Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

charged amino acids (aspartates) denominated the
“Ca
2þ
bowl.” The Ca
2þ
bowl has emerged as a good
candidate for the high-affinity Ca
2þ
-binding site.
Slo channels control a large variety of physiological
processes, including smooth muscle tone, neurosecretion,
and hearing. Despite being coded by a single gene, the
diversity of Slo channels is great. Regulatory
b
-subunits,
splicing, and metabolic regulation create this diversity
fundamental to the adequate function of many tissues.
Except in heart myocytes, Slo channels are almost
ubiquitously expressed among mammalian tissues, and
they play a variety of roles. In smooth muscle, local Ca
2þ
increases, called Ca
2þ
sparks, generate spontaneous
transient outward currents, which are generated by Slo
channels. This hyperpolarizes the membrane causing
muscle relaxation. In chromaffin cells, Slo channels are
important for rapid termination of the action potential.
In the cochlea of turtles, frogs and chicks, gradients of
spliced Slo channels, and
b
-subunits through an
interplay with voltage-dependent Ca
2þ
channels, they
are able to generate the different oscillatory responses in
the hair cells.
KCNQ CHANNELS
The first member of the KCNQ family to be cloned,
KCNQ1, was isolated by virtue of the fact that
mutations in this gene give rise to the most common
form of long QT syndrome, LQT1. Exploiting their
homology to KCNQ1, other members of this family
were subsequently cloned. KCNQ1 encodes the major
subunit of the cardiac I
Ks
channel, which is involved in
repolarization of the ventricular action potential.
KCNQ1 mRNA is expressed strongly in the heart,
with lower levels in pancreas, kidney, lung, placenta,
and ear.
It has been suggested that KCNQ2 and KCNQ3 is a
heteromer, an idea which is consistent with their
overlapping tissue distribution and the fact that
antibodies directed against KCNQ2 are able to
coimmunoprecipitate KCNQ3, and vice versa. The
heteromeric KCNQ2/KCNQ3 channel corresponds to
neurone’s M-channel. They are widely distributed
throughout the brain, being expressed at high levels
in hippocampus, chordate nucleus, and amygdala.
Mutations in KCNQ2 and KCNQ3 rise to a form of
idiopathic epilepsy known as benign familial neo-
natal convulsions.
K
V
CHANNELS
The vertebrate fast voltage-gated K
þ
delayed rectifier
channels fall into a variety of families (K
v
1–K
v
11)
according to their amino acid sequence similarity
(Figure 2). A channel from a fruit fly Drosophila was
the first K
þ
channel to be cloned (denominated in
vertebrates as K
v
1.1, the first number denoting
subfamily and the second, order of discovery). The
identification started with mutant flies called shakers,
which shake their legs while under ether anesthesia.
These flies lack a fast transient K
þ
current in
presynaptic terminals, so repolarization of the action
potential is delayed and the evoked release of
neurotransmitter from a single action potential
becomes enormous.
K
v
channels are found in a wide range of different
tissues and they have many different functions. Some
ideas of the diversity of roles of K
v
channels are shown in
Figure 2. For example, K
v
3.1 channels are found in some
neurons that are specialized to fire very short action
potentials at high rates, such as those of the auditory
system; K
v
1.3 channels are found in leukocytes playing a
role in inflammatory response; K
v
1.2 channels are found
in smooth muscle participating in contractil tone
regulation; and K
v
4.2 channels are expressed in myo-
cardium and their function is action potential pro-
longation in heart.
Defects in K
v
channels function therefore may
have profound physiological effects. Linkage studies
have identified the gene responsible for episodic ataxia
type-1. This gene encoded for K
v
1.1 channel, which is
expressed in the synaptic terminals and dendrites in
brain neurones.
SEE ALSO THE FOLLOWING ARTICLES
Ion Channel Protein Superfamily † Voltage-Sensitive
Ca
2þ
Channels
GLOSSARY
delayed rectifier AK
þ
channel that changes the membrane con-
ductance with a delay after a depolarizing voltage step.
gating The opening or closing of a channel in response to some
stimulus.
inward rectifier AK
þ
channel that opens when the membrane is
hyperpolarized.
long QT syndrome An inherited cardiac arrhythmia.
voltage sensor A channel structure able to detect changes in membrane
potential. In voltage-dependent ion channels the voltage-sensing
elements are located in the S4 segment.
FURTHER READING
Hille, B. (2001). Ions Channels of Excitable Membranes. Sinauer
Associates, Sunderland, Massachusetts.
Jiang, Y., Lee, A., Chen, J., Ruta, V., Cadene, M., Chalt, B. T., and
MacKinnon, R. (2003). X-ray structure of a voltage-dependent K
þ
channel. Nature 423, 33– 41.
Laine
´
,M.,Papazian,D.M.,andRoux,B.(2004).Critical
assessment of a proposed model of Shaker. FEBS Lett. 564,
257–263.
VOLTAGE-DEPENDENT K
1
CHANNELS 403
Orio, P., Rojas, P., Ferreira, G., and Latorre, R. (2002). New disguises
for and old channel: MaxiK channel
b
-subunits. News Physiol. Sci.
17, 156–161.
BIOGRAPHY
Ramon Latorre is Professor and Chair of the Laboratory of Biophysics
and Molecular Physiology, CECS. He holds a Ph.D. from the
University of Chile and received his postdoctoral training at the
Biophysics Laboratory in the National Institutes of Health, Maryland.
He is a Foreign Associate of the National Academy of the United States
of America. The main scientific interest of Dr. Latorre is the molecular
workings of ion channels.
Francisco Morera holds a degree in biochemistry from the Catholic
University, Chile and is at present a Ph.D. in the Austral University,
Valdivia, Chile.
404 VOLTAGE-DEPENDENT K
1
CHANNELS

Voltage-Sensitive Ca
2þ
Channels
Harald Reuter
University of Bern, Bern, Switzerland
Ion channels are integral, pore-forming transmembrane
proteins that serve to pass ions across biological membranes.
The channels differ in their ion selectivity, i.e., each type allows
certain ion species to permeate through the open channel pore.
Conformational changes of the proteins leading to openings or
closings of the channels are called “gating.” It is achieved
either by binding of ligands to special sites on the channels
(ligand-gated ion channels), or by changes in membrane
potential (voltage-gated ion channels). Calcium (Ca
21
) chan-
nels are voltage-gated and have a high selectivity for Ca
21
ions.
Opening of these channels by membrane depolarization leads
to an influx of Ca
21
ions into the cell. The resulting increase in
the intracellular Ca
21
concentration initiates essential physio-
logical functions, such as cardiac contraction, secretion of
hormones and neurotransmitters, gene transcription, or
repetitive electrical activity of neurons and the heart. Most of
these functions result from binding of Ca
21
to specific
intracellular proteins (e.g., calmodulin, troponin C) which
subsequently mediate the Ca
21
-dependent cellular responses.
The diversity of voltage-gated Ca
21
channels in surface
membranes of different cells is important for initiation of
specific signaling cascades within the cells. The various types of
Ca
21
channels are encoded by different genes and show tissue-
specific expression. They are targets for toxins and for drugs
used for therapeutic purposes.
Molecular Properties
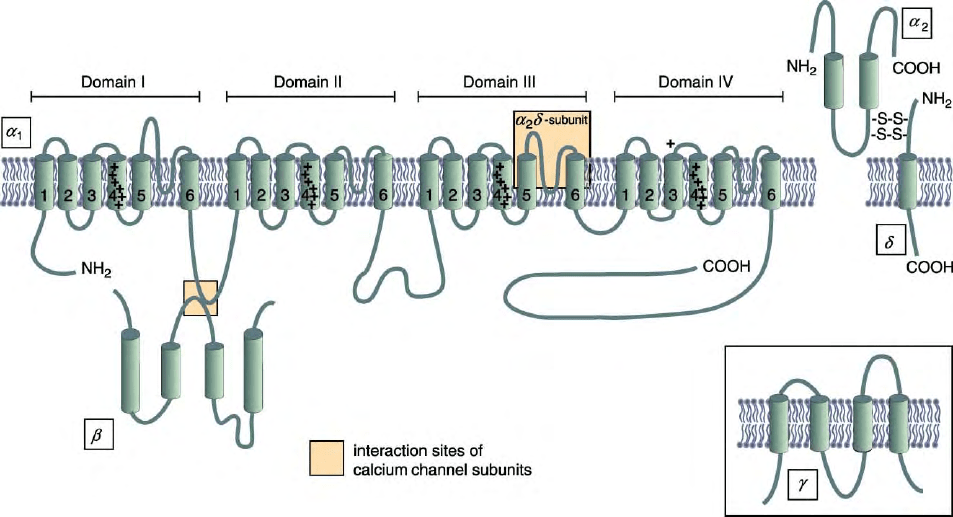
STRUCTURES
Voltage-gated Ca
2þ
channels are oligomeric protein
complexes composed of the voltage-sensitive, pore-
forming
a
1
-subunits, together with auxiliary disulfide-
linked
a
2
d
-subunits and intracellular
b
-subunits
(Figure 1). An additional transmembrane
g
-subunit is
associated with
a
1
S in skeletal muscles. The diversity of
Ca
2þ
channel types not only results from different genes
coding for the different channel subunits (10 for
a
1
, 4 for
a
2
d, and 4 for
b
), but also from multiple splice variants
of the subunits. The
a
1
-subunits consist of four
homologous domains (I–IV), each being composed of
six transmembrane
a
-helical segments (S1–S6). The
N and C termini are located intracellularly, and
the domains are linked together by intracellular loops.
In the folded structures of
a
1
-subunits, hairpin loops
between transmembrane segments S5 and S6 constitute
the channel pore. Segments S4 contain highly conserved,
positively charged amino acids that act as voltage-
sensitive sensors during gating of the channels. The
b
-subunits are involved in the regulation of the
channels’ gating properties and, like
a
2
d-subunits, play
a role in the functional expression of the channels in cell
surface membranes.
SELECTIVITY
Ca
2þ
channels are selective pathways for the movement
of Ca
2þ
ions down their electrochemical gradient into
the cell. The pore-forming structures of the channel
contain four negatively charged glutamate residues, each
being in a homologous position on the four domains.
The resulting ring of negative charges facing the pore
coordinates binding of an entering Ca
2þ
ion. A second
Ca
2þ
ion that enters the channel reduces the binding
affinity of the first one by electrostatic repulsion, thus
kicking it off from its binding site and letting it
move down its electrochemical gradient. Thus, the
selectivity of the channel for Ca
2þ
is critically dependent
on the correctly placed negative charges of glutamate
in the pore.
CALMODULIN BINDING
Two forms of autoregulation, Ca
2þ
-dependent inacti-
vation and facilitation of Ca
v
1.2 channels (Table I),
involve calmodulin (CaM). The subunit
a
1C of Ca
v
1.2
contains a consensus CaM-binding isoleucine-glutamine
(IQ)-motif in its cytoplasmic C-terminal tail. The IQ-
motif consists of 12 amino acids. Extensive mutations
within this sequence revealed structural determinants
for the two opposing forms of channel regulation. Two
short stretches of aminoacids upstream of the IQ-motif
seem to be sites for constitutive, Ca
2þ
-independent
binding of apoCaM. The IQ-motif binds CaM only in
the presence of Ca
2þ
. It represents the regulatory site for
Ca
2þ
-dependent inactivation, while facilitation may
require additional activation of Ca/CaMkinaseII.
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 405
PHOSPHORYLATION
Ca
2þ
channel function can be modulated by second
messenger-activated protein kinases (PKs) such as
cyclicAMP-dependent PKA, diacylglycerol-dependent
PKC, or Ca/calmodulin-dependent kinases. Although
the exact molecular mechanisms of these modulatory
pathways are not yet fully understood, they do depend
on phosphorylation by kinases of specific aminoacid
residues of the Ca
2þ
channel protein. Multiple intra-
cellular sites of phosphorylation have been identified on
a
2
1
- and
b
-subunits. However, which phosphorylation
sites are necessary and sufficient for different forms of
modulation of Ca
2þ
channel function by the various
protein kinases has not been clearly resolved.
GPROTEINS
Ca
2þ
channels of the Ca
v
2 family (Table I) that are
involved in neurotransmitter and hormone secretion are
inhibited by G proteins. There is excellent evidence that
the rapid inhibition of the channels results from direct
binding of the
bg
-subunits of G proteins to an identified
segment of the intracellular loop between domains I and
II (Figure 1), but other sequences in the C and N termini
of the
a
2
1
-subunits may also be important. Rapid
inhibition of the channels by this mechanism does not
require formation of soluble intracellular messengers.
The inhibition results from a membrane-delimited
pathway leading to the release of the
bg
-subunit of the
G-protein complex.
Ca
21
-Currents
PHYSIOLOGY
Depolarization of cell membranes during excitation
leads to spontaneous openings (activation) and closings
(inactivation) of Ca
2þ
channels. At a given voltage,
stochastic openings of multiple channels produce a
considerable flow of Ca
2þ
ions into a cell and can thus be
measured as an electrical current. The currents are
recorded by electrophysiological methods, at a level of
resolution where even the current through an individual
channel can be measured (patch-clamp method). This
allows detailed quantitative analyses of the kinetic
properties (gating) and selectivities of the channels.
Currents through different types of Ca
2þ
channels
have traditionally been classified as L-, P/Q-, N-, R-,
and T-type (Table I). This classification resulted from
functional and pharmacological analyses before the
channels were cloned and sequenced. A general over-
view of the distribution of the channels in different
tissues is summarized in Table I. The specific expression
of different Ca
2þ
channel types in different tissues is of
FIGURE 1 Oligomeric subunit complex of voltage-gated Ca
2þ
channels. The subunit composition consists of the pore-forming
a
1-subunit with
its four homologous domains, the intracellular
b
-subunit, and the disulfide-linked a2-subunit. The sites of interaction between the subunits are
indicated by the squares. An extra
g
-subunit is present in skeletal muscle Ca
v
1.1 complex (inset). Kindly prepared by Dr. R. D. Zuehlke, Bern.
406 VOLTAGE-SENSITIVE CA
21
CHANNELS
great physiological and pharmacological importance.
The localization of individual channels in association
with other cellular constituents governs specific signal-
ing pathways. For example, P/Q-,and N-type channels
are directly linked to the SNARE (Soluble N-ethylma-
leimide sensitive fusion protein Attachment protein
REceptor) protein complex in neural and endocrine
cells that is essential for secretion of neurotransmitters
and hormones. L-type channels that are predominant in
cardiac cells, are in close proximity to intracellular Ca
2þ
stores. When Ca
2þ
ions move through the channels
into these cells during excitation, they release more Ca
2þ
from the stores which eventually leads to contraction of
the heart. Thus, changes in the intracellular Ca
2þ
-
concentration are coding for important information of
diverse cellular functions. Autoregulatory mechanisms,
by which the channels inactivate in voltage- and Ca
2þ
-
dependent manners, prevent Ca
2þ
overload of the
cells. Ca
2þ
-dependent inactivation has been studied
most extensively in L-type Ca
2þ
channels, but
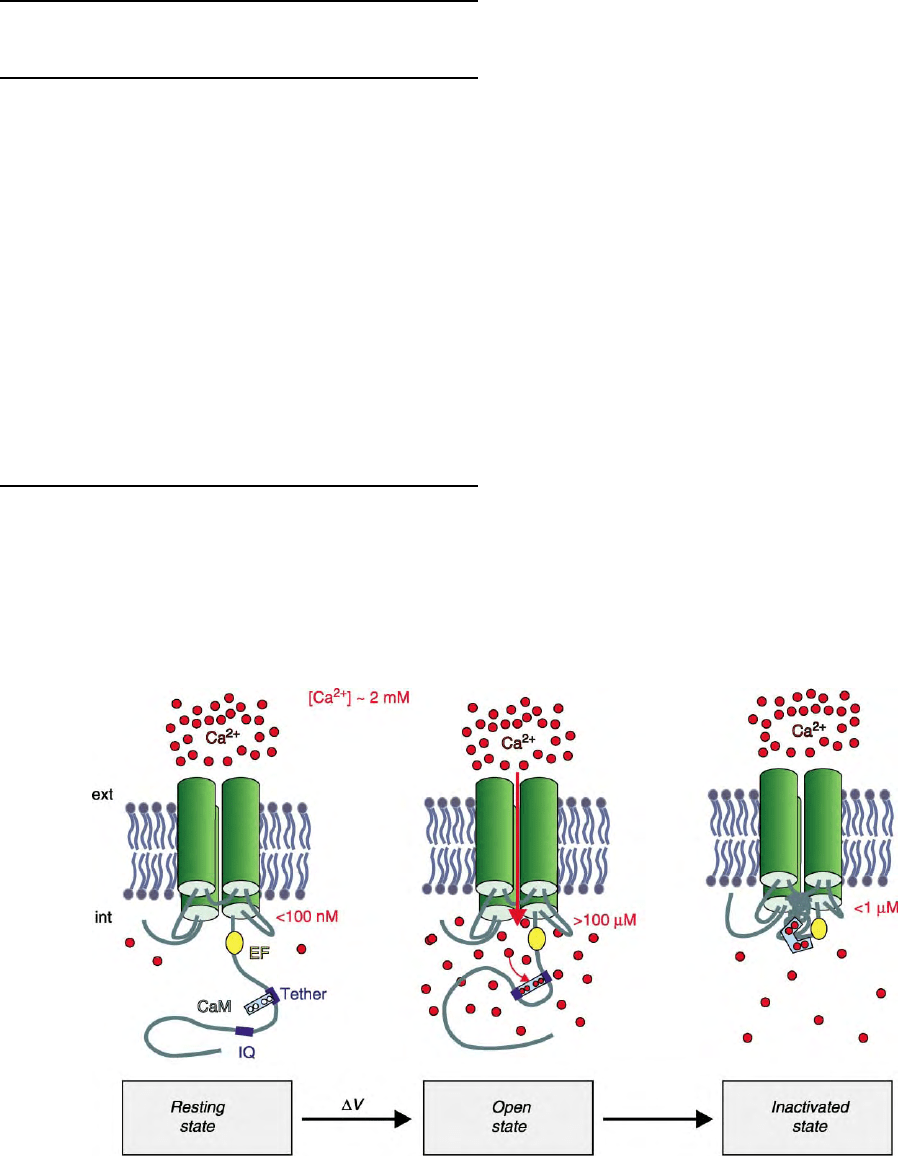
may also apply to other channel types. During the
flow of Ca
2þ
ions through the pore, they bind to
constitutively tethered apoCaM at the cytoplasmic C
terminus of the channel. Occupancy of CaM by Ca
2þ
triggers binding of its C-terminal lobe to the
nearby IQ-motif and accelerates inactivation of the
channel, possibly by disinhibiting voltage-dependent
inactivation (Figure 2).
FIGURE 2 Model of voltage- and Ca
2þ
-dependent gating of a Ca
2þ
channel. The model shows a schematic folding of the
a
1-subunit in Figure 1.
At membrane resting potential the pore is closed (resting state); upon depolarization (DV) the pore opens and Ca
2þ
ions move across the membrane
down their electrochemical gradient (open state); when the intracellular Ca
2þ
-concentration increases, Ca
2þ
binds to tethered calmodulin
(CaM) which then attaches itself to the regulatory IQ-motif and promotes closure of the pore (inactivated state). Kindly prepared by Dr. R. D.
Zuehlke, Bern.
TABLE I
Ca
21
Channels
Channel
types
a
Pore-
forming
a
-subunits
b
Tissue
distribution
Pharmacology
(blockers)
HVA
Ca
v
1.1 (L)
a
1S Skeletal muscle DHPs
Ca
v
1.2
a
1C Heart, smooth
muscle, CNS
Ca
v
1.3
a
1D CNS, kidney,
cochlea, glands
CA
v
1.4
a
1F Retina
Ca
v
2.1 (P/Q)
a
1A CNS
v
-Agatoxin
Ca
v
2.2 (N)
a
1B CNS
v
-Conotox.GVIA
Ca
v
2.3 (R)
a
1E CNS, heart,
glands
LVA
Ca
v
3.1 (T)
a
1G CNS, sinus node Mibefradil
Ca
v
3.2
a
1H CNS, heart,
kidney, liver
Ca
v
3.3
a
1I CNS
a
Channel types: HVA ¼ high-voltage-activated channels; LVA ¼
low-voltage-activated channels; letters in brackets refer to the
nomenclature of currents carried through the different channel types.
b
Pore-forming
a
1
-subunits encoded by identified genes have
multiple splice variants (not shown); CNS ¼ central nervous system;
DHPs ¼ Dihydropyridines.
VOLTAGE-SENSITIVE CA
21
CHANNELS 407
MODULATION
Modulation of Ca
2þ
channel activity by neurotransmit-
ters and hormones has become a major field of research.
The up-regulation of cardiac Ca
2þ
current (Ca
v
1.2) by
epinephrine was the first example of modulation of an
ion channel by a neurotransmitter. It has been shown to
be the result of activation of the cyclicAMP pathway
via
b
1
-adrenoceptors and subsequent phosphorylation
by protein kinase A of aminoacid (serine) residues
at intracellular sites of the channel. This causes
enhanced probability of openings of the channels,
resulting in largely increased Ca
2þ
currents. Other
examples of modulation mainly concern N- and
P/Q-type Ca
2þ
channels (Ca
v
2.1 and Ca
v
2.2; Table I).
They are inhibited by neurotransmitters, such as
norepinephrine. Release of G protein
bg
-subunits by
neurotransmitters shifts channel activation into a
reluctant state, thus causing a reduction of Ca
2þ
currents
during depolarization. These are just two examples of
modulation of Ca
2þ
channel activities. There are
several others that are not included under the scope of
this article.
PHARMACOLOGY
Ca
2þ
channels are important targets for toxins and
drugs. Although a full discussion of their effects is
beyond the scope of this article, a few of them are
listed in Table I. Dihydropyridines (e.g., nifedipine,
nimodipine) are relatively specific inhibitors of the
Ca
v
1 channel family, mainly Ca
v
1.2 type. Other drugs
acting on these channels are the phenylalkyamines
verapamil and the benzothiazepine diltiazem. They are
grouped together as “Ca-channel blockers,” and are
therapeutically used for the treatment of cardiovas-
cular diseases, notably hypertension and angina
pectoris. The toxins have no, or very limited,
therapeutic use. In some cases of otherwise untrea-
table pain, the N-type channel blocker
v
-conotoxin
GVIA has been successfully applied. Because of
unwanted side effects, the T-type channel blocker
mibefradil has been withdrawn from therapeutic use,
although it was quite effective in the treatment of
essential hypertension.
SEE ALSO THE FOLLOWING ARTICLES
Calcium Oscillations † Calcium Transport in Mito-
chondria † Calcium/Calmodulin-Dependent Protein
Kinase II † Intracellular Calcium Channels: NAADP
þ
-
Modulated † Ion Channel Protein Superfamily † Store-
Operated Membrane Channels: Calcium
GLOSSARY
apoCaM Ca
2þ
-free calmodulin.
electrical activity Results from ion flow through channels in
membranes.
electrochemical gradient Driving force for ions due to differences
in concentrations and electrical potentials across membranes.
G proteins Small intracellur proteins involved in signaling pathways.
oligomeric proteins Composition of several protein subunits.
protein kinases Enzymes that catalyze phosphate transfer from ATP
to proteins; protein kinases are activated by distinct second
messengers.
second messengers Intracellular molecules formed after receptor acti-
vation and involved in signaling (e.g. cyclic-AMP, diacylglycerol).
signaling cascade Information transfer within a cell.
FURTHER READING
Catterall, W. A. (2000). Structure and regulation of voltage-gated Ca
2þ
channels. Annu. Rev. Cell Dev. Biol. 16, 521– 555.
Hille, B. (2001). Ionic Channels of Excitable Membranes. Sinauer,
Sunderland, MA.
McDonald, T. F., Pelzer, S., Trautwein, W., and Pelzer, D. J. (1994).
Regulation and modulation of calcium channels in cardiac,
skeletal, and smooth muscle cells. Physiol. Rev. 74, 365–507.
Perez-Reyes, E. (2003). Molecular physiology of low-voltage-activated
T-type calcium channels. Physiol. Rev. 83, 117–161.
Reuter, H. (1996). Diversity and function of presynaptic calcium
channels in the brain. Curr. Opin. Neurobiol. 6, 331– 337.
Saimi, Y., and Kung, C. (2002). Calmodulin as an ion channel subunit.
Annu. Rev. Physiol. 64, 289–311.
BIOGRAPHY
Harald Reuter is Professor Emeritus and former Director of the
Institute of Pharmacology at the University of Bern, Switzerland. He
received his M.D. at the University of Mainz, Germany. Dr. Reuter first
described Ca
2þ
currents and the sodium/calcium-exchange transport in
the heart, and discovered regulation of Ca
2þ
channels by catechol-
amines and calmodulin. He is further interested in synaptic function
in the brain. He has received many awards for his discoveries and
has been elected a Foreign Associate of the National Academy of
Sciences, USA.
408 VOLTAGE-SENSITIVE CA
21
CHANNELS

Voltage-Sensitive Na
þ
Channels
William J. Brammar
University of Leicester, Leicester, UK
Voltage-sensitive sodium channels are the transmembrane
protein complexes that mediate the increased permeability to
Na
1
ions during the initial rising phase of the action potential
in most excitable cells. The channels are closed at resting
membrane potentials, but open in response to membrane
depolarization to become selectively permeable to Na
1
ions.
The open or activated state is transitory: channels are
inactivated and Na
1
permeability returns to the baseline
level within about a millisecond, allowing the repolarization
phase of the action potential that is controlled by K
1
channels.
A family of genes encoding multiple sodium channel
isoforms has been revealed by molecular cloning. Mutations in
genes encoding Na
1
channel subunits give rise to genetic
diseases affecting the function of skeletal muscles, the heart or
the brain. Sodium channel proteins include target-sites for the
action of a number of neurotoxins, insecticides, and local
anesthetics.
Isolation and Purification
of Sodium Channel Proteins
The first biochemical identification of sodium channel
proteins relied on specific covalent labeling of the
proteins with a photoreactive derivative of the north
African scorpion toxin. Denaturing polyacrylamide gel
electrophoresis of proteins derived from rat brain
synaptosomes revealed two labeled polypeptides of
260 and 36 kDa that were subsequently designated as
the
a
-and
b
1-subunits of the sodium channel. Purifi-
cation of the sodium channel solubilized from rat
brain revealed three polypeptides,
a
of 260 kDa,
b
1of
36 kDa and
b
2 of 33 kDa, in a 1:1:1 stoichiometry.
The
b
1-subunit is non-covalently associated with the
a
-subunit, whereas the
b
2-subunit is covalently attached
via disulfide bonds.
The sodium channels purified from rat skeletal
muscle sarcolemma or transverse tubules consist of a
260 kDa subunit and one or two subunits of 38 kDa.
In contrast, those purified from eel electroplax and
chicken heart contain only the 260 kDa component.
Reconstitution experiments, in which the purified
proteins were incorporated into phospholipid vesicles
to generate fully functional, neurotoxin-sensitive
sodium channels, proved the validity of the protein
purification methods based on neurotoxin-binding.
Primary Structures of Sodium
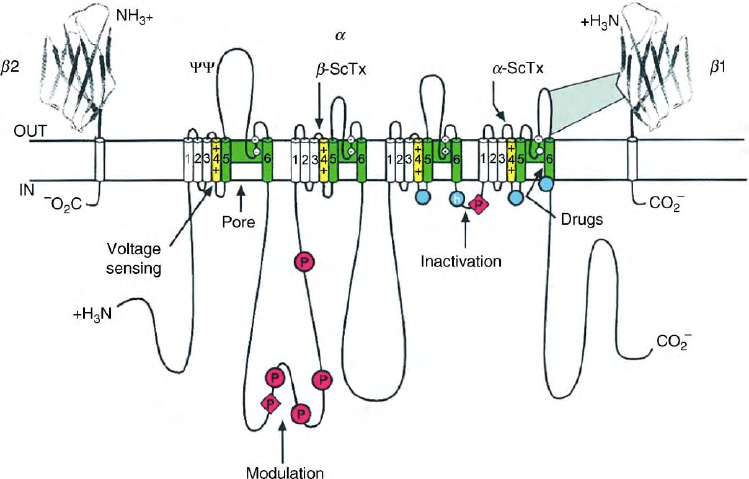
Channel Proteins
THE
a
-SUBUNITS OF SODIUM CHANNELS
Sequences encoding a complete Na
þ
channel protein
were first cloned as cDNAs derived from the mRNA of
the electroplax of the electric eel. The deduced sequence
of 1820 amino acids contains four internally homolo-
gous domains, each having six putative membrane-
spanning
a
-helical segments (Figure 1). In each
homology unit, five segments (S1, S2, S3, S5, and S6)
are predicted to be hydrophobic, membrane-spanning
helices. The interspersed S4 transmembrane segments
contain positively charged amino acids located at every
third position, forming an
a
-helix with a spiral of
positive charges.
The suggestion that the four S4 segments act as
voltage-sensors for the gating of the Na
þ
channel has
been confirmed by mutational alteration of the posi-
tively charged residues. The absence of an N-terminal
signal sequence suggests that the N terminus is
cytoplasmic.
The structural motif of four internally homologous
domains, first promulgated for the sodium channel of
the eel electroplax, is also evident in the voltage-gated
calcium and potassium channels and in other channels
within the large superfamily. Discernable sequence
similarity (, 32–37% identity) between the four hom-
ologous domains of the voltage-gated Na
þ
channels and
those of the voltage-gated Ca
2þ
channels reflects the
common ancestry of these two channel types.
Sequences encoding mammalian sodium channel
a
-subunits have been isolated using segments of the
eel electroplax Na
þ
channel cDNA as hybridization
probes and by screening expression libraries with
antibodies prepared against purified components.
DNA sequences encoding regions of brain Na
þ
channels
have been used in low stringency hybridizations to
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 409
isolate cDNAs encoding
a
-subunits from other tissues
such as skeletal muscle and heart. Although the different
channel
a
-subunits vary slightly in length and sequence,
they are clearly homologous and share fundamentally
similar structures.
THE
b
-SUBUNITS OF SODIUM CHANNELS
Both the
b
1 and
b
2 subunits of the voltage-sensitive Na
þ
channel are predicted to contain a single transmembrane
region, located near the intracellular C terminus,
and an extracellular domain containing a single
“immunoglobulin-like fold” (Figure 1). The latter
consists of two superimposed
b
-sheets held together by
hydrophobic interactions and is characteristic of the
family of cell adhesion molecules that mediate cell–cell
interactions. In such molecules, immunoglobin-like
folds bind extracellular proteins and influence inter-
actions with the extracellular matrix and with other cells
to affect cell shape and mobility. Rat brain Na
þ
channels
and the
b
2-subunit have been shown to bind to the
extracellular matrix proteins tenascin-C and tenascin-R,
leading to the suggestion that such interactions may
influence the formation of specialized areas of high Na
þ
channel density such as nodes of Ranvier.
Heterologous expression of cDNAs encoding Na
þ
channel
a
and
b
subunits has shown that the
b
1 and
b
2
subunits modulate channel-gating behavior. Expression
of the unaccompanied
a
-subunit of brain or skeletal
muscle Na
þ
channel in Xenopus oocytes produces
channels that activate and inactivate more slowly
than the native channels. Coexpression of
b
1 and
b
2
subunits with the
a
-subunit yields channels that gate
normally, and the accelerating effect of the
b
1-subunit is
mediated by the immunoglobulin-like fold in the extra-
cellular domain.
The Molecular Basis of Sodium
Channel Function
THE OUTER PORE AND
SELECTIVITY FILTER
The guanidinium-containing compounds tetrodotoxin
and saxitoxin, selective and reversible blockers of Na
þ
channels at nanomolar concentrations, have been
influential in allowing identification of the outer pore
and selectivity filter of the channel. These toxins are
membrane-impermeant and act only from the extra-
cellular side of the membrane to plug the selectivity filter
in the outer pore of the channel. Mutational studies have
identified a pair of amino acids, mostly negatively
charged, in analogous positions in all four domains of
FIGURE 1 Primary structures of the subunits of the voltage-gated sodium channel. The
a
,
b
1, and
b
2 polypeptide chains are shown to illustrate
their relationship to each other and to the cell membrane. The cylinders represent putative
a
-helices. The lengths of the intracellular and
extracellular loops are roughly proportional to the number of amino acid residues in the brain sodium channel subtypes. The extracellular domains
of the two
b
subunits are drawn as immunoglobulin-like folds. C shows the site of probable N-linked glycosylation: P in red circles, sites of
phosphorylation by (circles) and PKC (diamonds); the green regions are pore-lining segments; white circles represent the outer and inner rings of
amino acid residues that form the ion selectivity filter and the tetrodotoxin-binding site: yellow shows the S4 voltage sensors; h in a blue circle
represents the inactivation particle and blue circles the sites forming the receptor for the inactivation gate. Binding sites for
a
and
b
scorpion toxins
(
a
-ScTx and
b
-ScTx) are shown by the downward arrows. (Reproduced from Catterall, W. A. (2000). From ionic currents to molecular mechanism:
The structure and function of voltage-gated sodium channels. Neuron 26, 13–25, with permission from Elsevier).
410 VOLTAGE-SENSITIVE NA
1
CHANNELS
the rat brain Na
þ
channel
a
-subunit, that are important
for binding tetrodotoxin and saxitoxin (Figure 1). These
four pairs of amino acids were postulated to form outer
and inner rings that acted as both the receptor-site for
tetrodotoxin and saxitoxin and the selectivity filter in
the outer pore of the channel. The concept is strongly
supported by the finding that replacement of the four
amino acid residues in the inner ring by four glutamic
acid residues, their counterparts in voltage-gated Ca
2þ
channels, made the Na
þ
channels calcium-selective.
Cardiac Na
þ
channels bind tetrodotoxin with an
affinity that is 200-fold lower than that of the brain or
skeletal muscle channels, because of a replacement of an
aromatic amino acid by a cysteine residue in the outer
pore region of domain I. Cadmium is a high-affinity
blocker of cardiac Na
þ
channels because of its inter-
action with this same cysteine residue. Electrophysio-
logical measurements of the voltage-dependence of the
cadmium block of cardiac Na
þ
channels place the target
cysteine residue, and thus the selectivity filter, about 20%
of the way through the membrane electric field.
THE INNER PORE
Local anesthetics, such as lidocaine and procaine, are
generally lipid-soluble tertiary amine compounds that
inhibit propagated action potentials by blocking Na
þ
channels. A quaternary amine analogue of lidocaine,
QX-314, which is positively charged at all pHs and not
lipid-soluble, blocks Na
þ
channels only when applied to
the inside of the membrane. Blockage requires prior
opening of the channels by depolarization. Mutagenesis
studies demonstrated that the binding-site for local
anesthetics lies in the IVS6 transmembrane segment
(Phe1764 and Tyr1771 in rat brain type IIA channels).
Similar amino acid residues in the S6 transmembrane
segment of certain voltage-gated K
þ
channels act as
the targets for tetraethylammonium-based blockers.
These amino acids are located in an aqueous cavity
within the inner pore region of the K
þ
channel, by
extrapolation from a bacterial K
þ
channel whose
quaternary structure has been determined.
THE MECHANISM OF
VOLTAGE-DEPENDENT ACTIVATION
The voltage-dependent activation of sodium channels
involves the net outward movement across the mem-
brane electric field of , 12 electronic charges in the
channel protein. The S4 segments of the
a
-subunits, with
positively charged amino acids at every third position,
were predicted to act as the voltage-sensor and to
undergo outward movement on depolarization of the
membrane, initiating a conformational change associ-
ated with pore-opening.
Mutagenesis studies showed that neutralization of the
positive charges in the S4 domains reduced the steepness
of the potential-dependence of activation, equivalent to
reducing the gating charge. Physical movement of the S4
segments has been demonstrated by substituting
cysteine for the basic amino acids, then assessing the
availability of the individual cysteine-SH groups to
chemical modification by membrane-impermeant sulf-
hydryl reagents, before and after depolarization. Three
successive basic amino acids in the S4 segment of
domain IV (Arg1448, Arg1451, and R1454) move from
being inaccessible within the membrane to being
available for reaction from outside the membrane.
Similar outward movements of positively charged S4
segments have been demonstrated for voltage-sensitive
K
þ
channels. In this case it has been shown by
mutagenesis that the positive charges in S4 are paired
with negatively charged residues in either S2 or S3
segments, stabilizing the S4 segments in the membrane.
THE BASIS OF INACTIVATION
Native sodium channels spontaneously inactivate within
milliseconds of opening. The sensitivity of this rapid
inactivation to limited cytoplasmic applications of
proteases lead to the proposal of the ‘ball and chain’
hypothesis of inactivation, in which an intracellular
inactivation gate (‘the ball’), tethered by a flexible
‘chain’, was able to interact with the intracellular
mouth of the pore and block the channel. The
inactivation gate has been localized to a short intra-
cellular loop connecting regions III and IV. Antipeptide
antibodies directed against this sequence, cutting the
loop in this region and mutagenesis of a hydrophobic
triad of Ile, Phe, and Met in the region all prevent fast
inactivation. Voltage-dependent movement of this seg-
ment has been detected by the depolarization-induced
loss of accessibility to cytoplasmically applied reagents.
The receptor for the inactivation gate has been
delineated by scanning mutagenesis experiments. Mul-
tiple amino acid residues at the intracellular end of
transmembrane segment IVS6 and in intracellular loops
IIIS4-S5 and IVS4-S5 contribute to the receptor for the
inactivation the gate, allowing its binding to block the
pore of the channel (Figure 1).
Modulation of Channel Activity
Sodium channels in neurons and skeletal muscle are
susceptible to modulation by several protein kinases.
Cyclic AMP-dependent protein kinase A (PKA) phos-
phorylates sodium channel
a
-subunits in brain synapto-
somes and intact neurons on four sites in the
intracellular loop between domains I and II. The
consequence of phosphorylation by PKA is a reduction
VOLTAGE-SENSITIVE NA
1
CHANNELS 411
in sodium conductance, with little effect on the voltage-
dependence of activation and inactivation. Activation of
adenylate cyclase via dopamine acting at D1-like
receptors reduces action potential generation and peak
sodium currents in hippocampal neurons. The presence
of a protein kinase-anchoring protein, AKAP-15,
which brings PKA close to the sodium channel, is
essential for this regulation. Cocaine, which increases
dopaminergic neurotransmission by inhibiting dopa-
mine-uptake, also reduces sodium currents in nucleus
accumbens neurons.
Protein kinase C (PKC) also acts on sodium channel
a
-subunits, slowing channel inactivation and reducing
peak sodium currents. Phosphorylation of a site in the
inactivation gate is responsible for the slowing of channel
inactivation. The reduction in peak current is a con-
sequence of phosphorylation at sites in the intracellular
loop between domains I and II (see Figure 1).
The effects of PKA are enhanced by PKC-dependent
phosphorylation and by steady membrane depolariz-
ation, so that the information from three distinct
signaling pathways are integrated via the sodium
channels in the hippocampus and CNS.
The Genetics of Sodium Channels
THE GENE– PROTEIN FAMILY
OF
SODIUM CHANNELS
A large family of sodium channel genes and proteins,
containing at least ten members, has been recognized
through molecular cloning and heterologous expres-
sion to make characterizable protein products. The
nomenclature for sodium channels has been informal
and inconsistent, but a standardized system has recently
been proposed by a consortium of leading researchers
(see Table I).
The nine isoforms of the sodium channel
a
-subunit
that have been characterized are greater than 50%
identical in the transmembrane and extracellular
domains. The continuously variable pattern of
sequences is consistent with their constituting a single
family. The Na
V
1.1, Na
V
1.2, Na
V
1.3, and Na
V
1.7
a
-subunits are the most closely related. These four
channels are all highly tetrodotoxin-sensitive and
expressed in the central and peripheral nervous system.
The corresponding genes are all located on human
chromosome 2q23–24. The Na
V
1.5, Na
V
1.8, and
Na
V
1.9 channel
a
-subunits, all forming tetrodotoxin-
resistant sodium channels, are produced in heart and
neurons of the dorsal root ganglia from genes located on
chromosome 3p21–24. The skeletal muscle sodium
channel isoform, Na
V
1.4, formerly called type
m
1, is at
least 84% identical to the group of channels encoded on
chromosome 2, but has a more distant phylogenetic
relationship as determined by parsimony comparison.
Comparison across mammalian species shows that
the chromosome segments containing sodium channel
a
-subunit genes are paralogous segments containing
many sets of related genes, generated by large-scale
duplication events during early vertebrate evolution.
Several sodium channel
a
-subunits have been recog-
nized by molecular cloning and DNA sequencing, but
have not yet been characterized by functional expression
(Table I,Na
X
). These sequences have more than 80%
identity to each other and are phylogenetically closely
related to the group of sodium channel subunits encoded
on human chromosome 2q23 –24, where the human
SCN6A gene is located.
The genes encoding mammalian sodium channel
a
-subunits are complex, with more than 20 exons.
Splice variants of several sodium channel proteins have
been identified and more are likely to be found.
GENETIC DEFECTS AFFECTING
VOLTAGE-GATED SODIUM CHANNELS
Mutations in genes encoding sodium channel subunits
are associated with several inherited human diseases
characterized by hypersensitivity, including several
monogenic epilepsy syndromes. Mutations in the
human SCN1A gene have been associated with
familial generalized epilepsy with febrile seizures plus
(GEFSþ), an autosomal dominant syndrome character-
ized by febrile seizures (FS) and a variety of afebrile
generalized seizure types. De novo mutations in SCN1A
are a major cause of severe myoclonic epilepsy of
infancy (SMEI or Dravet syndrome). Benign familial
neonatal-infantile seizures have been ascribed to
mutations in the SCN2A gene.
Mutations in the SCN4A gene encoding the skeletal
muscle sodium channel
a
-subunit are the cause of the
hereditary disorders of sarcolemmal excitability, hyper-
kalemic periodic paralysis (HYPP or hyperPP) and
paramyotonia congenita (PMC). Mutations have been
shown to cause reduced rates of channel inactivation,
altered voltage-sensitivity or slowed coupling between
activation and inactivation. HYPP is a muscle disorder
that is prevalent in American quarter horses, where
selective breeding for muscular definition and hypertro-
phy has favored spread of the mutant SCN4A gene in the
gene-pool.
One of the causes of long QT syndrome, an inherited
cardiac arrhythmia, is mutation in the SCN5A gene,
leading to non-inactivating sodium currents that
prolong the plateau of the cardiac action potential.
The result is an elongated interval between the QRS
complex and the T wave in the electrocardiogram. The
mutations usually affect amino acid residues in the
inactivation gate or its receptor region, reducing the rate
of inactivation.
412
VOLTAGE-SENSITIVE NA
1
CHANNELS
