Leroy C., Rancoita P.-G. Principles Of Radiation Interaction In Matter And Detection
Подождите немного. Документ загружается.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
140 Principles of Radiation Interaction in Matter and Detection
Table 2.12 Energies of the absorption edges above 10 keV for ele-
ments with Z from 69 up to 100 from [Hubbell (1969)].
Element Z K-edge L
I
-edge L
II
-edge L
III
-edge
keV keV keV keV
Tm 69 59.380 10.121
Yb 70 61.300 10.490
Lu 71 63.310 10.874 10.345
Hf 72 65.310 11.274 10.736
Ta 73 67.403 11.682 11.132
W 74 69.508 12.100 11.538 10.200
Re 75 71.658 12.530 11.954 10.531
Os 76 73.856 12.972 12.381 10.868
Ir 77 76.101 13.423 12.820 11.212
Pt 78 78.381 13.883 13.272 11.562
Au 79 80.720 14.354 13.736 11.921
Hg 80 83.109 14.842 14.212 12.286
Tl 81 85.533 15.343 14.699 12.660
Pb 82 88.005 15.855 15.205 13.041
Bi 83 90.534 16.376 15.719 13.426
Po 84 93.112 16.935 16.244 13.817
At 85 95.740 17.490 16.784 14.215
Rn 86 98.418 18.058 17.337 14.618
Fr 87 101.147 18.638 17.904 15.028
Ra 88 103.927 19.236 18.486 15.444
Ac 89 106.759 19.842 19.078 15.865
Th 90 109.646 20.464 19.683 16.299
Pa 91 112.581 21.102 20.311 16.731
U 92 115.620 21.771 20.945 17.165
Np 93 118.619 21.417 20.596 17.614
Pu 94 121.720 23.109 22.253 18.054
Am 95 124.876 23.793 22.944 18.525
Cm 96 128.088 24.503 23.640 18.990
Bk 97 131.357 25.230 24.352 19.461
Cf 98 134.683 25.971 25.080 19.938
E 99 138.067 26.729 25.824 20.422
Fm 100 141.510 27.503 26.584 20.912
section and the emitted-electron angular distribution can be obtained following
Heitler’s treatment (Chapter V, Section 21 in [Heitler (1954)]) and using the cor-
rections by Bethe and Ashkin (Section 3 in [Bethe and Ashkin (1953)]).
In the non-relativistic region
∗
, the Born approximation can be used for inco-
ming photon-energies large compared with the ionization energy of the K-shell elec-
trons. The angular distribution of the emitted electrons is expressed by the K-shell
differential cross section per atom:
dτ
k,B
dΩ
= 4
√
2 r
2
e
Z
5
137
4
µ
mc
2
hν
¶
7/2
sin
2
θ cos
2
φ
(1 − β cos θ)
4
, (2.158)
where β is the velocity of the emitted electron in units of the speed of light, θ is the
angle between the directions of the incoming photon and the emitted electron, φ is
∗
This energy region is for hν ¿ mc
2
, where m is the rest mass of the electron.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 141
Table 2.13 Empirical-fit parameters to K-shell photoelectric cross
section above 200 keV from [Hubbell (1969)].
n a
n
b
n
c
n
p
n
1 1.6268 × 10
−9
−2.683 × 10
−12
4.173 × 10
−2
1
2 1.5274 × 10
−9
−5.110 × 10
−13
1.027 × 10
−2
2
3 1.1330 × 10
−9
−2.177 × 10
−12
2.013 × 10
−2
3.5
4 −9.1200 × 10
−11
0 0 4
the angle between the scattering plane
†
and the direction of the incoming radiation
polarization (see Chapter V, Section 21 in [Heitler (1954)]). From Eq. (2.158), we
note that photoelectrons are mostly emitted along the polarization direction of the
incoming radiation (i.e., θ =
1
2
π and φ = 0). While along the incoming-photon
direction (i.e., θ = 0), the differential cross section goes to zero, i.e., no photoelec-
tron is emitted in the very forward direction. Furthermore [see the denominator
of Eq. (2.158)], although the photoelectrons can also be emitted in the backward
hemisphere (i.e., with emission angles larger than 90
◦
), they will be emitted more
and more in the forward hemisphere (i.e., with emission angles lower than 90
◦
) as
the photon energy increases. The K-shell total photoelectric cross section can be
derived from Eq. (2.158) by neglecting the term β cos θ in the denominator, then,
integrating over the full solid angle and, finally, multiplying the result by a factor
2 to account for two K-shell electrons; thus, we have:
τ
k,B
= 2
Z
dτ
k,B
dΩ
dΩ
≈ 2
Z
4
√
2 r
2
e
Z
5
137
4
µ
mc
2
hν
¶
7/2
sin
2
θ cos
2
φ dΩ
= σ
T h
4
√
2
Z
5
137
4
µ
mc
2
hν
¶
7/2
, (2.159)
where σ
T h
= (8/3)πr
2
e
(' 6.6516 × 10
−25
cm
2
) is the classical Thomson scattering
cross section. For heavy elements or for incoming photon energies close to those
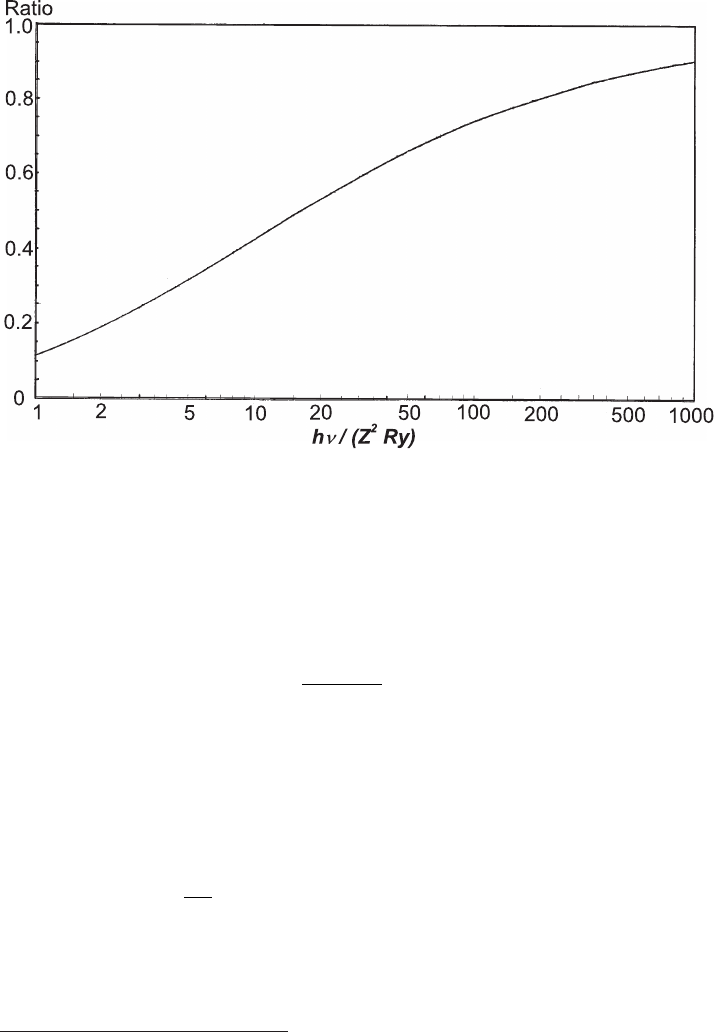
of absorption edges, the Born approximation is no longer valid and exact wave
functions must be used. Figure 2.49 shows the ratio between the τ
k
values, calcu-
lated with exact wave functions, and those of τ
k,B
[Eq. (2.159)] as a function of
hν/(Z
2
Ry).
There are a few direct measurements of total and K-shell photoelectric cross
sections in the region between 100 keV and 3 MeV. However, most of the theoretical
information was derived for the K-shell component of the cross section (see [Hubbell
(1969)]). The extensive results by Rakavy and Ron (1965) include almost all the
higher shells and cover the range between 1 keV and 2 MeV. In the energy range
between 10 and 200 keV, the calculations [Rakavy and Ron (1965)] are in very good
agreement with the experimental data. The uncertainties on K-shell cross sections
†
It is the plane determined by the directions of the incoming photon and the emitted electron.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
142 Principles of Radiation Interaction in Matter and Detection
Fig. 2.49 Ratio τ
k
/τ
k,B
as a function of E
ν
= hν/(Z
2
Ry) (adapted and reprinted with permission
from [Bethe and Ashkin (1953)]). τ
k,B
is calculated by means of Eq. (2.159).
are estimated to be about 2% in the region (0.2–100) MeV. Over the total photo-
electric cross section, the uncertainties are more likely to be about (3–5)% [Hubbell
(1969)]. Above 200 keV, an empirical formula for the K-shell photoelectric cross
section per atom is:
τ
k
≈ Z
5
4
X
n=1
a
n
+ b
n
Z
1 + c
n
Z
E
−p
n
γ
[b/atom], (2.160)
where E
γ
is the incoming photon energy in MeV and the parameters a
n
, b
n
, c
n
,
and p
n
are given in Table 2.13.
To a first approximation, for energies above K-shell binding energies, the total
photoelectric cross section τ
ph
per atom can be obtained multiplying τ
k
by the
ratios
§
derived by Kirchner and Davisson [Kirchner et al. (1930)]. These ratios can
be computed, within an accuracy of ± (2–3)%, by the formula [Hubbell (1969)]:
τ
ph
τ
k
≈ 1 + 0.01481 ln
2
Z − 0.000788 ln
3
Z. (2.161)
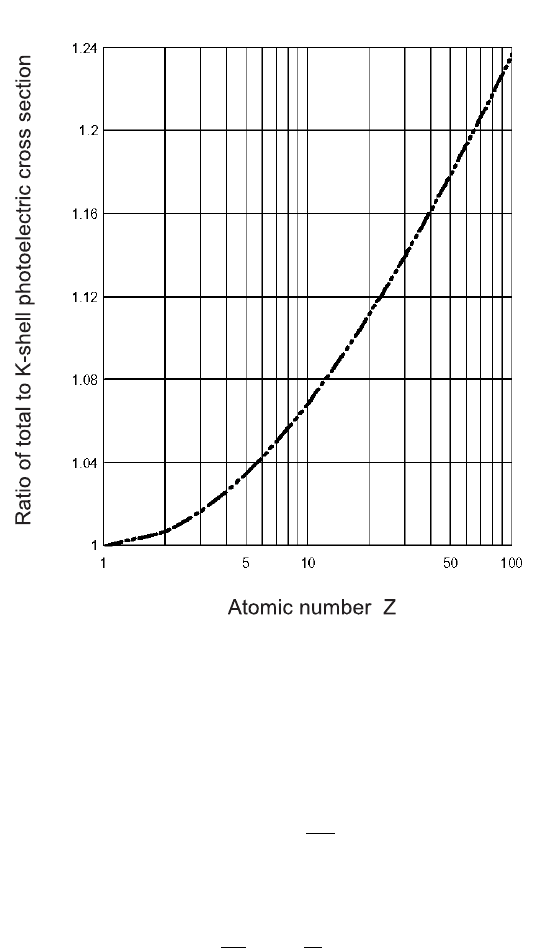
From Eq. (2.161), we note that, at large atomic numbers, the total photoelec-
tric cross section does not exceed the K-shell cross section by more than ≈ 25%
(Fig. 2.50). Other approximate empirical formulae
¶
for photoelectric cross sections
can be found and are based on calculations made by Pratt (1960).
§
These ratios are assumed to be almost energy independent.
¶
For instance, the reader can see Chapter 3, Section 7 in [Messel and Crawford (1970)].
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 143
Fig. 2.50 Ratio of the total over K-shell photoelectric cross section as a function of the atomic
number Z, from Eq. (2.161).
The contribution to the total linear attenuation coefficient due to photoelectric
effect is given by
µ
att,l,ph
= n
A
τ
ph
=
ρN
A
τ
ph
[cm
−1
] (2.162)
with τ
ph
in cm
2
/atom, while the total mass attenuation coefficient due to photo-
electric effect is:
µ
att,m,ph
=
n
A
ρ
τ
ph
=
N
A
τ
ph
[g
−1
cm
2
]. (2.163)
For instance, let us estimate the photon attenuation coefficients in Al absorber
at 20 keV incoming photon energy. At this energy, the photoelectric effect is the
dominant absorption process in low-Z media. τ
k,B
[computed from Eq. (2.159)]
is ≈ 3.11 × 10
2
b/atom, while the ratio τ
k
/τ
k,B
is ≈ 0.4 for hν/(Z
2
Ry) ≈ 8.7
(Fig. 2.49). Thus, τ
k
is about 1.24 ×10
2
b/atom. Using Eq. (2.161), the ratio of the
total to the K-shell photoelectric cross section is estimated to be ≈ 1.09. Therefore,

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
144 Principles of Radiation Interaction in Matter and Detection
the total photoelectric cross section becomes approximately
τ
ph
≈ 1.09 τ
k
= 1.35 × 10
2
b/atom.
Finally, from Eqs. (2.162, 2.163) the linear and mass attenuation coefficients are
≈ 8.1 cm
−1
and 3 g
−1
cm
2
, respectively. Similarly, we can calculate the attenuation
coefficients at 300 keV in Pb absorber. At this energy in Pb, the photon absorption
is almost due to the photoelectric interaction. τ
k
, computed by means of Eq. (2.160),
is about 0.86 × 10
2
b/atom. Therefore, from Eq. (2.161) the ratio of the total to
K-shell photoelectric cross section is estimated to be ≈ 1.22. Consequently, the total
photoelectric cross section becomes approximately
τ
ph
≈ 1.22 τ
k
= 1.05 × 10
2
b/atom.
From Eqs. (2.162, 2.163), the linear and mass attenuation coefficients are ≈ 3.5 cm
−1
and 0.3 g
−1
cm
2
, respectively.
Because the total photoelectric cross section depends on the atomic number Z
to a power close to 5, the photon absorption depends strongly on the medium for
photon energies for which the photoelectric process is dominant.
2.3.1.1 The Auger Effect
As previously discussed, there are processes (like the photoelectric effect) which
allow the emission of bound atomic electrons. However, when electrons are ejected
from an atomic shell, a vacancy is created in that shell leaving the atom in an
excited state. The atom with an electron vacancy in the innermost K-shell can
readjust itself to a more stable state by emitting one or more electrons instead of
radiating a single X-ray photon. This internal adjustment process is named after
the French physicist Pierre-Victor Auger, who discovered it in 1925 [Auger (1925)].
When an electron of the higher L-shell makes a transition to fill a K-shell electron
vacancy, the available amount of energy is the difference between the K-shell and
L-shell binding energies: B
e
(K) − B
e
(L). This energy can be released via a photon
(radiative emission) or absorbed by a bound electron of an higher shell, causing its
ejection. This soft electron is called Auger electron.
The probability of non-radiative transition, with emission of Auger electrons,
is larger for low-Z material (see for instance experimental data in [Burhop (1955);
Krause (1979)]). The Auger yield decreases with the atomic number Z, and at
Z ≈ 30 the probabilities of X-rays emission from the innermost shell and of the
emission of Auger electrons are almost equal. An empirical formula [Burhop (1955)]
for the K-fluorescence yield N
K
as a function of the atomic number is:
N
K
1 − N
K
= (−6.4 + 3.4Z −0.000103Z
3
)
4
× 10
−8
, (2.164)
where N
K
(the K-fluorescence yield) is the probability for emitting photons per
K-shell vacancy, 1 − N
K
is the Auger yield (i.e., the probability for ejecting Auger
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 145
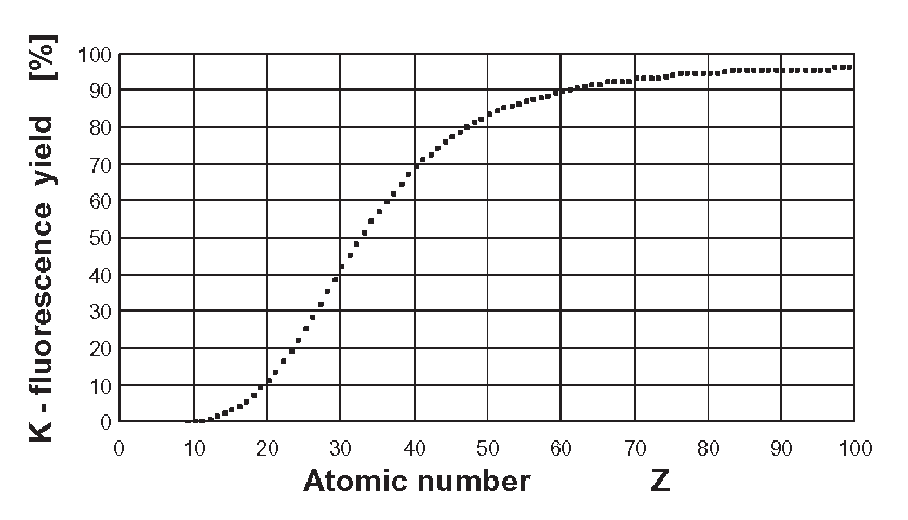
Fig. 2.51 Percentage of the K-fluorescence yield N
K
computed by means of Eq. (2.165) as a function of the atomic number Z.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
146 Principles of Radiation Interaction in Matter and Detection
electrons per K-shell vacancy). From Eq. (2.164), we have:
N
K
= (1 − N
K
)(−6.4 + 3.4Z − 0.000103 Z
3
)
4
× 10
−8
=
(−6.4 + 3.4Z − 0.000103 Z
3
)
4
× 10
−8
1 + (−6.4 + 3.4Z − 0.000103 Z
3
)
4
× 10
−8
. (2.165)
N
K
as a function of Z is shown in Fig. 2.51.
2.3.2 The Compton Scattering
The Compton effect is based on the corpuscular behavior of the incident radia-
tion and it is an incoherent scattering process on individual atomic electrons. These
electrons can be described as quasi-free, i.e., to a first approximation their binding
energies do not affect the interaction and can be neglected in calculations. Further-
more, it is considered as an inelastic process, although the kinematics description
of the reaction is that of an elastic collision.
The effect was observed for the first time by Compton (1922), who provided
a theoretical explanation, and it is depicted in Fig. 2.52. In the Compton effect,
an incoming photon of momentum hν/c interacts with a (quasi-)free electron at
rest. The scattered photon emerges at an angle θ
ν
with a momentum hν
0
/c, while
the electron recoils at an angle θ
e
with momentum ~p. By requiring momentum
conservation along the incoming photon direction, we have
hν
c
=
hν
0
c
cos θ
ν
+ p cos θ
e
,
from which we obtain
p
2
c
2
cos
2
θ
e
= h
2
(ν − ν
0
cos θ
ν
)
2
; (2.166)
Fig. 2.52 Compton scattering of an incident photon with incoming momentum hν/c onto a quasi-
free electron which emerges at an angle θ
e
. The photon is scattered at an angle θ
ν
with a momentum
hν
0
/c.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 147
while in the perpendicular direction, we get:
hν
0
c
sin θ
ν
= p sin θ
e
,
from which we find
p
2
c
2
sin θ
2
e
= h
2
ν
02
sin
2
θ
ν
. (2.167)
Due to energy conservation, we have
mc
2
+ hν = hν
0
+ mc
2
+ K
e
, (2.168)
where m and K
e
are the mass and kinetic energy of the electron, respectively. Equa-
tion (2.168) can be written as:
K
e
= hν − hν
0
= h(ν −ν
0
). (2.169)
Furthermore, see Eq. (1.8), the total electron energy is given by:
mc
2
+ K
e
=
p
p
2
c
2
+ m
2
c
4
⇒ m
2
c
4
+ K
2
e
+ 2mc
2
K
e
= p
2
c
2
+ m
2
c
4
,
from which we have
p
2
c
2
= K
e
(K
e
+ 2mc
2
). (2.170)
By summing Eqs. (2.166, 2.167) and substituting p
2
c
2
with the value given by
Eq. (2.170), we obtain
K
2
e
+ 2mc
2
K
e
= h
2
h
(ν − ν
0
cos θ
ν
)
2
+ ν
02
sin
2
θ
ν
i
= h
2
¡
ν
2
− 2νν
0
cos θ
ν
+ ν
02
¢
,
where we can substitute K
e
with the value obtained from Eq. (2.169):
h
2
(ν − ν
0
)
2
+ 2mc
2
h(ν − ν
0
) = h
2
¡
ν
2
− 2νν
0
cos θ
ν
+ ν
02
¢
⇒ h
2
ν
2
− 2h
2
νν
0
+ h
2
ν
02
+ 2mc
2
h(ν − ν
0
) = h
2
ν
2
− 2h
2
νν
0
cos θ
ν
+ h
2
ν
02
and, finally, we get
mc
2
h(ν − ν
0
) = h
2
νν
0
(1 − cos θ
ν
) . (2.171)
By introducing the Compton wavelength of the electron
λ
e
≡
h
mc
, (2.172)
considering that the photon wavelength and frequency are related by νλ = c and,
finally, by dividing both terms by νν
0
h
2
, we can rewrite Eq. (2.171) as the so-called
Compton shift formula:
4λ ≡ λ
0
− λ = λ
e
(1 − cos θ
ν
) . (2.173)
The quantity 4λ is called wavelength Compton shift. It increases as the photon
scattering angle θ
ν
increases. The maximum wavelength shift occurs for θ
ν
= 180
◦
,
i.e., for backward scattered photons for which 4λ = 2λ
e
.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
148 Principles of Radiation Interaction in Matter and Detection
The scattered photon energy depends on the photon scattering angle θ
ν
and it is
related to the incoming photon energy. The relationship can be derived by rewriting
Eq. (2.171) as:
hν = hν
0
+
h
2
mc
2
νν
0
(1 − cos θ
ν
) = hν
0
[1 + E (1 − cos θ
ν
)] ,
where
E ≡
hν
mc
2
is the reduced energy of the incoming photon. Thus, the fraction of the incoming
photon energy carried by the scattered photon is
hν
0
hν
=
1
1 + E (1 − cos θ
ν
)
, (2.174)
and, conversely, for the cosine of the photon scattering angle:
cos θ
ν
= 1 −
1
E
µ
hν
hν
0
− 1
¶
. (2.175)
From Eq. (2.174), for photons scattered in the forward direction, i.e., θ
ν
→ 0
◦
, we
have hν
0
→ hν independently of the incoming photon energy hν. In addition, at
very low energies for which E ¿ 1, the energy of the scattered photon becomes
hν
0
≈ hν, independently of the scattering angle θ
ν
. Under such circumstances, the
electron kinetic energy becomes negligible. From Eqs. (2.169, 2.174), the electron
kinetic energy can be rewritten as
K
e
= hν
E (1 − cos θ
ν
)
1 + E (1 − cos θ
ν
)
, (2.176)
or, equivalently, as a function of the recoil electron angle θ
e
:
K
e
= hν
2 E cos
2
θ
e
(1 + E)
2
− E
2
cos
2
θ
e
. (2.177)
The angles θ
e
and θ
ν
are related by:
tan θ
e
=
1
1 + E
cot
θ
ν
2
. (2.178)
In soft collisions, the maximum electron recoil angle is θ
e
= 90
◦
and is reached when
the photon is scattered at angle θ
ν
= 0
◦
, while for backward scattered photons, i.e.,
at the largest Compton shift [see Eq. (2.173)] where θ
ν
→ 180
◦
, the recoiling electron
is emitted at angle θ
e
→ 0
◦
. From Eqs. (2.176, 2.177), we note that, for backward
scattered photons, the electron kinetic energy reaches its maximum value:
K
e,m
= hν
2 E
1 + 2 E
.
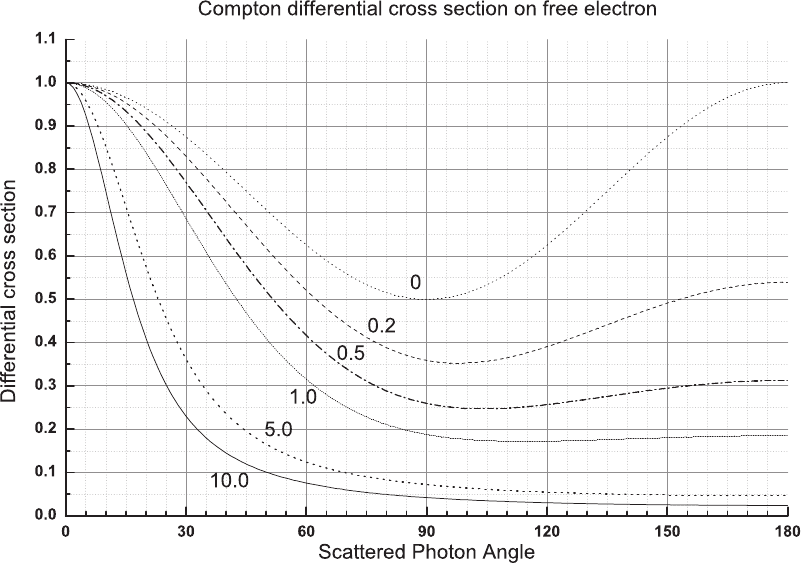
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Electromagnetic Interaction of Radiation in Matter 149
Fig. 2.53 Compton differential cross section on free electrons in units of r
2
e
as a function of the scattered photon angle θ
ν
, for reduced energies
E = 0, 0.2, 0.5, 1.0, 5.0, and 10. The curves were calculated by means of Eq. (2.180).
