Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


33.1
CHAPTER 33
SILVER OXIDE BATTERIES
Alexander P. Karpinski, Stephen F. Schiffer, and Peter A. Karpinski
33.1 GENERAL CHARACTERISTICS
The rechargeable silver oxide batteries are noted for their high specific energy and power
density. The high cost of the silver electrode, however, has limited their use to applications
where high specific energy or power density is a prime requisite, such as lightweight medical
and electronic equipment, submarines, torpedoes, and space applications. The characteristics
of the silver oxide secondary batteries are summarized in Table 33.1.
The first recorded use of a ‘‘silver battery’’ was by Volta with his now historic silver-zinc
pile battery, which he introduced to the world in 1800.
1
This battery dominated the scene in
the early nineteenth century, and during the next 100 years many experiments were made
with cells containing silver and zinc electrodes. All these cells, however, were of the primary
(nonrechargeable) type.
The first person to report a workable secondary silver battery was Jungner in the late
1880s.
2
Although he experimented in the early stages with iron /silver oxide and copper/
silver oxide batteries (which reportedly delivered as much as 40 Wh /kg), he settled on the
cadmium/ silver oxide battery for his experiments with electric car propulsion. The short
cycle life and high cost of these batteries, however, made them commercially unattractive.
During the next 40 years other scientists experimented with various electrode formulations
and separators, but without much practical success. It was the French professor Henri Andre´
who provided the key to the practical rechargeable zinc /silver oxide (silver-zinc) battery in
1941.
3,4
He described the use of a semipermeable membrane-cellophane—as a separator
which would retard the migration of the soluble silver oxide to the negative plate and also
impede the formation of zinc ‘‘trees,’’ or dendrites, from the negative to the positive plate,
the two major causes of cell short circuits.
In the 1950s, interest was revived in the silver-cadmium battery using the then newly
available silver-zinc and nickel-cadmium technologies. This provided improved cycle life
over the silver-zinc system. These batteries were first commercialized by Yardney Inter-
national Corporation. Later, Westinghouse Corporation reported the commercial application
of a silver-iron battery (see Chap. 25) in which they sought to ‘‘eliminate the zinc plate
problems with a trouble-free iron plate, ease the separator materials and life problem and
shift the deep discharge capacity stability to that limited by the silver plate.’’
5
The goal now,
as for the past two centuries, is to provide the high energy content and power capability of
the silver electrode in an improved-life, lower-cost commercially viable secondary battery.

33.2 CHAPTER THIRTY-THREE
TABLE 33.1 Advantages and Disadvantages of Silver Oxide Secondary Batteries
Advantages Disadvantages
Silver-zinc (zinc / silver oxide)
High energy per unit weight and volume High cost
High-discharge-rate capability Relatively low cycle life
Moderate-charge-rate capability Decreased performance at low temperatures
Good charge retention Sensitivity to overcharge
Flat discharge voltage curve
Low maintenance
Low self-discharge
Safe
Silver-cadmium (cadmium /silver oxide)
High energy per unit weight and volume
(approx. 60% of silver-zinc)
High cost
Decreased performance at low temperatures
Good charge retention
Flat discharge voltage curve
Low maintenance
Nonmagnetic construction
Safe
Silver-iron (iron / silver oxide)
High energy and power capability High cost
Good capacity maintenance Water and gas management requirements
Overcharge capability Not yet proven in field use
Zinc/ silver oxide batteries provide the highest energy per unit weight and volume of any
commercially available aqueous secondary batteries. They can operate efficiently at ex-
tremely high discharge rates, and they exhibit good charge acceptance at moderate rates and
low self-discharge. The disadvantages are low cycle life (ranging from 10 up to 250 deep
cycles, depending on design and use), decreased performance at low temperatures, sensitivity
to overcharge, and high cost. Rates as high as 20 times the nominal capacity (20C rate) can
be obtained from specially designed silver-zinc batteries because of their low internal im-
pedances. These high rates, however, must often be limited in time duration because of a
potentially damaging temperature rise within the cells.
Cadmium/ silver oxide batteries have been viewed as a compromise between the high
energy density but short life of the silver-zinc system and the long cycle life but low energy
density of the nickel-cadmium system. Their energy density is roughly 2 to 3 times higher
than that of nickel-cadmium, nickel-iron, or lead-acid batteries, with a relatively long cycle
life, especially during shallow cycling. Charge retention is excellent. In addition, the ability
to fabricate the cells without use of magnetic materials has made them the battery of choice
for several scientific satellite programs. The major disadvantage of the silver-cadmium system
is cost; the cost per unit energy is even higher than for the silver-zinc battery. In addition
their low-temperature discharge characteristics and their high-rate properties are not as good
as those of the silver-zinc system.

SILVER OXIDE BATTERIES 33.3
Iron /silver oxide batteries may provide high energy and power capability with long ser-
vice life under deep-discharge use. They are capable of withstanding overcharge and over-
discharge without damage and can provide good capacity maintenance with cycling. Dis-
advantages are, once again, cost and also the need for gas and water management in
overcharge applications. Their nominal load voltage of 1.1 V is comparable to that of the
silver-cadmium system, but lower than the 1.5-V level for silver-zinc. Sufficient data have
not been published for these batteries to date to permit complete characterization of their
properties.
All three systems also offer the advantages of long dry shelf life and of providing a flat
discharge voltage during the major portion of their discharge. This latter characteristic is
related to the fact that as the silver oxide is reduced to metallic silver during discharge, the
conductivity of the silver electrode increases and serves to counteract polarization effects.
33.2 CHEMISTRY
33.2.1 Cell Reactions
The overall electrochemical cell reactions for the silver-zinc, silver-cadmium, and silver-iron
systems, all of which use aqueous solutions of potassium hydroxide (KOH) for electrolyte,
can be summarized as follows:
discharge
—
AgO ⫹ Zn ⫹ H O Zn(OH) ⫹ Ag
—
22
charge
discharge
—
AgO ⫹ Cd ⫹ H O Cd(OH) ⫹ Ag
—
22
charge
discharge
—
4AgO ⫹ 3Fe ⫹ 4H O Fe O 4H O ⫹ 4Ag
—
2342
charge
These are simplified equations since there is still no general agreement on the detailed
mechanisms of these reactions or on the exact form of all the reaction products.
33.2.2 Positive-Electrode Reactions
The charge and discharge processes of the silver electrode in alkaline systems are of special
interest because they are characterized by two discrete steps which manifest themselves as
two plateaus in the charge and discharge curves. The reaction occurring at the silver electrode
at the higher (peroxide) voltage plateau is shown as
discharge
⫺
—
2AgO ⫹ HO⫹ 2e Ag O ⫹ 2OH
—
22
charge
and at the lower, monoxide, voltage plateau as
discharge
⫺
—
Ag O ⫹ HO⫹ 2e 2Ag ⫹ 2OH
—
22
charge
As shown, these reactions are reversible.
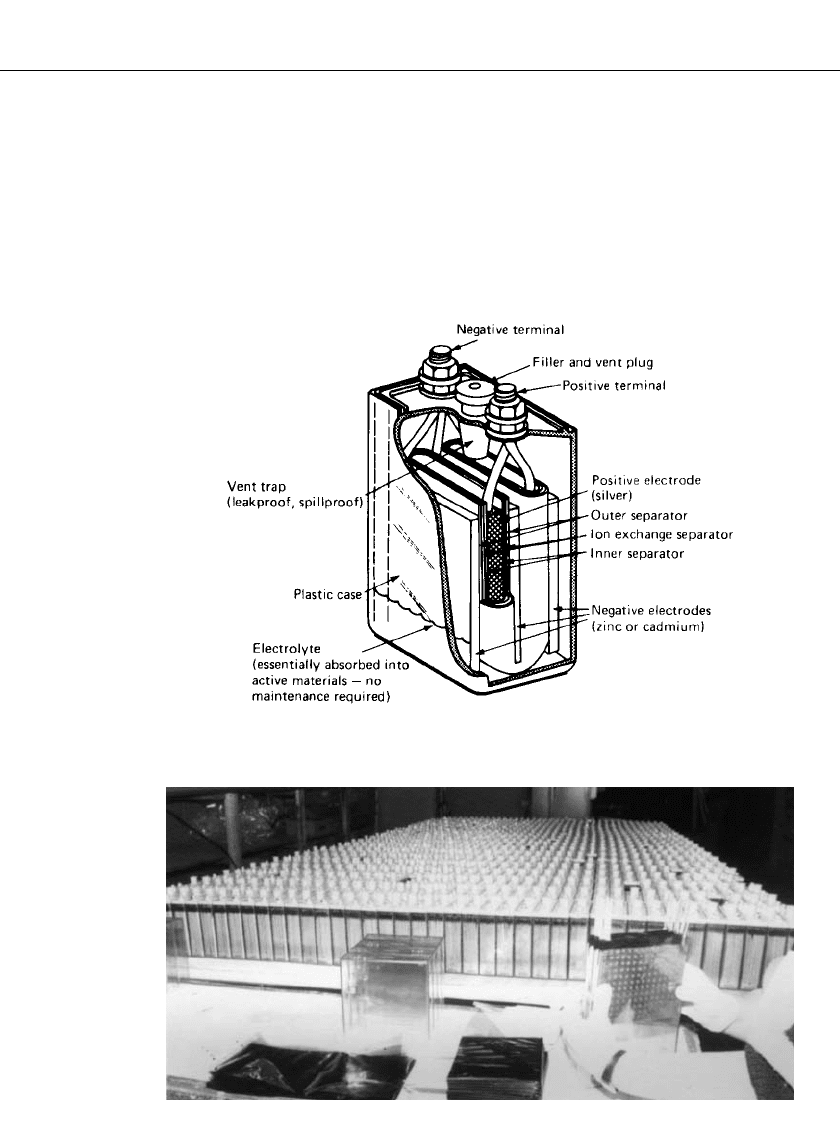
33.4 CHAPTER THIRTY-THREE
FIGURE 33.1 Cutaway view of typical prismatic zinc / silver oxide or
cadmium / silver oxide secondary cell.
FIGURE 33.2 Cell stack being assembled into cell case; model LR-190, 210-Ah silver-zinc
battery. (Courtesy of Yardney Technical Products, Inc.)
33.3 CELL CONSTRUCTION AND COMPONENTS
Secondary silver cells have been produced in prismatic, spirally wound cylindrical, and
button shape configurations. The most common shape is the prismatic cell. The construction
of a typical prismatic cell is shown in Fig. 33.1. This cell contains flat electrodes which are
wrapped with multiple layers of separator to provide mechanical separation and inhibit mi-
gration of the silver to the zinc plate and the growth of zinc dendrites toward the positive
plate. The plate groups are intermeshed, and the pack is placed in a tightly fitting case (Fig.
33.2). Because of the relatively short shelf life of the activated silver cells, they are usually
supplied by the manufacturers in the dry charged or dry unformed condition with filling kits
and instructions. The cells are filled with electrolyte and activated just prior to use. They
may also be supplied in the filled and ready-to-use condition if required by the user.

SILVER OXIDE BATTERIES 33.5
The mechanical strength of these cells is usually excellent. The electrodes are generally
strong and are fitted tightly into the containers. The cell containers are made of high-impact
plastics. Specific designs of these cells, when properly packaged, have met the high-shock,
vibration, and acceleration requirements of missiles and torpedoes with no degradation.
33.3.1 Silver Electrodes
The most common fabrication technique for silver electrodes is by sintering silver powder
onto a supporting silver grid. The electrodes are manufactured either in molds (as individual
plates or as master plates which are later cut to size) or by continuous rolling techniques.
They are then sintered in a furnace at approximately 700
⬚C.
Alternate techniques include dry processing and pressing, as well as slurry pasting with
a binder of AgO or Ag
2
O onto a grid. If pasted, the plates are often sintered, converting the
silver oxide into metallic silver and burning off the organic additives. The grid may be a
woven or expanded metal form of silver, or silver-plated copper.
After being cut to size and having wires or tabs hot-forged onto an appropriately coined
(compressed) area to carry current to the cell terminals, the electrodes are either electro-
formed (charged in tanks against inert counterelectrodes) before assembly into cells or as-
sembled into the cells in the metallic state and later charged in the cell.
Grid material, density, and thickness, electrical lead type and size; and final electrode
size, thickness, and density are all design variables which depend on the intended application
for the cells. The silver powder particle size may be varied, with the finer powders ap-
proaching the theoretical silver utilization of 2.0 g/ Ah. The use of very fine powder, however,
results in an initial voltage dip (typically less than 120 mS) at medium (C) to high discharge
rates.
33.3.2 Zinc Electrodes
Zinc electrodes are most widely made by dry pressing, by a slurry or paste method, or by
electrodeposition. In the dry-pressing method, a mixture of metallic zinc or zinc oxide,
binder, and additives is compressed around a metal grid; this is normally done in a mold.
The grid usually has the current-carrying leads prewelded in place. As the unformed powder
electrodes have little strength, one component of the separator system, the negative interse-
parator, is usually assembled around the electrode as part of the fabricating operation. Rolling
techniques have also been developed to permit continuous fabrication of dry-powder elec-
trodes.
6
In the paste or slurry method, a mixture of zinc oxide, binder, and additives is combined
with water and applied continuously to a carrier paper or directly to an appropriate metal
grid. Again, the negative interseparator is usually integral to provide needed physical
strength. After drying, multiple layers of these pasted slabs may be pressed together about
a pretabbed grid to form the final electrode. These plates may be assembled unformed into
the cell, or they may be electroformed in a tank against inert counterelectrodes.
Electrodeposited negative electrodes are manufactured by plating zinc in tanks onto me-
tallic grids. The plates must then be amalgamated and pressed or rolled to the desired thick-
ness and density, followed by drying.
The zinc electrode is acknowledged as the life-limiting component in both the silver-zinc
and the nickel-zinc systems. Accordingly, much work has been done in the area of additives
for these electrodes, both to reduce hydrogen evolution and to improve cycle life. The com-
mon additive to reduce hydrogen evolution has traditionally been mercury (1 to 4% of the
total mix), but this is being replaced, for personnel safety and enviromental reasons, by small

33.6 CHAPTER THIRTY-THREE
amounts or mixes of the oxide of lead, cadmium, indium, thallium, gallium,
7–11
and bis-
muth.
23
Many other (proprietary) additives have been introduced into the zinc electrode by
various manufacturers in attempts to increase life.
Zinc electrodes also suffer capacity loss, which results from ‘‘shape change,’’ or the
migration of materials from the sides and top to the center and bottom of the electrode.
Several approaches have been taken to improve the stability of the zinc electrode: (1) an
excess of zinc is used to compensate for losses during cycling, (2) oversized electrodes are
used on the basis that shape change starts on the electrode edges where current densities are
higher, (3) binders such as PTFE, potassium titanate, neoprene latex or other polymers are
used to hold the active materials together, and (4) electrolyte additives are used.
12–14
As is the case for the silver electrodes, the grid material, additives, and final electrode
size, thickness, and density are all design variables which depend on the final application.
33.3.3 Cadmium Electrodes
Most silver-cadmium cells contain cadmium electrodes that are manufactured by pressed-
power or pasting techniques. Although other methods have been used, such as impregnating
nickel plaque with cadmium salts, as is done for nickel-cadmium cells, the most common
method in silver-cadmium cells is to press or paste a mixture of cadmium oxide or cadmium
hydroxide with a binder onto a silver or nickel grid. These processes are similar to those
used for the pressed and pasted zinc electrodes.
33.3.4 Iron Electrodes
The iron electrodes used here are generally manufactured by powder-metallurgy techniques
(see Chap. 25).
33.3.5 Separators
The separators in the silver cells must meet the following major requirements:
1. Provide a physical barrier between positive and negative electrodes
2. Have minimum resistance to the flow of electrolyte and ions
3. Prevent migration of particles and dissolved silver compounds between positive and neg-
ative electrodes
4. Be stable in the electrolyte and cell operating environment
In general, secondary silver-zinc and silver-cadmium cells require up to three different sep-
arators, as shown in Fig. 33.1. The inner separator, or positive interseparator, serves both as
an electrolyte reservoir and as a barrier to minimize oxidation of the main separator by the
highly oxidative silver electrode. This separator is usually made of an inert fiber such as
nylon or polypropylene.
The outer separator, or negative interseparator, also serves as an electrolyte reservoir and
can also, ideally, stabilize the zinc electrode and retard zinc penetration of the main separator,
thus minimizing dendrite growth. Much work has been done to develop improved inorganic
positive electrode interseparators utilizing such materials as asbestos and potassium titanate.
Improvements in life have been reported as a result of this work.
7–10,11,15
This separator is
usually omitted in short and medium life cells.

SILVER OXIDE BATTERIES 33.7
The main, or ion exchange, separator remains the key to the wet life of the secondary
silver cell. It was Andre´’s
3
use of cellophane as a main separator that first made the secondary
silver cells feasible. The cellulosics (cellophane, treated cellophane, and fibrous sausage
casing) are usually employed in multiple layers as the main separators for these cells. Again,
much work has been done in recent years to develop improved separators utilizing such
materials as radiation-grafted polyethylene,
16
inorganic separators,
10,11,15,17,24
and other syn-
thetic polymer membranes. Improved cell life has been reported through use of these new
membranes either alone or in combination with cellulosics. Some of these have yet to be
applied extensively to commercial silver cells, however, because of drawbacks usually in-
volving high impedance, availability and cost.
33.3.6 Cell Cases
The cell cases must be chemically resistant to attack by the corrosive concentrated potassium
hydroxide electrolyte and to oxidizing effects of the silver electrodes. They must also be
strong enough to contain any internal pressure generated in the cells and to maintain struc-
tural integrity throughout the anticipated range of environmental conditions that will be
experienced by the cells.
The majority of secondary silver cells are assembled in plastic cases. The plastic most
commonly used is an acrylonitrile-styrene copolymer (SAN). This material is relatively trans-
parent and can be sealed easily by solvent cement or epoxy. However, its relatively low
softening temperature (80
⬚C) precludes it from use in some applications. A wide variety of
other plastics have been used for cell cases. Table 33.2 lists some of these materials and
gives their characteristics. Metal cases have been used for some sealed cell and button cell
applications; however, these present problems in sealing and in electrically isolating the
electrodes from the cases and are not used widely.
TABLE 33.2 Properties of Typical Plastic Cell Case and Cover Materials
Type (Trade name)
SAN
(SAN 35)
ABS
(Cycolac X-37)
ABS
(Cycolac GSM) Nylon (612)
Modified
PPO
(Noryl SE-1)
Polysulfone*
(P-1700)
Specific gravity 1.07 1.06 1.04 1.07 1.08 1.24
Transparency Yes No No No No Yes
Tensile strength, 10
3
lb/in
2
11.5 7.0 6.3 8.8 9.2 10.2
Flexural strength, 10
3
lb/in
2
14.0 12.0 10.7 11.0 15.0 15.4
ZOD impact strength, ft
lb
per inch notched
0.45 3.0 7.0 0.85 3.5 1.3
Hardness, Rockwell R 83 109 105 114 119 120
Heat-deflecton temperature
at 264 lb / in
2
.F
220 230 192 150 255 345
Dielectric strength, V/ mil 460 500 427 400 500 425
Thermal conductivity,
Btu
in/(h ft
2
⬚F)
0.87 2.38 1.55 1.5 1.5 1.8
* Glass or teflon filled (typically 10%) is also used to increase strength.

33.8 CHAPTER THIRTY-THREE
33.3.7 Electrolyte and Other Components
The electrolyte used in secondary silver cells is generally an aqueous solution (35 to 45%
concentration) of potassium hydroxide (KOH). Lower concentrations of electrolyte provide
lower resistivity and thus a higher voltage output under load as well as a lower freezing
point. Concentrations below 45% KOH, however, are more corrosive to the cellulosic sep-
arators typically used in silver-based batteries and are not used for extended wet-life appli-
cations. Table 33.3 depicts the critical parameters of various KOH solutions. Various addi-
tives such as zinc oxide, lithium hydroxide, potassium fluoride, potassium borate, tin, and
lead have been used to reduce the solubility of the zinc electrode.
14
Since potassium hydroxide readily combines with carbon dioxide in the air to form po-
tassium carbonate, thus reducing conductivity, cell vents are usually covered with a vent cap
or a low-pressure relief valve.
Cell terminals are typically made of steel or brass and are almost always silver- or nickel-
plated to improve conductivity and corrosion resistance.
TABLE 33.3 Physical and Electrical Characteristics of KOH Solutions
%KOH
Specific
gravity
at
15.6
⬚C
Conductivity
at 18
⬚C,
⍀
⫺
1
cm
⫺
1
Specific
heat at
18
⬚C,
cal/
g
⬚C
Freezing
point,
⬚C
Boiling point,
⬚C
at 760 mm
Hg
at 100 mm
Hg
Vapor pressure,
mm Hg
at
20
⬚C
at
80⬚C
Viscosity, cP.
at
20
⬚C
at
40⬚C
0
5
10
15
20
25
30
35
38
40
45
50
1.0000
1.0452
1.0918
1.1396
1.1884
1.2387
1.2905
1.3440
1.3769
1.3991
1.4558
1.5143
0.170
0.310
0.420
0.500
0.545
0.505
0.450
0.415
0.395
0.340
0.285
0.999
0.928
0.861
0.801
0.768
0.742
0.723
0.707
0.699
0.694
0.678
0.660
0
⫺3
⫺8
⫺14
⫺23
⫺36
⫺58
⫺48
⫺40
⫺36
⫺31
⫹6
100
101
102
104
106
109
113
118
122
124
134
145
52
52.5
53
54
56
59
62
66
69
71
80
89
17.5
17.0
16.1
15.1
13.8
11.9
10.1
8.2
7.0
6.2
4.5
2.6
355
342
327
306
280
250
215
178
156
140
106
70
1.00
1.10
1.23
1.40
1.63
1.95
2.42
3.09
3.70
4.16
5.84
8.67
0.66
0.74
0.83
0.95
1.10
1.31
1.61
1.99
2.35
2.59
3.49
4.85
33.4 Performance Characteristics
33.4.1 Performance and Design Tradeoffs
The secondary silver batteries provide high energy capability combined with minimum
weight and volume. The advantages and disadvantages of the various systems have been
described earlier in this chapter. The performance of the batteries for specific application
will depend on the internal design and history of the cells. It is rare that one can select an
‘‘off-the-shelf’’ battery that will meet all the requirements of a specific application.
Starting with the basic parameters, the cell design will consist of a series of compromises
to obtain the most favorable combination of voltage, electrical capacity, and cycle life char-
acteristics within the allowable battery weight and volume.
Assuming, for example, a nominal 1.5 V per silver-zinc battery at low current densities
(0.01 to 0.03 A /cm
2
) and lower voltages at higher currents, the designer selects the number
of cells for the application. The problem is increased if high current pulse loads are required
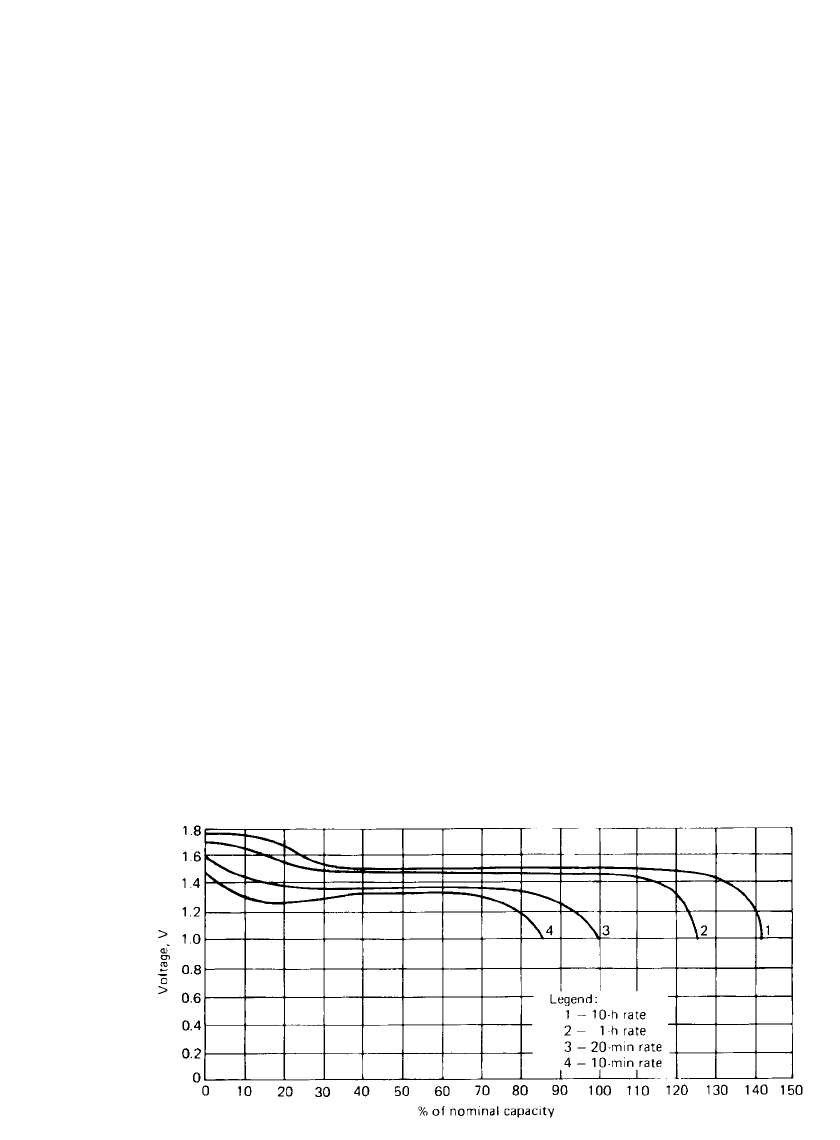
SILVER OXIDE BATTERIES 33.9
FIGURE 33.3 Typical discharge curves of silver-zinc battery at various rates at 20⬚C.
and the battery must provide voltage above the minimum allowable at the high rate, while
not exceeding the maximum allowable voltage at initial low rates. The size of the cell is
then chosen by dividing the allowable volume by the calculated required number of cells.
The voltage, current, electrical capacity, and cycle life requirements must then be reviewed
in conjunction with the allowable weight and the environmental conditions that the battery
must be able to withstand. Each of these will be a factor in determining the choice of
separator material for the cell. The stability and number of layers of separator must be
sufficient to provide the desired wet life under these conditions while having a resistance
low enough to prevent undue voltage drop at the high current load. Each of these require-
ments is also a factor in choosing the number of electrodes within the cell. As the number
of electrodes (and thus the active electrode area) is increased, the current density during any
discharge (Amperes per square centimeter) is decreased, raising the output voltage. It should
be noted that a cell design optimized for high discharge rates will, by nature of the design,
have a reduced capacity under low discharge rates. This is a result of having many electrodes
each having to be wrapped with the required number of layers of separators. Given a fixed
internal volume, it follows that in such a high-rate cell, less space is available for active
electrode material.
The cell must also be designed to contain enough active electrode material (such as silver
and zinc) to supply the required electrical capacity for the desired number of cycles. Theo-
retically,2gofsilver and 1.2 g of zinc are required in the cell for each Ampere-hour of
electrical capacity desired. Since these values are the theoretical capability of the pure ma-
terial, and since some of the active materials will go into solution with each charge-discharge
cycle, the designer must work with higher values—on the order of 3.5 g of silver and 3.0 g
of zinc per nominal Ampere-hour for long cycle-life cells. Other design variables, such as
silver powder particle size, will also ultimately affect cell performance.
Because of these considerations, the performance curves shown in the following sections
must be viewed as general characteristic of the systems and not necessarily of specific
batteries for a specific application.
33.4.2 Discharge Performance for Zinc /Silver Oxide Batteries
The open-circuit voltage of the zinc /silver oxide battery is 1.82 to 1.86 V. The discharge is
characterized by two discrete steps, the first corresponding to the divalent oxide and the
second to the monovalent oxide, as shown in Fig. 33.3. The flat portion of the curves is
referred to as ‘‘plateau voltage.’’ This voltage is rate-dependent; at high rates the voltage
steps may be obscured.
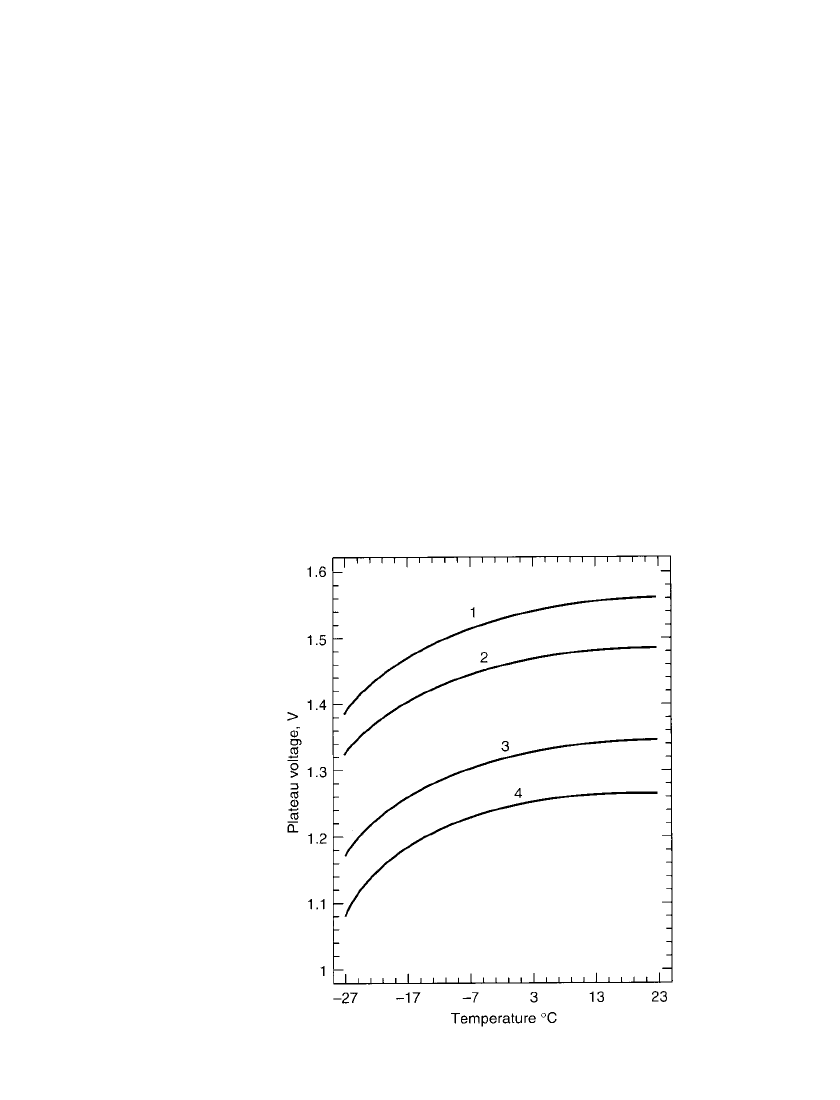
33.10 CHAPTER THIRTY-THREE
FIGURE 33.4 Typical effect of temperature on plateau
voltage for high-rate silver-zinc battery (operated without
heaters). Curve 1–10-h rate; curve 2–1-h rate; curve 3–20-
min. rate; curve 4–10-min. rate.
The performance of the battery at various discharge rates and temperatures can be seen
in Figs. 33.4 to 33.6, which show the effect on plateau voltage and capacity. The high-rate
capability of the zinc/silver oxide battery is a complex process which can be characterized
as the result of the electrical conductivity of the silver grid and the conductivity of the
positive electrode as it is discharged, as well as the thin multiplate design of the cell. The
performance of the battery falls off with decreasing temperature, particularly below
⫺20⬚C.
Heating the battery with external heaters or by retaining the internal heat generated during
the discharge can improve the performance at low ambient temperatures.
The performance characteristics of the zinc/ silver oxide battery are summarized in Figs.
33.7 and 33.8, which can be used to determine the capacity, service life, and voltage under
a variety of discharge conditions. These figures present typical performance data. Perform-
ance differences can occur for each specific design and even for each battery, depending on
cycling history, state of charge, storage time, temperature, and other conditions of use.
Figures 33.3–33.8 are specifically for high-rate (HR) designs. For many applications,
tradeoffs can be made to provide longer life at the expense of somewhat lower energy density.
Alternative low-rate (LR) designs contain additional layers of separator, meaning, of neces-
sity, fewer electrodes with higher impedance and lower capacity within a given volume.
Typically, the LR battery cannot be discharged at higher than the 1-h rate and will provide
about 3 to 5% lower average voltage and capacity than its HR counterpart at the 1-h rate.
However, the LR batteries do provide substantial wet shelf life and cycle life advantages
(see Table 33.4).
