Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

FURTHER READING
Bash, R., and Lohr, D. (2001). Yeast chromatin structure and
regulation of GAL gene expression. Prog. Nucl. Acids Res. Mol.
Biol. 65, 197–259.
Johnston, M. (1987). A model fungal gene regulatory mechanism: The
GAL genes of Saccharomyces cerevisiae. Microbiol. Rev. 51,
458–476.
Johnston, M., and Carlson, M. (1992). Regulation of carbon and
phosphate utilization. In The Molecular and Cellular Biology of the
Yeast Saccharomyces Vol. II, pp. 193–281. Cold Springs Harbor
Laboratory Press, Cold Springs Harbor, New York.
Lohr, D. (1997). Nucleosome transactions on the promoters of the
yeast GAL and PHO genes. J. Biol. Chem. 272, 26795–26798.
Lohr, D., Venkov, P., and Zlatanova, J. (1995). Transcriptional
regulation in the yeast GAL gene family: A complex genetic
network. FASEB J. 9, 777–787.
Reece, R., and Platt, A. (1997). Signalling activation and repression of
RNA polymerase II transcription in yeast. BioEssays 19,
1001–1010.
BIOGRAPHY
Dennis Lohr is a Professor in the Department of Chemistry and
Biochemistry at Arizona State University, with principal interests in the
relationships between chromatin structure and gene expression in
eukaryotes. He holds a Ph.D. from the University of North Carolina
(Chapel Hill) and was a postdoctoral associate in the Biochemistry and
Biophysics Department at Oregon State University, in the lab of
K. VanHolde. At Oregon State, he carried out most of the initial
characterizations of yeast chromatin structure and in his own lab
carried out the first detailed structural analyses of a single copy
eukaryotic promoter, the GAL1–10 promoter, and its accompanying
gene, GAL1.
Ralph Bash is a postdoctoral Research Associate at the Arizona
Biodesign Institute and the Department of Physics and Astronomy at
Arizona State University with interests in chromatin structure,
chromatin function and molecular imaging. He holds a Masters from
San Jose State University and a Ph.D. from Arizona State University,
where he studied intrinsic DNA bending on GAL genes.
YEAST GAL1–GAL10 SYSTEM 433

Zinc Fingers
Mark Isalan
European Molecular Biology Laboratory (EMBL), Heidelberg, Germany
Zinc fingers are small, compact protein subunits, with
structured peptide chains folding around chelated zinc ions.
Functionally, these subunits carry out a wide variety of tasks
within cells by providing stable structural scaffolds and driving
critical binding interactions, especially between proteins,
DNA, and RNA. Zinc fingers are particularly important in
gene regulation, where many proteins employ them to bind
DNA in a sequence-specific manner, so as to activate or inhibit
particular genes. Although many families of zinc fingers exist,
each with a characteristic fold or structure, perhaps the best
studied is the “classical” (“Cys
2
–His
2
”
or “C2H2”) type.
Classical zinc fingers are extremely versatile, as can be seen by
their abundance in nature: over 700 proteins contain such
domains in the human genome alone, making them the second
most common human protein motif. Zinc fingers may be seen
as convenient molecular building blocks, often used in clusters,
whose modular nature allows them to be conveniently
duplicated, altered, and shuffled by evolution. In a biotechnol-
ogy context, zinc fingers may be re-engineered to have novel
binding properties with numerous applications in both
research and medicine.
Zinc Finger Structures
and Families
CLASSICAL ZINC FINGERS
History
The zinc finger motif was discovered in 1985 by Miller,
McLachlan, and Klug in a protein responsible for
controlling gene expression: the Xenopus transcription
factor IIIA (TFIIIA). Originally, nine novel consecutive
repeats were found, each , 30 amino acids in length.
Since every repeat had a conserved pair of cysteines and
histidines at defined positions, Klug proposed that these
residues could chelate a zinc ion, stabilizing an
independent structural domain, the “zinc finger.”
Notably, the term “finger” was derived from the
finger-like appearance of the sequences when drawn
schematically around a zinc ion, as in Figure 1(A). The
repeats found in TFIIIA are now used as a definition
of the classical zinc finger, where individual fingers
conform to the archetypal consensus sequence shown
in Figure 2.
Secondary Structure and Hydrophobic Core
Structural studies revealed that the classical Cys
2
–His
2
finger is a simple and compact unit based on an
antiparallel
b
-hairpin packed against an
a
-helix
(Figure 1(B)). In addition to chelating zinc, classical
fingers are stabilized at the core by a hydrophobic cluster
of residues, converging from three fixed positions (see
Figures 1 and 2). Moreover, this hydrophobic triad is
highly conserved, often consisting of tyrosine, phenyl-
alanine, and leucine. By contrast, zinc fingers may
withstand extensive amino acid substitutions at other
positions, without significantly altering the overall
structure.
The Role of Zinc
Small protein domains are inherently unstable and, even
though the
bba
fold found in zinc fingers can form
around hydrophobic cores alone, the stability of the fold
is dramatically improved by zinc chelation. Such
stability cannot be obtained using the disulfide
bridge – a common extracellular alternative – because
of the highly reducing environment inside cells. Con-
sequently, intracellular proteins have solved the problem
of stabilizing small domains by utilizing metal-binding
sites. Zinc is ideally suited for this purpose as it has a
single oxidation state, a fixed and distinguishable
ionic radius, and can accommodate both nitrogen and
sulfur ligands.
Evolution
Zinc fingers may have evolved originally from peptides
which maintained a simple
bba
structure (similar to that
shown in Figure 1(B)) independently of zinc. In any case,
the
bba
fold is one of the smallest possible units of
protein tertiary structure, and may therefore be one of
the oldest. Zinc fingers are very abundant in nature and
the huge variety found in various organisms, from yeast
to human, may in part be due to their modular nature.
z
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 435
In this regard, not only are zinc fingers independently
folding structural building blocks, but they are often
encoded by single exons. Indeed, exon shuffling and gene
duplication may account for why zinc fingers have
spread so widely during evolution.
Genomic Abundance
The recent initial drafts of the human genome have
revealed the extent to which classical zinc fingers are
used by cells: there are 706 genes containing one or more
classical zinc fingers in humans, totaling several thou-
sand fingers, and making them the second most common
human protein family. It is remarkable that only
immunoglobulin domains are more abundant in
humans, and indeed only slightly more so, being found
in 765 genes. By contrast, the Drosophila melanogaster
fly has 357 classical zinc finger genes, actually making
these the most common protein family in this organism.
Despite the fact that zinc finger usage varies from
F
Q
AB
CD
EF
C
R
I
C
M
R
N
F
S
R
S
D
D
L
T
T
H
I
R
T
H
T
G
E
KP
hf
K
C
F
N
C
G
K
G
A
H
I
R
N
C
R
A
:C
V
N:
E
R
Y
C
A
V
C
N
D
Y
A
S
G
Y
H
Y
V
W
S
C
E
G
C
K
A
F
FK
G
R
C
D
I
C
N
S
C
E
C
K
R
Y
C
A
N:
A
K
C
K
L
SC:
Q
K
K
P
N
W
E
K
L
L
K
K
C
C
C
C
C
C
C
H
A
F
Y
V
L
P
L
A
M
S
Y
N
S
P
D
E
L
I
P
R
E
A
V
T
A
M
N:
I
T
R
W
I
R
Q
N
P
T
K
V
P
V
E
S
V
V
H
T
I
E
S
D
S
E
F
:C
:C
N:
:C
N:
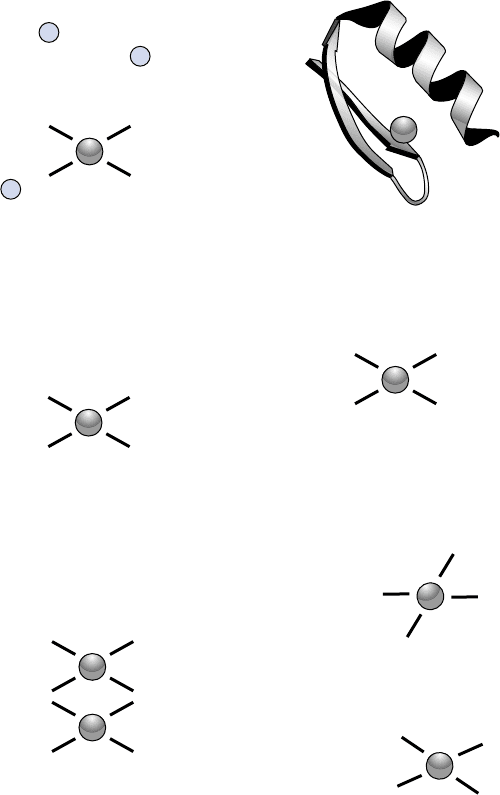
FIGURE 1 Examples of zinc finger families and folds. (A) A classical Cys
2
–His
2
zinc finger is drawn schematically as a string of amino acids
(1-letter abbreviations), with cysteine (C) and histidine (H) pairs chelating the central zinc ion. Three circled residues (F, F, L) come together in the
structure to form a hydrophobic core. The finger shown is Finger 2 from the mouse transcription factor Zif268. (B) Representation of the actual
bba
structure of a classical Cys
2
–His
2
zinc finger. Note the position of the hydrophobic core (h
f
). (C) A Cys
4
steroid-receptor zinc finger from the
estrogen receptor. Note that this protein actually contains two contiguous Cys
4
zinc finger repeats, forming a single structural unit. (D) A Cys
3
–His
zinc finger from a viral Nucleocapsid protein. (E) A yeast GAL4 domain. (F) The “ring” finger fold.
436 ZINC FINGERS
organism to organism, these motifs are clearly a wide-
spread feature in all eukaryotic cells.
NON-CLASSICAL ZINC FINGERS
Although zinc-binding proteins have been known for
many years, the usual definition of zinc fingers maintains
that they are distinct from other zinc-binding protein
subregions by virtue of being autonomously folding,
functional mini-domains. Following the discovery of the
classical Cys
2
–His
2
zinc finger, many other classes of
“zinc finger” have emerged. Although these form a
diverse collection, often with radically different folding
topologies, these proteins are often referred to in the
literature as zinc fingers. Therefore some well-studied
examples are outlined here below.
Cys
4
Zinc Fingers
One of the best-characterized nonclassical zinc fingers is
the Cys
4
type, including the steroid-hormone receptor
family. The estrogen receptor (Figure 1(C)) was the first
protein of this type to be elucidated structurally and the
gene contains two Cys
4
zinc finger repeats, forming a
single structural unit that is quite unlike the independent
classical zinc fingers of TFIIIA. Functionally, the steroid
receptors form dimers, that bind hormones like estro-
gen, and then mediate activation of gene expression
through sequence-specific DNA-binding interactions in
the promoter regions of target genes. Further examples
of proteins containing Cys
4
finger motifs include other
nuclear hormone receptors, such as the glucocorticoid
and retinoic acid receptors. Many Cys
4
proteins function
as transcription factors, such as the elongation factor
TFIIS and the adenoviral E1A transactivator.
Cys
3
–His Zinc Fingers
The Cys
3
–His zinc finger is another variant defined by
its characteristic combination of cysteine and histidine
residues. For example, the nucleocapsid protein, found
in certain viruses such as HIV, contains Cys
3
–His
fingers, with various nucleic acid binding properties
that facilitate hybridization and the viral life-cycle
(Figure 1(D)). Other examples of Cys
3
–His proteins
include MetRS and the first finger in the LIM domain.
Multi-Zinc Proteins
Moving beyond the simpler zinc finger domains, which
fold around a single zinc ion, there exist larger more
complex folds, each employing two or more zinc ions.
Examples include the yeast GAL4 domain (Figure 1(E))
and the “ring” finger (Figure 1(F)). Note that although
these larger domains are not quite as prevalent as the
single-zinc modules, they nonetheless form quite abun-
dant families. For example, the “ring” finger family
ranks as the 15th most abundant domain in the human
genome, with 210 members, while another fold, the
“PHD” finger, ranks 34th with 84 members.
Zinc Finger Functions
INTERACTIONS WITH DNA AND RNA
Since their discovery, zinc fingers have been associated
with binding nucleic acids, namely, DNA and RNA.
Indeed, the archetypal zinc finger protein, TFIIIA, is
bi-functional, having distinct modes of binding which
allow it to bind both to the DNA internal control region
of 5S RNA genes and to the transcribed RNA gene
product. In this sense, zinc fingers are master gene
regulators, with the potential to influence both tran-
scription and translation.
Structure of Zinc Finger Bound to DNA
The majority of our knowledge of zinc finger functions
has focused on their ability to bind specific DNA
sequences. The first structure of zinc fingers bound to
DNA, that of the DNA-binding domain of the mouse
transcription factor Zif268, was solved by Carl Pabo
and colleagues in 1991. The structure revealed that each
of the three classical zinc fingers occupies the major
groove, wrapping around the DNA for almost one turn
of the double helix (Figure 3A). Base specific contacts
occur primarily from consecutive turns of the zinc finger
a
-helices to the guanine-rich strand of the DNA.
DNA Recognition Helix
Essentially, classical zinc fingers use the
a
-helix as a
“reading head” to recognize specific DNA sequences.
Contacts from amino acids consist primarily of electro-
static interactions and hydrogen bonds, although
hydrophobic interactions also play a role. Convention-
ally, the amino acids in the
a
-helix are numbered
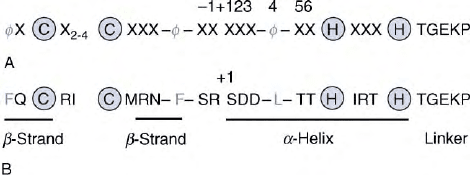
FIGURE 2 Zinc finger amino acid sequences. (A) Consensus
sequence of a classical Cys
2
–His
2
zinc finger, where X is any amino
acid and
f
represents hydrophobic residues (typically Y, F, and L).
Zinc-chelating cysteine and histidine residues are circled.
a
-helical
positions (numbered relative to position þ 1) are indicated above the
sequence. (B) The sequence of Finger 2 of Zif268, showing conformity
with the consensus sequence.
ZINC FINGERS 437
relative to a defined first position, Position þ 1
(see Figures 2 and 3(B)). Since positions 2 1, þ 3, and
þ6 are all to be found on the external face of the helix,
these can form a binding surface that is especially useful
in DNA recognition because the spacing of the residues
matches the register of the base pairs. It should be noted,
however, that contacts from other positions, especially
position þ2, are also important in DNA recognition and
that not all zinc finger–DNA interactions are as regular
as the canonical structure of Zif268.
DNA Recognition “Code”
The most striking feature of the canonical Zif268
structure is the presence of regularly spaced one-to-one
contacts between amino acids and DNA bases, from the
same fixed helical positions in each finger (Figure 3B). By
studying mutations at these positions, it has emerged
that classical zinc fingers are a special case in terms of
protein–DNA interactions. Unlike for most DNA-
binding proteins, these positions do seem to fit a
residue-base code-like pattern. Positions 2 1, þ 2, þ 3,
and þ 6inthe
a
-helix are particularly important
(Figure 3B), and some major residue-base associations
are outlined in Figure 3C.
As a note of caution, it is important to mention that
the code is not absolute, and is certainly an over-
simplification of the interplay between different residues
in the protein and between factors in the DNA structure,
such as base stacking and unwinding. Nonetheless,
thinking about zinc finger DNA interactions in this way
can give a valuable approximation into the possible
binding modes of a given zinc finger protein.
RNA Recognition
Unlike the double helical scaffold of DNA, RNA has
much more complex folds, with every RNA presenting a
unique binding surface. Nonetheless, zinc fingers have
the versatility to accommodate such structures, with
contacts from all over the finger framework. Like DNA
recognition, electrostatic interactions and hydrogen
bonding play a major role, with amino acids
contacting either RNA bases or the phosphodiester
backbone. Note that in RNA recognition there is a
greater potential for hydrophobic interactions, with
aromatic residues such as Tyr, Phe, and Trp stacking
around extruding bases.
NON-NUCLEIC ACID INTERACTIONS
Protein–Protein Interactions
Zinc fingers are not limited to controlling gene
expression, but also play an important cellular role in
mediating specific protein–protein interactions.
ACGCCCA G
GC GGTGCGG
Glu 3
His 3
Thr 6
Glu 3
Arg 6
Arg 6
C
Arg-1
Arg-1
Arg-1
Asp2 Asp2
Asp2
3´4´5´6´7´8´9´10´
32
4
5678910
11´
F1 F2 F3
3´
5´
5´
3´
F1
F2
F3
A
C
B
Position in Zn finger
-1
+3
ACDEFGH KLMNPQRSTVWY
G
A
T
C
G
A
T
C
+6
G
A
T
C
I
Base specified
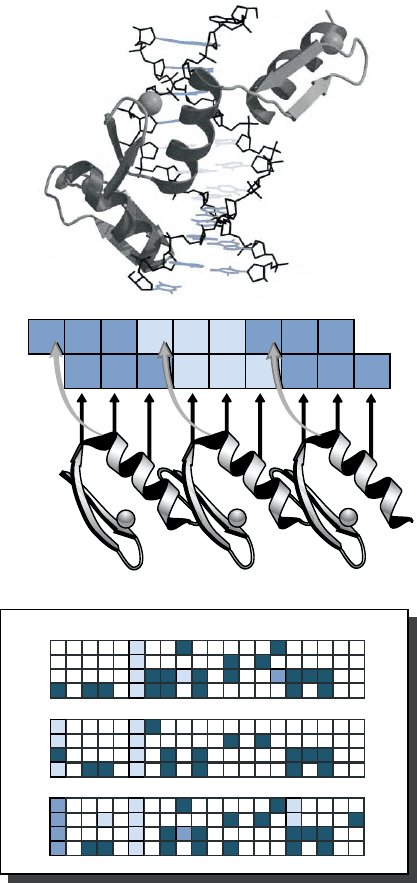
FIGURE 3 Interactions between zinc fingers and DNA. (A) Side view
of Zif268 binding DNA (Pavletich and Pabo (1991). Zinc finger-DNA
recognition: crystal structure of a Zif268-DNA complex at 2.1A
˚
.
Science 252, 809–817. The three zinc fingers (F1–F3) bind the major
groove of the DNA. (B) Schematic diagram of a simple model of
recognition between the three zinc fingers of Zif268 and the triplet
subsites of an optimized DNA-binding site. Arrows indicate contacts
by recognition residues with bases in each DNA strand. Note that most
contacts are to the G-rich strand. Since the N-terminal finger contacts
the 3
0
end of the DNA and the C-terminal finger the 5
0
end, binding is
said to be antiparallel. (C) Zinc finger DNA-recognition code. The grid
summarizes common associations between amino acid residues (A–Y)
and the bases they face in a classical zinc finger DNA-binding site.
Darker shading of boxes indicates stronger associations. Note that
contacts from overlapping C-terminal zinc fingers (especially from
position 2) can significantly alter the coding specificity shown here
for position þ 6.
438 ZINC FINGERS
Whereas nucleic acid recognition usually requires
clusters of fingers, cytoplasmic proteins often contain
only one or two fingers. This is possibly because a single
protein-binding finger has sufficient affinity to bind
effectively, given the more variable binding surface
presented by other proteins.
Lipid Binding
Recently, the known binding repertoire of zinc fingers
has been extended to include lipid recognition. This is
exemplified by the FYVE zinc finger family, that is found
in a wide range of eukaryotic cells. These nonclassical
fingers have a complex structure consisting of multiple
loops, two twin-stranded
b
-sheets and an
a
-helix
stabilized by a pair of zinc ions. Functionally, the
domains bind to phosphatidylinositol 3-phosphate
(PI3P) in cellular membranes, commonly found on
endosomes, and mediate further recruitment of a range
of proteins.
ZINC FINGERS IN BIOTECHNOLOGY
Classical zinc fingers are the ideal scaffold for protein
engineering. They are small and versatile, withstanding
extensive mutations without destroying their basic
structure. Moreover, individual finger modules may be
linked in tandem repeats to allow recognition of
extended target surfaces such as DNA grooves. Novel
DNA-binding zinc fingers have been routinely engin-
eered for nearly a decade and are beginning to
demonstrate their utility in biotechnical applications.
By fusing with suitable effector domains, effective
transcription activators and repressors can be syn-
thesized that recognize specific DNA sequences, typi-
cally 9–18 bp long. These constructs can up-regulate or
down-regulate specific genes in a cell and have been used
in a wide variety of contexts, including as antiviral
agents and angiogenesis-promoting factors.
SEE ALSO THE FOLLOWING ARTICLES
Aminopeptidases † Calpain † DNA Polymerase 1,
Eukaryotic † DNA Sequence Recognition by Proteins †
Inorganic Biochemistry † Nucleotide Excision Repair,
Bacterial: The UvrABCD System † Opioid Receptors †
Phosphatidylinositol Bisphosphate and Trisphosphate †
Phosphatidylinositol-3-Phosphate † Retinoic Acid
Receptors
GLOSSARY
classical finger A zinc finger of the TFIIIA type, where a zinc ion is
coordinated by two cysteines and two histidines (“Cys
2
–His
2
”or
“C2H2”).
protein motif A small structural element of a protein with a charac-
teristic fold or shape.
TFIIIA A transcription factor protein from Xenopus laevis, in which
zinc fingers were originally discovered.
transcription factor A protein involved in gene regulation that helps
to activate or repress the expression of a particular gene.
zinc finger A small structural protein motif consisting of peptide chain
folded around a central chelated (coordinated) zinc ion.
FURTHER READING
Beerli, R. R., and Barbas, C. F., III (2002). Engineering polydactyl zinc-
finger transcription factors. Nat. Biotechnol. 20, 135– 141.
Berg, J. M., and Shi, Y. (1996). The galvanization of biology: A
growing appreciation for the roles of zinc. Science 271,
1081–1085.
Choo, Y., and Isalan, M. (2000). Advances in zinc finger engineering.
Curr. Opin. Struct. Biol. 10, 411– 416.
Klug, A., and Schwabe, J. W. (1995). Protein motifs 5. Zinc fingers.
FASEB J. 9, 597–604.
Lander, E. S., et al. (2001). Initial sequencing and analysis of the human
genome. Nature 409, 860–921.
Mackay, J. P., and Crossley, M. (1998). Zinc fingers are sticking
together. Trends Biochem. Sci. 23,1–4.
Pabo, C. O., Peisach, E., and Grant, R. A. (2001). Design and selection
of novel Cys
2
His
2
zinc finger proteins. Annu. Rev. Biochem. 70,
313–340.
BIOGRAPHY
Mark Isalan is a fellow of the Wellcome Trust, UK, and is currently at
the European Molecular Biology Laboratory (EMBL), Heidelberg. His
principal research interests include combinatorial and design
approaches to protein engineering, gene regulation, and gene net-
works. He holds a Ph.D. from the University of Cambridge, where he
trained at the MRC Laboratory of Molecular Biology, developing
methods to engineer novel DNA-binding zinc fingers.
ZINC FINGERS 439

Editors-in-Chief
William J. Lennarz
State University of New York at Stony Brook, Stony Brook,
New York, USA
Section: Lipids, Carbohydrates, Membranes and Membrane Proteins
WILLIAM J. LENNARZ received his B.S. in Chemistry
from Pennsylvania State University and a Ph.D. in
Organic Chemistry from the University of Illinois.
Subsequently he carried out postdoctoral work at
Harvard with Konrad Bloch on fatty acid biosynthesis.
In 1962 he was appointed Assistant Professor at Johns
Hopkins in the Department of Physiological Chemistry.
After promotion to Associate Professor in 1967, and full
Professor in 1971, he remained at Hopkins until 1983. At
that time, he was appointed Robert A. Welch Professor
and Chair of the Department of Biochemistry and
Molecular Biology at the University of Texas Cancer
Center, M.D. Anderson Hospital. In 1989 he became a
Leading Professor and Chair of the Department of
Biochemistry and Cell Biology at SUNY at Stony
Brook. In 1990 he founded and became Director of the
Institute for Cell and Developmental Biology at Stony
Brook.
Dr. Lennarz has served on many national and
international committees. He has served as President
of the Biochemistry Chairman’s Organization, President
of the American Society for Biochemistry and Molecular
Biology and President of the Society for Glycobiology.
He was a member of the Executive Committee of the
International Union of Biochemistry and Molecular
Biology for almost a decade.
He has presented special lectures at the University of
Notre Dame, the NIH, the University of West Virginia,
Johns Hopkins University, Florida State University, the
University of California at San Diego, the University of
Arkansas, Indiana University and the Medical College
of Virginia.
He is a member of the National Academy of Sciences.
The focus of his early work was on lipids and bacterial
cell surfaces. More recent efforts have been in the
structure, biosynthesis and function of cell surface
glycoproteins. The biosynthesis studies initially were
carried out in liver and oviduct, but these efforts now are
focused in yeast. The functional studies have concen-
trated on the role of cell surface glycoproteins in
fertilization and early development in the sea urchin
and, more recently, the frog. For over 30 years Dr.
Lennarz’ research has been supported by federal sources,
primarily the National Institutes of Health. Recently he
was appointed Distinguished Professor and Chair of his
department.
M. Daniel Lane
The Johns Hopkins University, School of Medicine, Baltimore,
Maryland, USA
Section: Metabolism, Vitamins and Hormones
M. DANIEL LANE received B.S. and M.S. degrees in
1951 and 1953 from Iowa State University and a Ph.D.
in 1956 from the University of Illinois. He was a Senior
Postdoctoral Fellow with Professor Feodor Lynen at the
Max-Planck Institute Fur Zellchemie in Munich.
Following faculty positions at Virginia Polytechnic
Institute and New York University School of Medicine,
he joined the faculty at the Johns Hopkins University
School of Medicine in 1969 and served as DeLamar
Professor and Director of the Department of Biological
Chemistry from 1978 to 1997. He is presently Distin-
guished Service Professor at Johns Hopkins. In 2002 he
received an honorary degree, Doctor of Humane Letters,
from Iowa State University.
Dr. Lane was elected to membership in the National
Academy of Sciences (in 1987) and was elected as a
Fellow of the American Academy of Arts and Sciences
(in 1982) and of the American Society of Nutritional
Sciences (in 1996). He received the Mead Johnson
Award from the American Society for Nutritional
Sciences in 1966 for his research on biotin-dependent
enzymes and in 1981, the William C. Rose Award from
the American Society for Biochemistry and Molecular
Biology for his work on the insulin receptor. In
1990–1991 Lane served as President of the American
Society of Biochemistry and Molecular Biology. He has
presented many named lectureships (including the
i
Feodor Lynen Lecture in Germany in 1999) and served
on numerous editorial boards including the Journal of
Biological Chemistry and the Annual Reviews of
Biochemistry. Currently he is Associate Editor for
Biochemical and Biophysical Research Communi-
cations.
Dr. Lane has published 280 research papers in major
scientific journals. His early work focused on various
enzymatic CO
2
fixation reactions, notably the mechan-
isms by which the B-vitamin, biotin, functions in
enzymes to catalyze carboxylation. Dr. Lane’s work on
the regulation of acetyl-CoA carboxylase, the key
regulatory enzyme of fatty acid synthesis, led him to
his present interests which are to understand the basic
mechanisms of lipogenesis, adipogenesis and the con-
sequence of aberrations in these processes, most notably
obesity. Research currently underway in his laboratory
focuses on: (1) the genes that signal stem cell “commit-
ment” to the adipocyte lineage and subsequent differ-
entiation into adipocytes, and (2) the mechanisms by
which the region of the brain, known as the hypothala-
mus, monitors and controls the drive to eat.
ii
EDITORS-IN-CHIEF

Associate Editors
Ernesto Carafoli
Universita
`
degli Studi di Padova, Padova, Italy
Section: Bioenergetics
ERNESTO CARAFOLI earned his M.D. degree at the
University of Modena in Italy in 1957. After postdoc-
toral studies in the Laboratory of Albert L. Lehninger at
Johns Hopkins University in the mid 1960s he returned
to his home institution in Italy where he worked until
1973, when he was appointed Professor of Biochemistry
at the Swiss Federal Institute of Technology (ETH) in
Zurich. He returned to Italy in 1998 as a Professor of
Biochemistry at the University of Padova, where he now
also directs the newly founded Venetian Institute of
Molecular Medicine (VIMM).
Dr. Carafoli became interested in calcium as a
signaling agent during his post-doctoral days at Johns
Hopkins. When he arrived there his main interests were
in mitochondrial bioenergetics and it was thus natural
for him to expand them to the newly discovered area of
mitochondrial calcium transport. He was involved in
most of the early discoveries in the field, and he
continued to work on mitochondria and calcium after
his return to Italy and until he moved to the ETH. There
his interests still remained focused on calcium, but the
emphasis shifted to the proteins that transport it across
membranes and to those that process its signal. His
favorite object of study became the calcium pumps,
especially that of the plasma membrane, an enzyme
which is essential to the regulation of calcium homeo-
stasis and thus to the well being of cells. His
contributions on the enzyme, especially after he purified
it in 1979, have helped establishing most of its properties
and have clarified important problems of mechanism,
regulation and structure.
Dr. Carafoli has authored or co-authored about 450
peer-reviewed articles and reviews, and has edited or
co-edited about 20 books. He has served on the Editorial
or Advisory Boards of several periodicals and has
organized about 30 International Workshops and
Symposia. He has been featured as a plenary or
honorary lecturer at numerous events ranging from
specialized Workshops to International Symposia and
Congresses. Dr. Carafoli’s honors and awards include
several international prizes and medals, memberships in
several Academies, and three honorary degrees.
Don W. Cleveland
University of California, San Diego, La Jolla, CA, USA
Section: Cell Architecture and Function
DON W. CLEVELAND has been a longstanding
contributor to the elucidation of regulation of assembly
of mitotic spindles and chromosome movement and
how errors in these contribute to the chromosome loss
characteristic of human tumors. He discovered the
tubulin gene families encoding the major subunits of
microtubules and the first mammalian example of
control of gene expression through regulated RNA
instability. He identified components required for
microtubule nucleation and anchoring during
spindle assembly. He identified the first human centro-
meric protein (CENP-B). He then discovered CENP-E,
the centromere-associated, microtubule-motor that he
showed to be essential for chromosome attachment and
for activation and silencing of the mitotic checkpoint, the
cell cycle control mechanism that prevents errors of
chromosome segregation in mitosis.
Dr. Cleveland has also been a leading force in
dissecting the disease mechanism for major human
neurodegenerative disorders. He initially purified and
characterized tau, the microtubule-associated protein
that assembles aberrantly in human dementias includ-
ing Alzheimer’s disease and Pick’s disease. He
established that the extreme asymmetry of neurons
acquired during development is achieved with a
deformable array of interlinked neurofilaments, micro-
tubules and actin. He showed that disorganization of
neurofilament arrays caused selective death of motor
neurons in mice and humans. He also demonstrated
that neuronal death could also arise by a toxicity of
mutant superoxide dismutase unrelated to its normal
activity, thereby uncovering the mechanism underlying
the major genetic form of amyotrophic lateral
sclerosis. He showed that this toxicity could be
i
sharply ameliorated by lowering the content of
neurofilaments.
Dr. Cleveland is currently Head, Laboratory for Cell
Biology in the Ludwig Institute for Cancer Research and
Professor of Medicine, Neurosciences and Cellular and
Molecular Medicine at the University of California at
San Diego. He is also the Editor of the Journal of Cell
Biology and Current Opinion in Cell Biology.
Jack E. Dixon
University of California, San Diego School of Medicine,
La Jolla, CA, USA
Section: Protein/Enzyme Structure, Function, and Degradation
JACK E. DIXON earned his Ph.D. in Chemistry at the
University of California, Santa Barbara in 1971 and did
his postdoctoral training in Biochemistry at the Univer-
sity of California, San Diego.
Dr. Dixon is a pioneer and leader in the structure and
function of the protein tyrosine phosphatases (PTPases).
He demonstrated that the unique catalytic mechanism of
the PTPases proceeds via a novel cysteine-phosphate
intermediate. He discovered the first dual-specificity
phosphatase, which led to the identification of the cell
cycle protein, p80
cdc25
, as a phosphatase. He also
showed that the bacteria responsible for the plague or
“black death” harbor the most active PTPase ever
described. He and his colleagues went on to demonstrate
that this PTPase gene product is essential for the
pathogenesis of the bacteria. Dr. Dixon and his
colleagues determined X-ray structures for both tyrosine
and dual specificity phosphatases. Dr. Dixon also found
that sequences outside of the PTPase catalytic domain
could function to direct the subcellular localization of
the PTPases and to restrict their substrate specificity.
This is now a widely acknowledged regulatory paradigm
for the PTPases. Recently, his laboratory demonstrated
that the tumor suppressor gene, PTEN, which
shares sequence identity with the PTPases, catalyzes
the dephosphorylation of a lipid second messenger,
phosphatidylinositol 3,4,5-trisphosphate (PIP3). This
represents the first example of a PTPase dephosphor-
ylating a lipid second messenger. PIP3 activates the
protein kinase, AKT, which plays a critical role in
controlling the balance between apoptosis and cell
survival. The loss of the PTEN gene elevates PIP3 levels
leading to constitutive activation by AKT and oncogen-
esis. Recently, Dr. Dixon in collaboration with Nikola
Pavletich determined the X-ray structure of PTEN. Their
structure– function studies explain the PIP3 substrate
specificity of PTEN and also provide a rationale for
many of the mutations seen in human cancers. Earlier in
his career, Dr. Dixon adopted the tools of molecular
biology as they became available in the 1970s, and his
laboratory was among the first to use synthetic
oligonucleotides to isolate and extensively characterize
cDNAs encoding peptide hormones.
Dr. Dixon is Professor of Pharmacology, Cellular and
Molecular Medicine and Chemistry and Biochemistry
and Dean of Scientific Affairs at the University of
California, San Diego. He is a member of the National
Academy of Sciences, the Institute of Medicine and the
American Academy of Arts and Sciences. Dr. Dixon was
the recipient of the 2003 William C. Rose Award from
the American Society for Biochemistry and Molecular
Biology.
John H. Exton
Howard Hughes Medical Institute, Vanderbilt University School of
Medicine, Nashvillie, TN, USA
Section: Signaling
JOHN H. EXTON was born and educated in New
Zealand where he received his medical training and a
Ph.D. in Biochemistry from the University of Otago in
1963. He did postdoctoral work at Vanderbilt Univer-
sity under Charles R. Park and Earl W. Sutherland, and
became an Investigator of the Howard Hughes Medical
Institute in 1968 and Professor of Physiology in 1970.
He is presently Professor of Molecular Physiology and
Biophysics, Professor of Pharmacology and a Hughes
Investigator at Vanderbilt.
Dr. Exton’s research initially focused on the changes
in carbohydrate metabolism in liver during diabetes and
treatment with various hormones using the perfused rat
liver as the experimental system. His work concen-
trated on gluconeogenesis and identified the enzymatic
reactions that were under control by insulin, epineph-
rine (adrenaline), glucagon and glucocorticoids, and
demonstrated the importance of cyclic AMP in the
regulation of these reactions. The role played by the
supply of substrates, especially of alanine, was also
shown.
Dr. Exton then turned his attention to the action of
epinephrine (adrenaline) and demonstrated that many of
its actions were not mediated by cyclic AMP but by
calcium ions. This led to study of the breakdown of
inositol phospholipids by phospholipase C that underlay
the increase in calcium. Later this resulted in the
discovery of G
q
, a novel G protein that activated
phospholipase C. Further studies demonstrated that
agonists caused the breakdown of another phospholipid
(phosphatidylcholine) by another phospholipase (phos-
pholipase D). Current work is focused on the physio-
logical role of phospholipase D.
Dr. Exton has authored over 350 scientific articles
and is presently an Associate Editor of the Journal of
Biological Chemistry. He has served on many scientific
review groups and as a reviewer for many journals. He
has won numerous awards, most notably the Lilly
ii
ASSOCIATE EDITORS
