Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

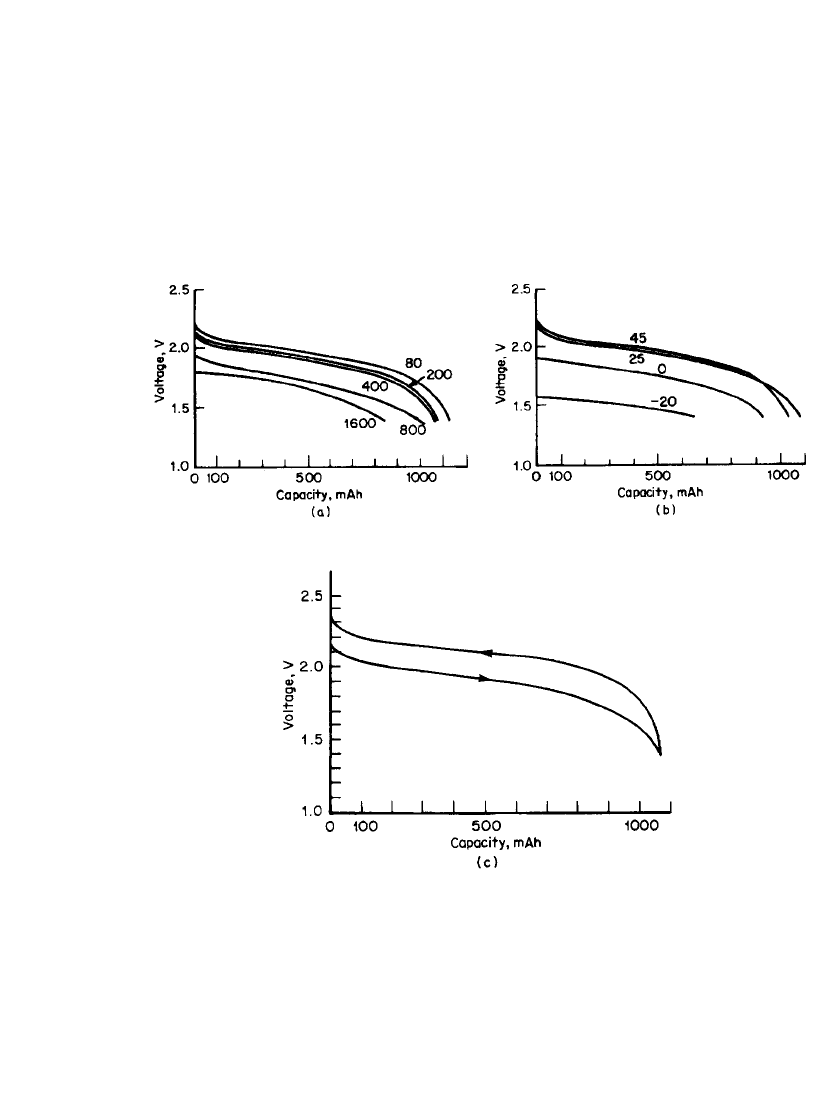
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.31
Lithium/ Niobium Selenide (Li/ NbSe
3
) Batteries. This cathode material is attractive be-
cause it can accept (intercalate) three lithium ions per molecule. The cathode can also be
made without any binder and conducting diluent because of its soft, fibrous nature and high
electronic conductivity. A AA-size cylindrical battery can deliver about 1.1 Ah with an
average voltage of 2.0 V. Figure 34.18 shows the discharge characteristics of a Li/NbSe
3
battery at various discharge rates and temperatures.
40
The battery has a mildly canted dis-
charge profile. An important characteristic of this system is its high-rate discharge capability
(up to the 2C rate). A typical discharge and charge cycle is shown in Fig. 34.18c. More than
200 cycles were obtained at 75% depth of discharge.
The development of this system was terminated despite its technical promise.
FIGURE 34.18 Discharge characteristics of the Li / NbSe
3
AA-size battery. (a) Discharge curves
at 20⬚C (mA). (b) Discharge curves at different temperatures (⬚C) at 200 mA. (c) Typical discharge
and charge curve for the Li/ NbSe
3
AA-size battery. Discharge: 400 mA; charge: 80 mA.

34.32 CHAPTER THIRTY-FOUR
Lithium/ Lithiated Cobalt Dioxide (Lu/ Li
x
CoO
2
) and Lithium/ Lithiated Nickel Dioxide
(Li/Li
x
NiO
2
) Batteries. These batteries are characterized by a high cell voltage, high en-
ergy density, good low-temperature performance, relatively high charge-discharge rates, and
moderate cycle life. The net charge and discharge reactions of the Li /Li
x
CoO
2
cell can be
represented by the cell reaction:
discharge
⫹
—— —
xLi ⫹ L CoO LiCoO
———
1
⫺
x 2
2
charge
The Li /Li
x
CoO
2
battery is manufactured in a fully discharged state and thus requires a charge
prior to its usage. When used in combination with oxidation-resistant ester-based solvents
(methyl formate or methyl acetate), the battery can reach full charge at 4.2 to 4.3 V de-
pending on the cycle history of the cell.
A Li/Li
x
CoO
2
battery that utilizes an ester-based electrolyte displays a flat discharge at
3.85 to 3.95 V in a cathode compositional range of between x
⫽ 0.5 and x ⫽ 1.0, depending
on the discharge rate conditions.
Batteries have been built in cylindrical spirally wound configurations in AA (300 to 600
mAh), D (4–6 Ah), and larger sizes. The electrolyte consists of LiAsF
6
/LiBF
4
in methyl
formate, methyl acetate, or a mixture of both. Figure 34.19a shows the discharge character-
istics of a 5-Ah D-size battery. The effect of various charging procedures on the discharge
performance is illustrated in Fig. 34.19b for a 30-Ah battery. Figure 34.19c shows the cycle
life characteristics of a D-size battery. The battery also has good storage capability. The
effects of storage on both initial capacity after storage and permanent capacity loss are
summarized in Table 34.14. Capacity losses of batteries stored at temperatures up to 35
⬚C
in the charged state can be fully recovered on the first charge.
41
The charge and discharge reactions of the Li/ Li
x
NiO
2
battery are similar to those of the
lithiated cobalt oxide system:
discharge
⫹
—— —
xLi ⫹ Li NiO LiNiO
———
1
⫺
x 2
2
charge
Batteries have been build in a spirally wound cylindrical D size. Table 34.15 lists the design
parameters. This D-cell battery employed an alloy anode of 85% Li-15% Al. Figure 34.20a
shows the discharge characteristics of the battery at several discharge rates and Fig. 34.20b
shows the cycle life characteristics. The declining capacity of the cell with cycling is attrib-
uted to increasing impedance of the cathode.
42
While there is currently no commercial production, the Li /Li
x
CoO
2
and Li/Ni
x
CoO
2
sys-
tems continue to receive attention for military applications because of their high specific
energy and energy density.
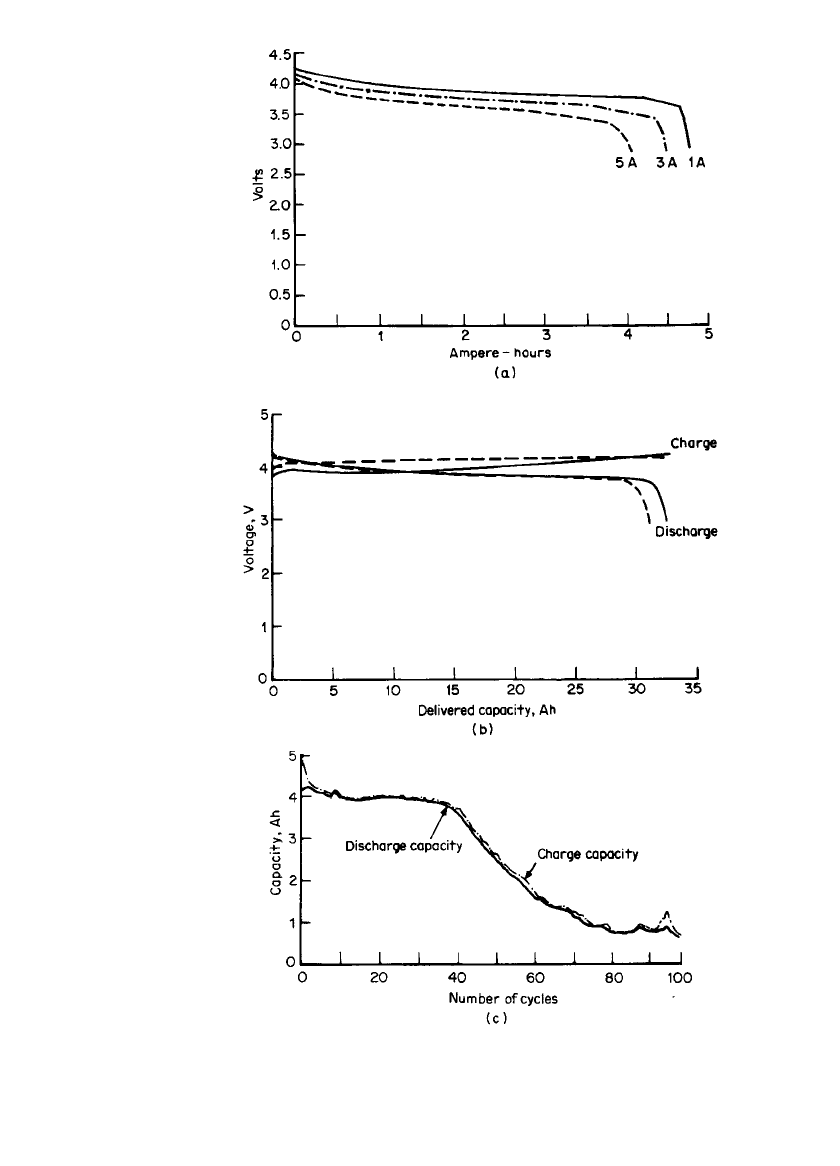
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.33
FIGURE 34.19 (a) Discharge characteristics of a 5-Ah Li /Li
x
CoO
2
D-
size battery at 20⬚C. (Delivered specific energy at 1.0 A, 70 Wh/ lb.) (b)
Effect of charging rates on discharge performance of 30-Ah Li / Li
x
CoO
2
battery at 20⬚C. - - -, constant potential at 4.20 V [current limit at 5.0
mA/cm
2
(C / 2)]. ——, constant current at 1.0 mA / cm
2
(C / 10). Discharge
rate at C /6. (c) Cycle life characteristics of a Li/ Li
x
CoO
2
D-size battery.
Charge at 300 mA, 4.3 V. Discharge at 3 A, 3.0 V. (From Alliant Tech-
systems.).
34.34 CHAPTER THIRTY-FOUR
TABLE 34.14 Effect of Storage at 20⬚C on Capacity Retention of Charged Li/ Li
x
CoO
2
D-Size Batteries
Charge and
prestorage
test conditions
Average
capacity
of first
5 cycles, Ah
Discharge
capacity
after 15-day
(360-h)
storage, Ah
Capacity
loss
during
storage
period, %
Average
capacity of
next 5
cycles
after storage,
Ah
Permanent
capacity
loss due to
storage, %
4.3 V charge 5.1 4.6 9.8 5.2 0
(0.5 F / M)
4.1 V charge 4.05 3.65 9.9 4.0 1
(0.4 F / M)
4.1 V float charging 4.05 4.2 0 4.0 1
TABLE 34.15 Design Features of Li/ LiNiO
2
D-Size Batteries
Positive electrode (supported on aluminum grid):
89.5% LiNiO
2
, 2% graphite, 5% EC600 carbon,
0.015 in (0.38 mm)
Porosity 40%
Loading 75 mg /cm
2
Negative electrode (four copper wires running length of electrode):
15% Al-85% Li
0.004 in (0.10 mm)
Electrode capacity ratio:
Li anode
⫽ 3
LiNiO cathode
2
Electrolyte:
1.2M LiAsF
6
in PC /EC /DMC
18 ml
Separator:
Celgard 2400 (1 ply)
Polyethylene (2 ply)
General:
Working area 900 cm
2
Allowance for expansion 5%
Nickel-plated steel hardware
Molybdenum pin and TA-23 glass-to-metal seal
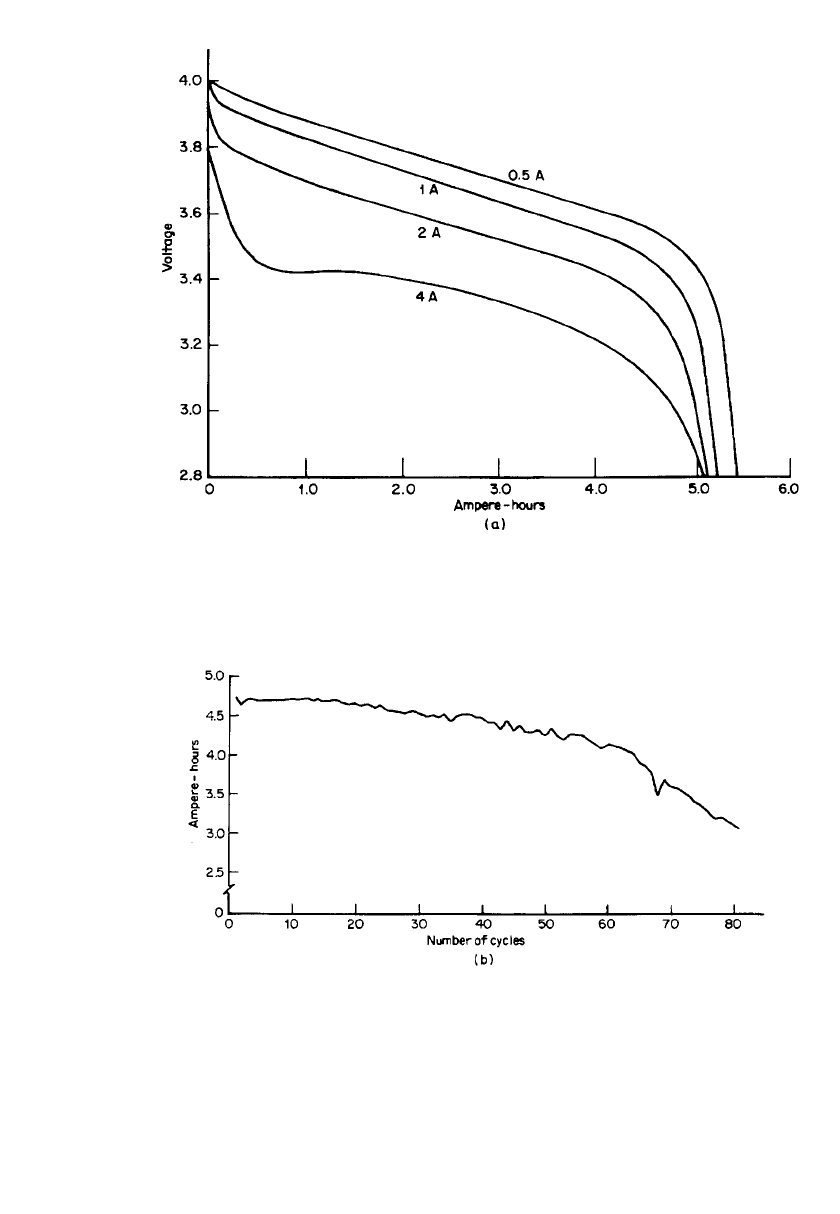
RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.35
FIGURE 34.20 (a) Discharge characteristics of a Li / Li
x
NiO
2
D-size battery. Charge at 0.3 A
to 4.1 V. (b) Cycle life characteristics of a Li/ Li
x
NiO
2
D-size battery. Discharge at 2 A to 2.8 V.
Charge at 0.4 A to 4.1 V. Taper to 0.1 mA. (From Ref. 42.)

34.36 CHAPTER THIRTY-FOUR
34.4.2 Polymer Electrolyte Cells
The polymer electrolyte lithium batteries contain all solid-state components: lithium as the
anode material, a thin polymer film as a solid electrolyte and separator, and a transition metal
chalcogenide or oxide, or a sulfur-based polymer as the cathode material. These features
offer the potential for improved safety because of the reduced activity of lithium with the
solid electrolyte, flexibility in design as the cell can be fabricated in various sizes and shapes,
and high energy density.
Figure 34.9 shows a schematic cross section of a solid polymer electrolyte cell. The
cathode and electrolyte are coated onto a current collector to form a thin sheet, called the
cathode laminate. The lithium metal foil is applied to the cathode laminate to form a layered
structure, with the solid polymer separating the lithium from the cathode. These cells are
designed in extremely thin components with high surface areas to minimize the internal
resistance and compensate for the lower conductivity of the polymer electrolyte. The thick-
ness depends on the specific cell design and required capacity. A thicker laminate delivers
a higher capacity per unit area of electrode, but with lower efficiency at the higher current
drains.
43
The early batteries used polyethylene oxide (PEO)-based electrolytes containing a lithium
salt, which have an appreciable conductivity at about 100
⬚C but low conductivity at room
temperature. Later new polymeric electrolyte materials, such as PEO copolymers, PEO
blends, plasticized PEO electrolytes, and gelled electrolytes, with better conductivity were
developed. Some of the cathode materials investigated for these batteries are TiS
2
,VO
x
,
V
2
O
5
,Li
x
CoO
2
and sulfur-based polymers.
The solid polymer electrolyte (SPE) battery has been considered for a wide range of
applications, from small portable electronics up to and including electric vehicles.
34.4.3 Lithium Batteries Using PEO-based Electrolytes
In the late 1970s, polymer electrolyte materials were proposed for use in solid-state battery
designs.
44
A considerable development effort has resulted in a number of review arti-
cles
45–47
describing the status of such batteries in some detail. The unique aspect of these
batteries is that the electrolyte is a solid flexible film comprised of a polymer matrix and an
ionic salt complexed into the matrix. Thin-film solid-polymer electrolyte batteries offer the
possibility of an intrinsically safe battery design in combination with good high-rate capa-
bility.
Polyethylene oxide was the first material utilized as the matrix material. Initially, it was
necessary to operate the cells at elevated temperatures (100
⬚C) to obtain adequate conduc-
tivities (10
⫺
3
S/ cm), but a variety of polymeric electrolytes that may be useful at normal
ambient temperatures were subsequently developed. This polymer electrolyte battery is based
on thin-film components that incorporate large area electrolyte and electrode layers. A gen-
eral cell can be schematized as:
⫹
Li 具A典/Li ion-conductor /Li 具B典
xz
where Li
x
具A典 is a metallic lithium anode or a low-voltage lithium-intercalation anode (neg-
ative electrode), the Li
⫹
ion conductor is a lithium salt-polymer complex, and Li
z
具B典 is a
high-voltage lithium-intercalation cathode (positive electrode). Usually, the polymer com-
ponent of the electrolyte layer is also the binder for the electrode layers. In addition, the

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.37
polymer component may be gelled by the addition of low molecular weight (liquid) solvents
to enhance its ionic conductivity. Such a battery system shows two major advantages with
respect to standard batteries that use inert porous separators:
1. The electrode and electrolyte layers are laminated (usually by heating and pressing the
layers), thus allowing various battery shapes without loss of contact.
2. Even if a low molecular weight (liquid) plasticizer is added to obtain high conductivity
at ambient and sub-ambient temperatures, there is no free liquid present in the battery
thus preventing any leakage problems.
Examples of the battery systems under development or available on the marketplace are
indicated in Table 34.16. They can be conveniently classified into three categories:
1. Positive and negative intercalation electrodes (lithium-ion) with a gelled polymer electro-
lyte (PEO-based).
2. Positive and negative intercalation electrodes (lithium-ion) with a porous polymer sepa-
rator layer filled with a liquid electrolyte (PVDF-based).
3. Positive intercalation electrode-lithium anode (lithium-metal) with a dry polymer electro-
lyte layer.
The first and the second categories, the latter of which (PVDF-based systems) does not
properly belong to the class of polymer electrolyte batteries, are covered in Chap. 35. In this
chapter, the attention is focused on the lithium metal, dry polymer electrolyte systems.
The search for high energy density batteries, especially for electric vehicle applications,
has led to the consideration of the use of lithium metal anodes. No other anode can attain
the same electrochemical performance in terms of specific energy as metallic lithium. How-
ever, safety issues as well as poor cycle life performance have prevented the commercial
success of lithium-metal batteries with the exception of primary batteries. At the present
time, there are three major programs aimed at the development of lithium-anode, dry-polymer
electrolyte batteries for electric vehicles (Table 34.17). Within these R&D programs, sub-
stantial successes have been obtained in terms of cyclability of the lithium-polymer electro-
lyte interface
51
as well as the whole battery system.
50
However, Argotech’s battery NPS-
24V80 (Table 34.18) appears to be the only system close to commercialization. This battery,
designed for telecommunication applications (especially for outside plants), is a fall-out of
the EV battery module developed within the USABC program.
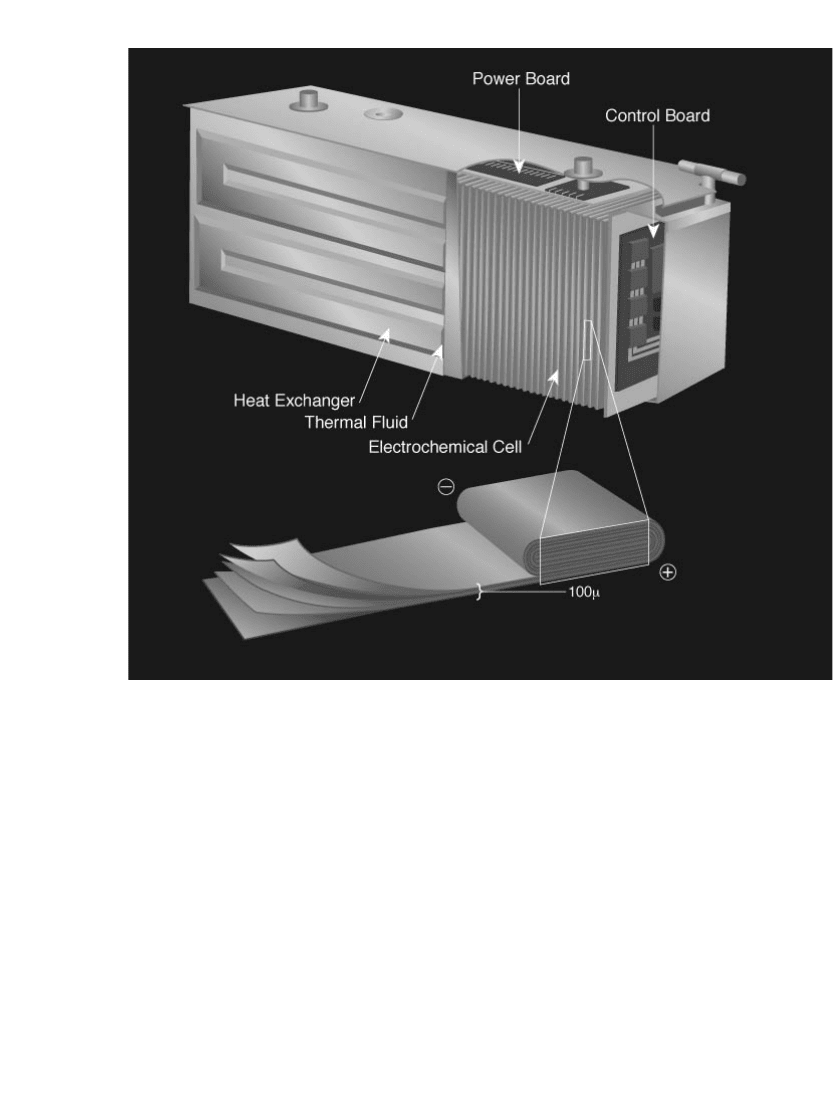
Figure 34.21 illustrates the internal design of the lithium polymer cell. The cell is made
by laminating five thin layers including an insulating layer, a lithium foil anode, a solid
polymeric electrolyte, a transition metal oxide cathode (VO
x
) and a current collector. The
unit cell is made by winding the laminated film into a jelly roll (as in Fig. 34.21) or a flat
roll (preferred for multiple cell battery assembly) structure. These cells are then assembled
in series and in parallel arrangements to form modules of different sizes and shapes for
numerous applications. As an example, Fig. 34.22 illustrates the design of a lithium-polymer
battery module for electric vehicle applications. Unit laminated cells are packaged into a
stack of flat cells to create a module. The cells can be connected in parallel and /or series
arrays within a single container to build the desired module capacity and voltage. The pack-
aging also provides the mechanical, electrical, and thermal controls required for operation.
Each module is a fully functional battery system, including intelligent control and monitoring
electronics that interface with the battery pack controller.

34.38
TABLE 34.16 Status of Lithium and Lithium-Ion Polymer Battery Development (
a
from Ref. 48;
b
from Ref. 49)
Manufacturer Country Cathode Anode Electrolyte
Voltage
V
Energy Specific
density energy
Wh/ L Wh / kg Application
Matsushita Battery
a
Japan LiCoO
2
Graphite PVDF gel 3.7 250/ 125 Cellular
Sony
a
Japan LiCoO
2
Graphite PVDF gel 3.7 245/ 125 Cellular, PC
Japan Storage
Battery
a
Japan LiCoO
2
Graphite PVDF gel 3.6 210/ 125 Cellular, MiniDisc
Hitachi Maxell
a
Japan LiCoO
2
Graphite PEO gel 3.6 130 / 90 Cellular, PC
Sanyo
a
Japan LiCoO
2
Graphite PEO gel? 3.6 200/ 120 Cellular
Toshiba
a
Japan LiCoO
2
Graphite PVDF gel 3.6 245/ 115 Cellular, PC
Yuasa Corp.
a
Japan LiCoO
2
Coke PEO gel 3.6 165 / 95 Cellular, MiniDisc
Hirion/ Mitsubishi
Chem.
a
Japan LiCoO
2
Graphite PEO gel 3.7 280 / 130 Cellular, PC
Ultralife
a
US LiMn
2
O
4
Graphite PVDF gel 3.7 185/ 105 Cellular, PC
Valence
a
US LiMn
2
O
4
Graphite PVDF gel 3.7 220/ 110 PC
Thomas & Betts
(HET)
a
US LiCoO
2
Graphite PVDF gel 3.7 220/ 120 Cellular
Lithium
Technology
a
US LiCoO
2
Graphite PVDF gel 3.6 240/ 125 PC
ElectroFuel
a
Canada LiCoO
2
Graphite PVDF gel 3.6 435/ 175 PC
Argotech
b
(HydroQuebec/ 3M)
Canada VO
x
Li-metal Dry PEO 24 (2.6) 110 / 97 Telecommunications
facilities
Shubila
a
Malaysia LiCoO
2
Graphite PVDF gel 3.6 215/ 120 Cellular

RECHARGEABLE LITHIUM BATTERIES (AMBIENT TEMPERATURE) 34.39
TABLE 34.17 Major Lithium Metal Anode, Dry Polymer Electrolyte (PEO-based), Battery
Technology Development Projects (from Ref. 50)
Sponsor Project leaders Cathode Application Status
USABC (USA) IREQ-3M-ANL VO
x
EV 2.4 kWh
prototypes
Bollore´ Tech.
EDF (France)
CEREM VO
x
,Li
y
MnO
x
EV Prototypes
MICA-MURST
(Italy)
ENEA VO
x
,Li
y
MnO
x
EV Scaling-up to
1 kWh
prototypes
TABLE 34.18 Specifications of Argo-Tech’s Batteries from Ref. 49
NPS-24V80 EV module
Nominal voltage
24 V /Module
2.65 V /Cell
20 V
Rated capacity 80 Ah (@ C /8) 119 Ah (@ C / 3)
Rated energy 1.9 kWh (@ C /8) 2.4 kWh @ C / 3)
Maximum discharge current 20 A 365 A
Float voltage
27.9 V /Module
3.1 V /Cell
N/A
Energy density 110 WhL
⫺
1
220 WhL
⫺
1
Specific energy 97 Whkg
⫺
1
155 Whkg
⫺
1
Length 394 mm Application specific
Width 176 mm Application specific
Height 251 mm Application specific
Volume 17.4 dm
3
ca.11dm
3
Weight 19.9 kg 15.7 kg
Ambient operating temperature
⫺40 to 65⬚C N/A
Storage temperature
⫺40 to 65⬚C N/A
Float life at 40
⬚C Over 10 years N /A
Cycle life at 60
⬚C (80%
DOD)
200 cycles 600 cycles
FIGURE 34.21 Laminated lithium-polymer electrolyte cell assembly. (From the Proceedings of EVS 16,
reprinted by permission of the publisher, EVAAP.)
34.40 CHAPTER THIRTY-FOUR
FIGURE 34.22 Design of a Lithium Polymer Battery (LPB) module for EV applications. The module
includes an enclosure which provides mechanical support and thermal insulation along with control hardware
and vehicle interfaces. (From the Proceedings of EVS 16, reprinted by permission of the publisher, EVAAP.)
The specification given for the stationary application NPS-24V80 battery are given in
Table 34.18 and compared with the parent EV-module. A typical discharge curve is depicted
in Figure 34.23. The battery is able to deliver 80 Ah capacity at a 10 A discharge rate in a
60
⬚C temperature environment. The excellent performance at high ambient temperature is
one of the major advantages of this system. The unit cells operate at 40 to 60
⬚C, and do not
contain any liquid: The battery is designed to have no degradation in non-ventilated and
warm environments (for example: telecommunication centers). It contains no liquid, thus it
is hermetically sealed, and dry-out during operation and liquid leakage are impossible, and
therefore no maintenance is required. In addition, the battery shows very good safety char-
acteristics. Overcharging tests showed no gas generation.
53
Once more, no liquid leakage is
possible even when the module is accidentally crushed. Finally and most important, water
immersion tests on entire crushed cells showed only a very slow reaction.
