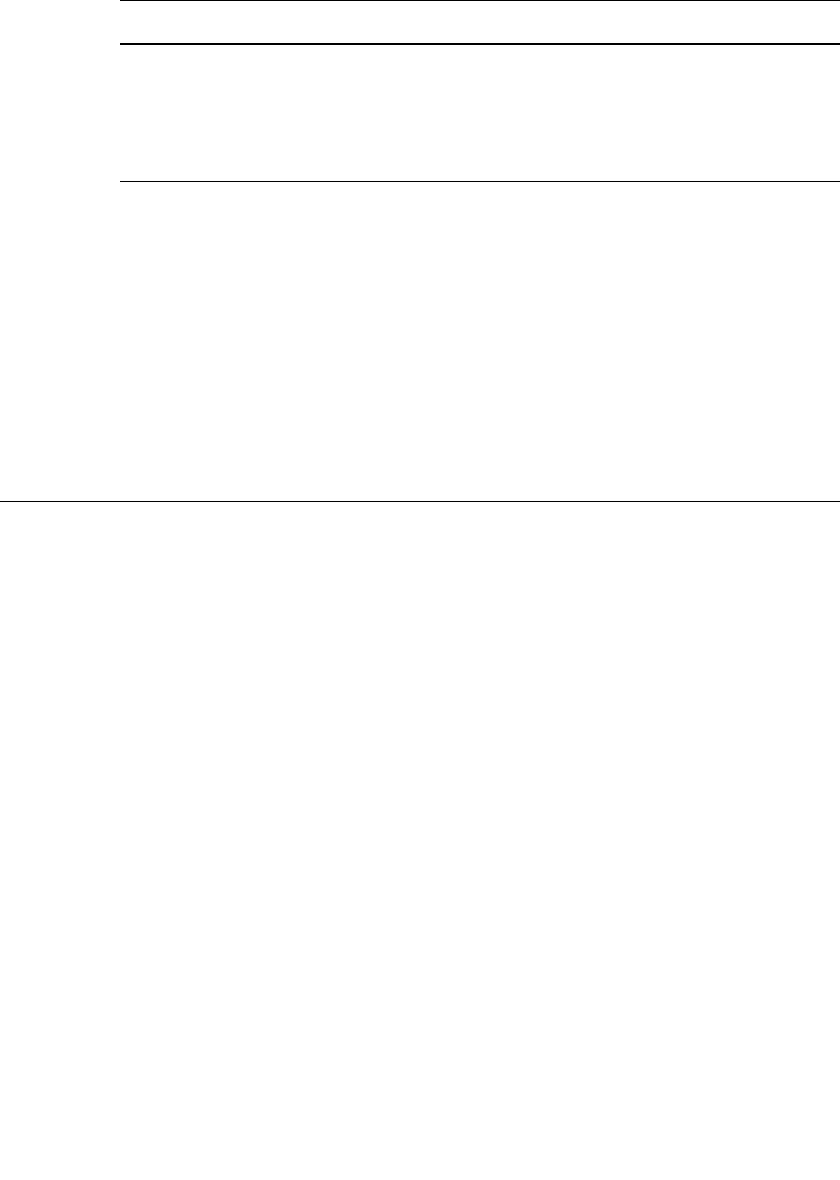
characteristics. These are summarized in Fig. 34.1a for batteries with lithium metal negative
electrodes (the anode during discharge) and in Fig. 34.1b for batteries with other materials,
such as lithium alloys and lithiated carbon.*
A variety of materials have been investigated for the positive electrode (the cathode during
discharge), such as intercalation solid compounds, soluble inorganic cathodes, and polymeric
materials. Liquid aprotic organic and inorganic electrolytes are used in many cells. Solid
polymer electrolytes are also popular as they may provide a safer design because of their
lower reactivity with lithium. These materials are identified in Fig. 34.1.
Rechargeable lithium batteries have been introduced into the market on a limited scale.
Coin cells, using lithium-aluminum anodes, are available for special applications mainly for
low-power portable applications where they can be conveniently recharged, in some instances
by solar cells. A small cylindrical cell, using a lithium anode, was briefly, and perhaps
prematurely, introduced in the 1980s for consumer electronics applications, but was with-
drawn when safety problems arose. More recently, the lithium-ion cell, which has a safety
advantage over other lithium secondary cells as it does not contain lithium in a metallic
form, has been marketed as a power source for consumer electronics such as cellular phones
and camcorders. This technology has become dominant in the market.
The shipment, use, and disposal of rechargeable lithium batteries are regulated, as for the
primary lithium batteries, by international organizations, government agencies, and quasi-
government institutions because of the concern for potential safety problems with lithium
batteries, particularly if they are physically or electrically abused.
1
*As discussed in Chap. 1, the electrodes in a rechargeable battery are referred to as
negative and positive. Sometimes they are referred to as anode and cathode, respectively, the
appropriate terminology for the cell during discharge.
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.3
FIGURE 34.1 Lithium rechargeable batteries (a) with metallic lithium as negative electrode, (b) with
lithium alloys or lithiated carbon negative electrode.

34.4 CHAPTER THIRTY-FOUR
TABLE 34.2 Negative Electrode Material
Material
Voltage range
vs. lithium,
V
Theoretical
specific capacity,
Ah/ g Comments
Li metal 0.0 3.86 Lithium foils readily available
LiAl 0.3 0.8 Generally brittle foils,
difficult to handle
Li
0.5
C
6
(coke)
LiC
6
(MCMB (b), or graphite)
0.0–1.3
0.0–0.1
0.185
冎
0.372 (a)
Used for lithium-ion cells
LiWO
2
LiMoO
2
LiTiS
2
0.3–1.4
0.8–1.4
1.5–2.7
0.12
0.199
冧
0.266
Possible use for lithium-ion
cells
(a) Based on weight of carbon only.
(b) Mesocarbon microbeads.
34.2 CHEMISTRY
The objective of the rechargeable lithium battery development is to produce batteries that
have high energy density, high power density, good cycle life and charge retention, and to
provide this performance reliably and safely. The selection of cell components and designs
is necessarily a compromise to achieve the optimum balance. Many of the characteristics
and criteria for selection are similar to those for primary lithium cells covered in Chap. 14.
The process, however, is even more complex for rechargeable batteries since the reactions
that occur during recharge affect all of the characteristics and the performance on subsequent
cycling.
The different types of lithium rechargeable batteries identified in Fig. 34.1 can be clas-
sified conveniently into five categories:
1. Solid-cathode cells using intercalation compounds for the positive electrode, a liquid
organic electrolyte, and a metallic lithium negative electrode.
2. Solid-cathode cells using intercalation compounds for the positive electrode, a polymer
electrolyte, and a metallic lithium negative electrode.
3. Cells using intercalation compounds for both the positive and the negative electrodes and
a liquid or polymer electrolyte (lithium-ion cells) (see Chap. 35).
4. Inorganic electrolyte cells, which use the electrolyte solvent or a solid redox couple for
the positive and lithium metal for the negative active material.
5. Cells with lithium-alloy anodes, liquid organic or polymer electrolytes, and a variety of
cathode materials, including polymers. This technology has been used mainly in small
flat or coin cells.
The components and reactions of typical examples of these types of rechargeable lithium
batteries are summarized in Sec. 34.3.
34.2.1 Negative Electrodes
Typical negative electrode materials for rechargeable lithium batteries are listed in Table
34.2. Of these, lithium is the lightest and most electropositive, and it has a high specific
capacity, 3.86 Ah/ g. It is also more easily handled than the other alkali metals.

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.5
While metallic lithium has the highest specific capacity, it is more reactive than lithium-
aluminum and other alloys. These have been used mainly in small flat or coin cells, as it is
difficult to scale up to larger and spirally wound designs because most lithium alloys are
brittle and cannot be extruded into thin foils. Another approach is the lithium ion battery
which uses a carbon material for the negative electrode. These are attractive from a safety
viewpoint as reactive metallic lithium is not present in the cell. A suitable intercalation
compound is selected for the positive electrode for these cells so that a high cell voltage is
obtained. The cell operates by the lithium ions shuttling back and forth between the elec-
trodes during the discharge-charge cycle. No metallic lithium is plated during the charge and
no metallic lithium is present in the cell.
Lithium Metal. The search for high-energy-density batteries has inevitably led to the use
of lithium, as the electrochemical characteristics of this metal are unique. A number of
batteries, both primary and rechargeable, using a lithium anode in conjunction with inter-
calation cathodes, were developed which had attractive energy densities, excellent storage
characteristics, and, for rechargeable cells, a reasonable cycle life. Commercial success has
eluded all but the primary batteries due to persistent safety problems.
The difficulties associated with the use of metallic lithium stem from its reactivity with
the electrolyte and the changes that occur after repetitive charge-discharge cycling. When
lithium is electroplated, during recharge, onto a metallic lithium electrode, it forms a mossy
and in some cases a dendritic deposit with a larger surface area than the original metal.
While the thermal stability of lithium metal foil in many organic electrolytes is good, with
minimal exothermic reaction occurring up to temperatures near the melting point of lithium
(181
⬚C), after cycling the surface area of the lithium increases significantly with a corre-
sponding increase in the reactivity. This lowers the thermal stability of the system, with the
result that cells become increasingly sensitive to abuse as they are cycled.
Another contributing effect is the inability of attaining 100% lithium cycling efficiency.
This happens because lithium is not thermodynamically stable in the organic electrolytes and
the surface of the lithium is covered with a film of the reaction products between the lithium
and the electrolyte. Every time the lithium is stripped and replated during discharge and
charge, a new lithium surface is exposed and then passivated with a new film, consuming
lithium. Because of the mossy deposit, some lithium becomes electrochemically unreactive
on repeated cycling. In order to obtain a reasonable cycle life, a three- to fivefold excess of
lithium is required.
The failure to control the surface area of the lithium anode remains a problem, limiting
the commercialization of lithium anode cells with liquid organic electrolytes. Given this
problem, an alternate solution is to use an electrolyte, such as a solid polymer electrolyte,
which is less reactive with lithium. This is covered in Sec. 34.2.3.
Carbon Materials. In the lithium-ion cell, carbon materials, which can reversibly accept
and donate significant amounts of lithium (Li:C
⫽ 1:6) without affecting their mechanical
and electrical properties, can be used for the anode instead of metallic lithium. Carbon
material is used as an anode in lithium-ion cells since the chemical potential of lithiated
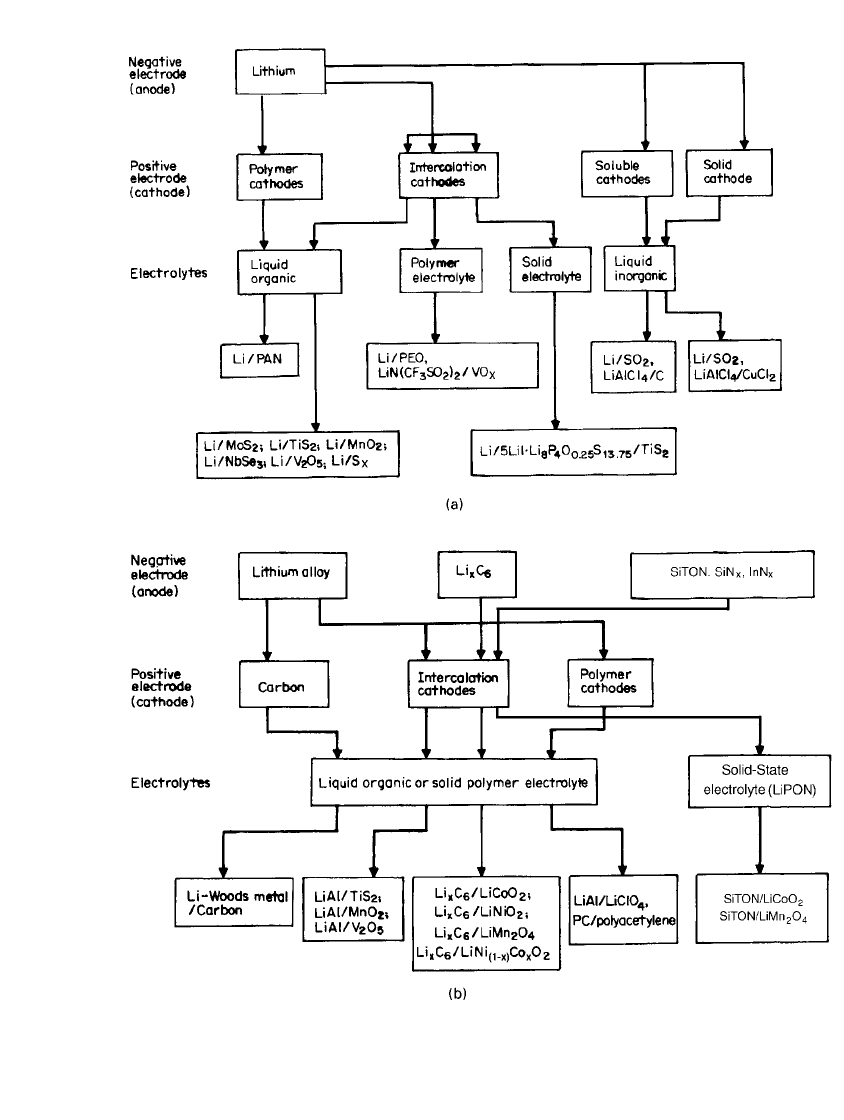
carbon material is close to that of metallic lithium, as shown in Fig. 34.2. Thus an electro-
chemical cell made with a lithiated carbon material will have almost the same open-circuit
voltage as one made with metallic lithium. In practice, the lithium-ion cell is manufactured
in a fully discharged state. Instead of using lithiated carbon material, which is air-sensitive,
the anode is made with carbon and lithiation is carried out by subsequent formation of the
cell.
The specific energy or capacity of the lithium-ion system depends largely on the type of
carbon materials used, the lithium intercalation efficiency, and the irreversible capacity loss
associated with the first charge process. Table 34.3 lists the properties of some of these
carbon materials. Coke-type carbon, having physical properties such as ash content
⬍0.1%,
surface area
⬍10 m
2
/g, true density ⬍2.15 g/cm
3
, and interlayer spacing ⬎3.45 A
˚
, were
used in first-generation lithium-ion system. These types of carbon materials can provide about
34.6 CHAPTER THIRTY-FOUR
FIGURE 34.2 Electrochemical potential of some Li-
intercalation compounds vs. Li metal. PPY ⫽ polypyrrole.
PVF ⫽ polyvinyl furan.
TABLE 34.3 Physical Properties of Coke and Graphite Anodes for Lithium-Ion Cells
Item Coke Graphite
Type Petroleum
coke
Synthetic,
KS-15
Synthetic,
KS-44
Isotopic,
EC-110
Natural
Structure Disordered Ordered layer structure
Physical parameters:
Interlayer spacing d002, A
˚
3.46 3.35 3.35 3.34 3.34
Crystalline size L
c
,A
˚
46 900 ⬎1000 ⬎1000 ⬎2000
Surface area, m
2
/g 6 14 10 10 —
Density, g /cm
3
2.14 2.255 2.248 — —
Ash content, % 0.08 0.05
⬍0.1 — —
185 mAh /g capacity (corresponding to LiC
12
). By controlling the temperature of the heat
treatment, carbon material having specific properties such as density and interlayer spacing
can be prepared. Doping with nitrogen, boron, or phosphorus can increase the capacity of
coke type materials to 350 mAh/ g. Graphitic carbons having an interlayer spacing of 3.36
A
˚
can also deliver 350 mAh /g capacity. The capacity delivered by different types of carbon
materials, including natural and synthetic graphite, in ethylene carbonate-based electrolytes
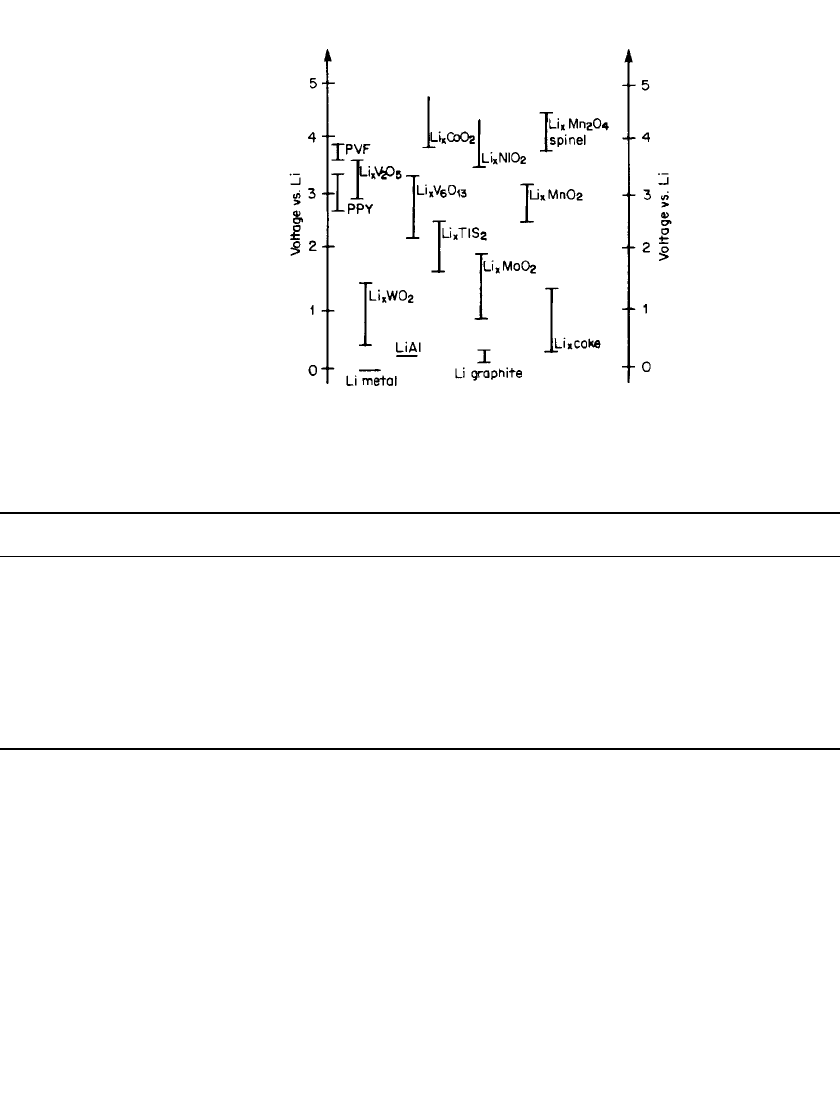
on the first discharge is shown in Fig. 34.3.
2
This figure shows the advantage of the graphite
materials, which have a flatter discharge and a higher capacity than the coke materials.
Mesocarbon Microbeads (MCMB), which are also graphitized, have also been employed in
lithium ion batteries.
During the first electrochemical intercalation of lithium into the carbon, some lithium is
irreversibly consumed forming a solid electrolyte interface (SEI) and cannot be recovered in
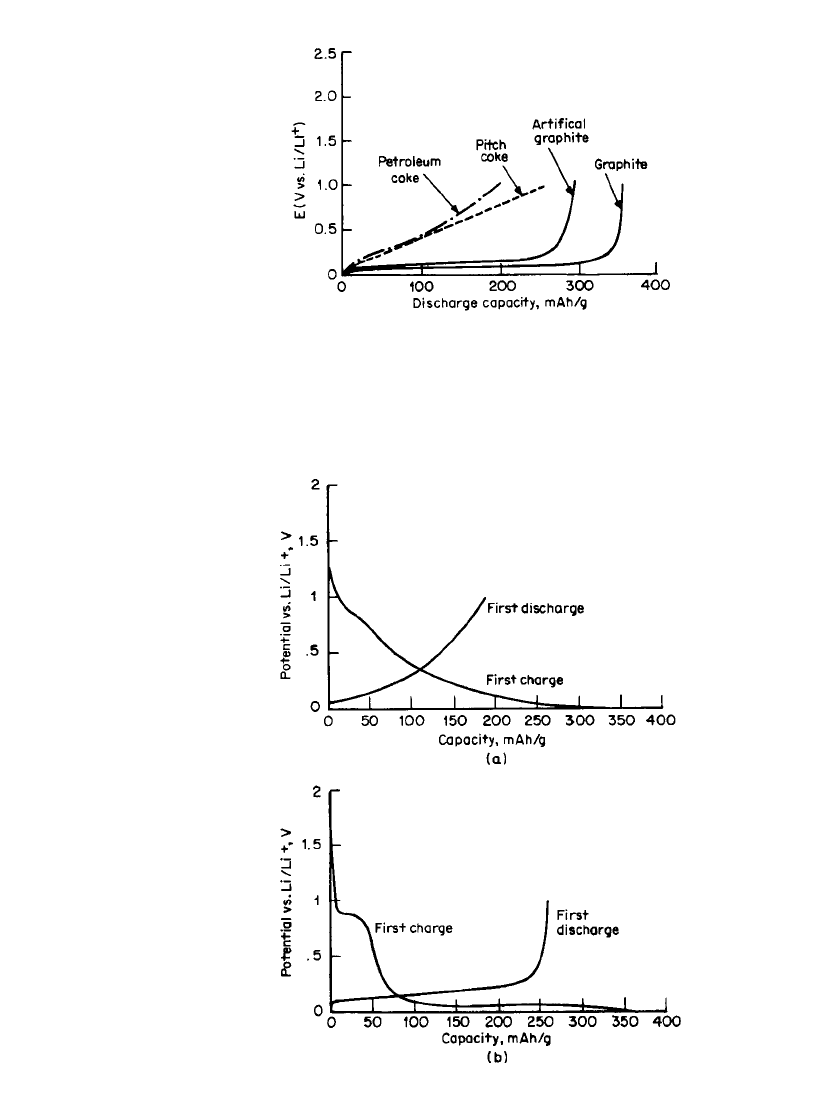
the following discharge, resulting in a loss of capacity. (See Chap. 35.) Figure 34.4 shows
the voltage profile of the first charge and discharge of petroleum coke and graphite electrodes
versus a lithium electrode, respectively.
3
This irreversible capacity, which depends on the
electrolyte solution and the type of carbon material, is explained on the basis of the reduction
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.7
FIGURE 34.3 First discharge curves of carbon materi-
als.
FIGURE 34.4 Representation of irreversible capacity asso-
ciaed with the first charge /discharge process. (a) Coke. (b)
Artificial graphite.
34.8 CHAPTER THIRTY-FOUR
of the electrolyte solution and the formation of a SEI layer on the Li
x
C interface.
4
When the
film is sufficiently thick to prevent electron tunneling, the electrolyte reduction is suppressed
and the electrode can then be cycled reversibly. The first step is, therefore, critical in order
to obtain a uniform passivating film. Very little loss usually occurs after the first intercalation.
The capacity on the second and subsequent cycles is about the same, and the lithium inter-
calation during charge and discharge is nearly 100% reversible.
The theoretical capacity of metallic lithium is much higher than that of lithiated carbon
material having a composition of LiC
6
. The advantage of the higher capacity of metallic
lithium is reduced because a three- to fivefold excess of lithium is required in rechargeable
batteries having metallic lithium anodes to achieve a reasonable cycle life. The comparison
is shown in Table 34.4.
TABLE 34.4 Comparison of Usable Specific Capacity (Ah/ kg)
and Capacity Density (Ah /L) for Lithiated Carbon vs. Lithium
Metal Anodes
Characteristics Li metal LiC
6
Theoretical specific capacity, Ah/ kg 3862 372
Theoretical capacity density, Ah/ L 2061* 837
Practical specific capacity, Ah/ kg
Fourfold excess of lithium 965 372
95% active material in carbon electrode 965 353
Practical capacity density, Ah / L
Fourfold excess of lithium 515 837
Porosity of carbon electrode (50%) 515 418
* Density of lithium ⫽ 0.534 g / cm
3
.
Transition Metal Compounds. Transition metal compounds having layered structures into
which lithium ions can be intercalated and deintercalated during charge and discharge and
with electrochemical potentials close to those of lithiated carbon materials have also been
used as the negative electrode in lithium-ion cells. Figure 34.2 shows the electrochemical
potentials of some lithiated transition metal compounds. The electrochemical potentials of
Li
x
WO
2
,Li
x
MoO
2
, and Li
x
TiS
2
are close to that of lithiated carbon and distinctly different
from the values for Li
x
Mn
2
O
4
,Li
x
CoO
2
, and Li
x
NiO
2
.WO
2
, MoO
2
,orTiS
2
can then be used
as anodes and LiMn
2
O
4
, LiCoO
2
, or LiNiO
2
as cathodes. Cells of these types have been
developed using TiS
2
anodes and LiCoO
2
cathodes in an organic electrolyte.
7
34.2.2 Positive Electrodes
There is a wide choice of materials that can be selected for the positive electrodes of lithium
batteries. However, many of these involve reactions which cannot be readily reversed and
are limited to primary nonrechargeable batteries. The best cathodes for rechargeable batteries
are those where there is little bonding and structural modification of the active materials
during the discharge-charge reaction.
8
Intercalation Compounds. The insertion or intercalation compounds are among the most
useful cathode materials. In these compounds, a guest species such as lithium can be inserted
interstitially into the host lattice (during discharge) and subsequently extracted during re-
charge with little or no structural modification of the host.

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.9
TABLE 34.5 Key Requirements for Positive-Electrode
Intercalation Material (Li
x
MO
z
) Used in Rechargeable Lithium
Cells
High free energy of reaction with lithium
Wide range of x (amount of intercalation)
Little structural change on reaction
Highly reversible reaction
Rapid diffusion of lithium ion in solid
Good electronic conductivity
No solubility in electrolyte
Readily available or easily synthesized from low-cost reactants
The intercalation process involves three principal steps:
1. Diffusion or migration of solvated Li
⫹
ions
2. Desolvation and injection of Li
⫹
ions into the vacancy structure
3. Diffusion of Li
⫹
ions into the host structure
The electrode reactions which occur in a Li/ Li
x
(HOST) cell, where (HOST) is an inter-
calation cathode, are
⫹
yLi ↔ yLi ⫹ ye at the Li metal anode
⫹
yLi ⫹ ye ⫹ Li (HOST) ↔ Li (HOST) at the cathode
xx
⫹
y
leading to an overall cell reaction of
yLi
⫹ Li (HOST) ↔ Li (HOST)
xx
⫹
y
A number of factors have to be considered in the choice of the intercalation compound,
such as reversibility of the intercalation reaction, cell voltage, variation of the voltage with
the state of charge, and availability and cost of the compound. Table 34.5 lists the key
requirements for the intercalation materials and Table 34.6 presents some of the character-
istics of the intercalation and other compounds that have been used in lithium rechargeable
batteries. The electrochemical potentials of several lithium intercalation compounds versus
those of lithium, metal and the variation of voltage with the amount of intercalation are
shown in Fig. 34.2.
Transition metal oxides (MnO
2
, LiCoO
2
, LiNiO
2
,VO
x
), sulfides (MoS
2
,TiS
2
), and selen-
ides (NbSe
3
) have been used in rechargeable lithium batteries. The lithiated transition metal
oxides (such as LiCoO
2
, LiNiO
2
, and LiMn
2
O
4
) are attractive materials used as the cathode
in the lithium-ion rechargeable cell. LiCoO
2
, LiNiO
2
and LiNi
1
⫺
x
Co
x
O
2
have a layered struc-
ture, where lithium and transition metal cations occupy alternate layers of octahedral sites
in a distorted cubic close-packed oxygen-ion lattice. The layered metal oxide framework
provides a two-dimensional interstitial space, which allows for each removal of the lithium
ions. The layered structure can be prepared by high-temperature treatment of a mixture of
lithium hydroxide or other salts and the metal oxide in air,
9
700
⬚
C
—
Co O ⫹ 2LiOH → 2LiCoO ⫹ HO
23 2 2
700
⬚
C
1
– —
2NiO ⫹ 2LiOH ⫹ O → 2LiNiO ⫹ HO
22 2 2

34.10 CHAPTER THIRTY-FOUR
TABLE 34.6 Positive-Electrode Materials and Some of Their Characteristics
Material
Average
voltage
vs. lithium,*
V
Lithium/
mole
Practical
specific
energy, †
Wh/ kg Comments
MoS
2
1.7 0.8 230 Naturally occurring
MnO
2
3.0 0.7 650 Inexpensive
TiS
2
2.1 1 550 Costly
NbSe
3
1.9 3 450 Costly
LiCoO
2
3.7 0.5 500 Good for lithium-ion system
LiNiO
2
3.5 0.5 480 Good for lithium-ion system
LiMn
2
O
4
3.8 0.8 450 Good for lithium-ion system, safe
VO
x
2.3 2.5 300 Good for SPE system
V
2
O
5
2.8 1.2 490 Good for SPE system
SO
2
3.1 0.33 220 Good for pulse power applications,
safety issues
CuCl
2
3.3 1 660 Good for pulse power applications,
safety issues
Polyacetylene 3.2 1 340 For polymer electrodes
Polypyrrole 3.2 1 280 For polymer electrodes
* At low rates.
† Based on cathode material only and average voltage and lithium / mole as shown.
Spinel LiMn
2
O
4
may be obtained by heating a mixture of appropriate amounts of Li
2
CO
3
and MnO
2
at 800⬚C in air.
5,10
The LiMn
2
O
4
spinel framework possesses a three-dimensional
space via face sharing octahedral and tetrahedral structures, which provide conducting path-
ways for the insertion and extraction of lithium ions.
The removal and insertion of the lithium ion for lithiated transition metal oxides (M) are:
⫹
LiMO Li MO ⫹ xLi ⫹ xe
2(1
⫺
x)2
The reversible value of x for LiCoO
2
and LiNiO
2
is approximately 0.5, and the value is
greater than or equal to 0.85 for lithiated manganese oxide. Thus although the theoretical
capacity of LiCoO
2
and LiNiO
2
(274 mAh/ g) is almost twice as high as that of LiMn
2
O
4
,
the reversible capacity of the three cathode materials are in the same range. Figure 34.5
compares the reversible discharge capacity of the three cathode materials. It is to be noted
that lithium-ion cells made with LiMn
2
O
4
require a higher charge voltage to achieve full
capacity. These batteries are typically charged to 4.2 V vs. 4.1 V for Co and 4.0 V for Ni.
The diffusion coefficients of lithium ion (D
Li
⫹
) in the three lithiated metal oxides are
shown in Table 34.7. The diffusion steps play a significant role in the mechanistic aspects
of the intercalation process.
Polymers. Electronically conductive polymers may also be used as cathode materials in
rechargeable lithium batteries. The most popular polymers are polyacetylene, polypyrrole,
polyaniline, and polythiophene, which are made conductive by doping with suitable anions.
The discharge-charge process is a redox reaction in the polymer. The low specific energy,
high cost, and their instability, however, make these polymers less attractive. They have been
used in small coin-type batteries with a lithium-aluminum alloy as the anode.
Another potential approach is an all-polymer cell using a cation-doped polymer for the
anode, a solid polymer electrolyte, and a polymer for the cathode. These cells can be fab-
ricated in thin sections and in a variety of shapes but, again, most of the development has
been in small coin sizes. No progress on these systems has been reported recently.
