Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

transcription factor. The AF-2 domain exists at the
extreme COOH terminus while the AF-1 domain is
located in the N-terminal region (or A/B domain) of the
receptors (Figure 2). The AF-1 domain is a constitutive
(i.e., hormone-independent) activation domain. In the
VDR, the N-terminal A/B domain is truncated compared
to other nuclear receptors and thus an analogous
constitutive AF-1 domain is lacking in the VDR.
In order for VDR to regulate transcription of target
genes, it must recognize and bind to DNA in the
promoter regions of vitamin D-responsive genes. It does
so through a specialized DNA-binding domain (DBD)
located near the amino terminus of the receptor
(Figure 3). This DBD is required for the VDR to bind
selectively and with high affinity to specific DNA
sequences termed vitamin D-responsive elements
(VDREs). The minimal DBD of the VDR that mediates
VDR–DNA interactions resides between amino acid
residues 22 and 113 in the human sequence. There are
nine cysteine residues within the DBD that are conserved
throughout the family members. The first eight of these
cysteines (counting from the N terminus) tetrahedrally
coordinate two zinc atoms to form two zinc finger DNA-
binding motifs. Mutation of the first eight of the nine
cysteine residues to serines eliminates VDR binding to
both nonspecific and specific DNA sequences and
eliminates VDR-mediated transactivation. Indeed, inac-
tivating mutations within the DBD of the human VDR
are responsible for the rare inherited disorder termed
vitamin D-resistant rickets type II. These patients
express a nonfunctional VDR that cannot bind to
DNA, and they exhibit classical symptoms of vitamin
D deficiency that are not corrected by providing an
external source of vitamin D.
LIGAND-BINDING DOMAIN (LBD)
The large carboxyl-terminal ligand-binding domain
(LBD) of VDR is organized into 12
a
-helices. As the
name suggests, the LBD is responsible for high-affinity
binding of the 1,25(OH)
2
D
3
ligand, exhibiting equili-
brium binding constants of the order of 10
210
to
SRCs
Ac
LXXLL
Med
220
RNA Pol II
Ac
Ac
Ac
Ac
Ac
Ac
Ac
Ac
TBP
LXXLL
VDRE
p300
SRCs
p300
NCoA62/
SKIP
D
RXR
VDR
VDRE
RXR
VDR
D
NCoA62/
SKIP
VDR
D
D
VDR
VDR
D
VDRE
VDRE
RXR
VDR
RXR
VDR
VDR
RXR
SRCs
p300
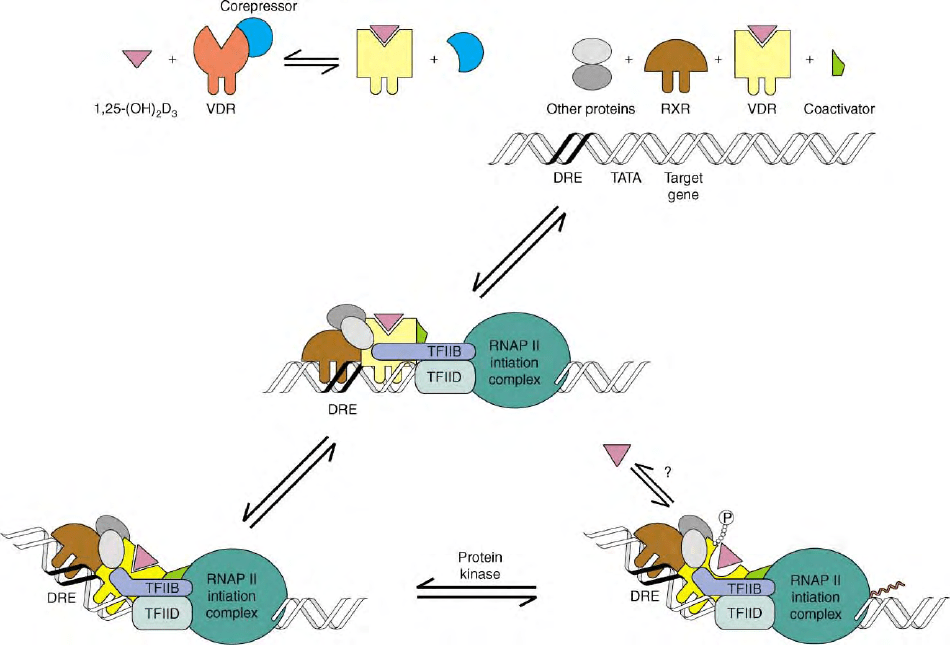
FIGURE 1 Mechanism of action of the vitamin D receptor. VDR
binds to its ligand, 1,25(OH)
2
D
3
, heterodimerizes with RXR, and
binds to a DR-3 type VDRE in the promoter of 1,25(OH)
2
D
3
-
responsive genes. VDR occupies the proximal or 3
0
half-site while RXR
occupies the distal or 5
0
half-site. VDR/RXR then recruits coactivators
such as SRCs and p300/CBP. The HAT activity of these coactivators
loosens the chromatin structure, allowing for a more transcriptionally
permissive environment. SRCs and p300/CBP dissociate, allowing
for binding of NCoA62/SKIP and the Mediator-D multimeric
complex. This latter complex is thought to recruit RNA Pol II and
the core transcriptional machinery to initiate active transcription of
the target gene.
VITAMIN D RECEPTOR 379

10
211
M. Both the 1-hydroxyl and 25-hydroxyl moieties
are crucial for efficient recognition by VDR, and the
nonhydroxylated, inactive parent vitamin D
3
compound
does not bind appreciably to the VDR.
In addition to hormone binding, the LBD is required
for several other aspects of receptor function, particu-
larly in mediating protein –protein interactions. One
important protein– protein contact is the heterodimer-
ization of VDR with the retinoid X receptor (RXR). As
mentioned above, VDR– RXR heterodimer formation is
generally required for high-affinity interaction of the
receptor with VDREs and an extensive heterodimeriza-
tion interface that coalesces around helices 10 and 11
in the LBD of VDR mediate protein–protein contacts
with RXRs.
The LBD also mediates association of VDR with
comodulatory proteins. Many of these interactions
require at the extreme C-terminus of the receptor,
where the AF-2 domain is located (Figure 2). The AF-2
domain is highly conserved throughout the hormone
receptor superfamily and its main structural feature is
that of an amphipathic
a
-helix. Removing 25 amino
acids from the C-terminus of hVDR (D403–427), which
contains the AF-2 domain, results in a complete loss of
1,25(OH)
2
D
3
/VDR-activated transcription. This loss of
function is not due to altered binding of RXR, VDRE, or
hormone and the mutant receptor was appropriately
targeted to the cell nucleus. Thus, the AF-2 domain of
VDR, corresponding to helix 12, plays a central role in
1,25(OH)
2
D
3
-activated transcription mediated by VDR.
Molecular Mechanism of
Transcriptional Control by VDR
VDR INTERACTION WITH VITAMIN D
R
ESPONSIVE ELEMENTS
VDR modulates transcription by binding to specific
VDREs, in the promoter regions of responsive
genes. VDREs from a variety target genes have been
identified and on this basis a consensus VDRE may be
generally described as an imperfect direct repeat of a
core hexanucleotide sequence,G=AGGTG=CA; with a
spacer region of three nucleotides separating each half
element (also termed DR-3 for direct repeat with a
three nucleotide spacer). The mechanism of VDR
binding to VDREs is reflected in the direct repeat
nature of the element. For example, purified VDR
alone does not bind to a VDRE with high affinity.
Rather, RXR is required for high-affinity binding of
VDR to VDREs. The asymmetry of the directly
repeating motif causes the VDR–RXR heterodimer
to bind to the VDRE with a defined polarity, with
RXR occupying the 5
0
half site and VDR occupying
the 3
0
half site.
The natural VDREs identified thus far provide only
a snapshot of the DNA sequences that mediate the
transcriptional effects of the VDR. Variations on
the DR-3 motif for VDREs have been identified in
the elements that mediate vitamin D responsiveness
in the calbindin D
9k
and calbindin D
28k
genes.
Moreover, several synthetic elements with large
spacer regions and inverted arrangements can
mediate vitamin D responsiveness under certain
conditions. It is likely that the affinity of VDR for
these atypical elements may vary from that of the
classic DR-3 motif adding yet another level of
regulatory complexity to the process of VDR-mediated
gene expression.
FIGURE 2 Functional domains of the VDR. A/B, amino terminal
region; DBD, DNA- binding domain containing two zinc-finger motifs;
LBD, ligand-binding domain; AF-2, activation function-2 domain
encompassing helix 12.
Helix II
P
G
D
F
C
P
F
N
G
D
CC
Q
A
C
C
G
GT
E
G
C
Zn
G
F
H
Zn
CC
Helix I
V
Helix III
T-box
P-box
D-box
DPLSTSAAMAEM NH
2
DRNVPRI
T
A
R
D
F
N
A
M
KGFFRRSMKRKALFT
R
R
HR
I
T
K
D
N
RLKRCVDIGMMKEFILTDEE
V
-
FIGURE 3 Schematic of the DNA-binding domain of the VDR. The cysteines responsible for coordinating the zinc atoms are shown in red.
380 VITAMIN D RECEPTOR
One important role for the 1,25(OH)
2
D
3
ligand in
the transactivation process is to dramatically enhance
both the formation of the VDR–RXR heterodimer and
the subsequent interaction of the heterodimer with the
VDRE (Figure 1). Upon ligand binding, there is a clear
1,25(OH)
2
D
3
-dependent decrease in the formation of
VDR homodimers and a concomitant increase in VDR
heterodimerization with RXR. These ligand-induced
changes in VDR–RXR interactions are likely due to
altered conformations of the VDR that disrupts weak
homodimers of unliganded VDR and promotes
liganded VDR heterodimerization with RXR. The
interaction of VDR and RXR generates a heterodimeric
complex that is highly competent to bind DR-3 like
VDREs and subsequently affect the transcriptional
process. Thus, the VDR–RXR heterodimer is the
primary active complex involved in controlling DR-3
VDRE-driven promoters.
COMMUNICATION BETWEEN VDR AND
THE
TRANSCRIPTIONAL MACHINERY
The regulation of VDR-mediated transcription involves
a complex series of macromolecular interactions occur-
ring in a temporally coordinated fashion (Figure 1).
Other transcriptional components that associate with
the liganded VDR/RXR heterodimer may be classified
into two general categories: general transcription factors
and the comodulatory proteins. The interaction of VDR
with the first group results in direct contacts with the
preinitiation complex (PIC), which may facilitate
assembly or recruitment of the PIC and thereby
stimulate transcription by RNA Pol II. Transactivator
interaction with the PIC may occur via adapter proteins
such as the TBP-associated factors (TAFs) or via direct
interaction with other core transcription factors. In this
regard, TFIIB may be a key target since it is known
to interact directly with VDR and augment vitamin
D-activated transcription in transient gene expression
studies. Indeed, differences in the activities of NH
2
-
terminal VDR isoforms are attributed to differential
interactions with TFIIB thus, supporting an important
role for the VDR–TFIIB interaction in determining the
overall transactivation potential of the liganded VDR.
In addition to direct contacts with the general
transcription machinery, the liganded VDR is also linked
to the transcriptional PIC by the NR comodulatory
factors. The general functional properties of the NR
comodulators are their ability to interact with nuclear
receptors and control their transcriptional responsive-
ness to ligand. NR comodulators are classified either as
coactivators or corepressors, and they aid in the
induction or repression, respectively, of ligand/recep-
tor-mediated transcription. NR coactivators may func-
tion as macromolecular bridges between the receptor
and the transcriptional machinery that aid in the
assembly or promote the stability of the preinitiation
complex. Moreover, several coactivator proteins either
possess intrinsic histone acetyltransferase (HAT) activi-
ties, or recruit other proteins that possess HAT activity
(e.g., CBP/P300). Acetylation of histones near the
promoter presumably results in a loosening of chroma-
tin structure and greater accessibility of the promoter for
the transcriptional machinery (Figure 1). The best-
characterized coactivators are the steroid receptor
coactivator (SRC) family of nuclear receptor coactiva-
tors that includes three members at present: SRC-1
(NCoA-1), SRC-2 (GRIP-1, TIF2, NCoA-2), and SRC-3
(pCIP, RAC3, ACTR, AIB-1, TRAM-1).
Structural studies of related receptors provide insight
into the mechanism of ligand-induced interaction of
VDR with SRC coactivators. It is hypothesized that the
1,25(OH)
2
D
3
ligand promotes coactivator interaction
by inducing a repositioning of the AF-2 activation helix,
or helix H12 (Figure 4). In the unliganded state, the AF-2
domain (helix H12) projects out away from the globular
core of the LBD, while in the liganded state the AF-2
domain is folded over onto the LBD globular core
domain. One outcome of helix H12 folding is
the creation of a platform or protein interaction surface
through which coactivator proteins such as SRCs
effectively dock with the VDR. The domain in the
coactivator proteins, which is responsible for this
interaction is termed the nuclear receptor or NR box
andisanamphipathic
a
-helix composed of the
consensus core sequence LXXLL. This interaction is
essential for transactivation mediated by the VDR.
A large multiprotein complex called Mediator D is
another coactivator required for VDR-mediated tran-
scription (Figure 1). Mediator D is also known as
vitamin D receptor interacting protein (DRIP), thyroid
receptor activating protein (TRAP) complex, and the
mammalian mediator complex. The Mediator D com-
plex is composed of at least ten different proteins
anchored by Med220, which interacts directly with
ligand-activated VDR/RXR heterodimers through one
of two LXXLL motifs. DRIP is essential for in vitro
VDR-mediated transcription from chromatinized tem-
plates. However, as the DRIP complex does not contain
any SRCs and does not possess HAT activity, it appears
to potentiate NR-mediated transcription through dis-
tinct mechanisms. In particular, DRIP directly recruits
the RNA polymerase II holoenzyme to 1,25(OH)
2
D
3
-
activated VDR, indicating that DRIP may serve as a
bridge between VDR and the core transcriptional
machinery. Indeed, chromatin immuno-precipitation
studies show that DRIPs enter transcriptional complexes
at a later time than SRC coactivators, supporting
the nonredundant roles of these coactivator classes in
NR-mediated transactivation.
Another protein named NCoA62/SKIP was identified
as a VDR and NR coactivator, and as such, it augments
VITAMIN D RECEPTOR 381

VDR- and other NR-activated transcriptional processes
in transient reporter gene assays. On the basis of its
primary amino acid sequence and its function,
NCoA62/SKIP is a unique protein. One key difference
is the lack of LXXLL motifs which are characteristic of a
large variety of coactivators including the SRCs,
CBP/P300 and the Mediator D coactivator complex. In
addition, NCoA62 interacts with VDR in a ligand- and
AF2-independent manner. Although NCoA62/SKIP
does not interact directly with SRC coactivators,
NCoA62/SKIP, VDR, and SRCs form a ligand-depen-
dent ternary complex. NCoA62/SKIP also functions
cooperatively with SRC coactivators to augment VDR-
activated transcription and it is recruited to vitamin D
responsive target genes in a distinct, temporal manner
from the SRC coactivators. Presently, the mechanisms
involved in this synergistic action of these two coacti-
vator classes are unknown.
Summary
Research spanning the 1980s and 1990s reveals that
1,25(OH)
2
D
3
-mediated transcription is more complex
than the simple binding of the receptor to DNA and the
recruitment of RNA Pol II to initiate transcription.
VDR/RXR-activated transcription involves complex
interactions that may occur in a spatially distinct and
temporally coordinated fashion to increase the rate at
which 1,25(OH)
2
D
3
-responsive genes are transcribed
and at which the resulting RNA transcript is processed.
A model for VDR-mediated transcription is proposed
which incorporates numerous properties of VDR that
were discussed in this section (Figure 1). The initial event
in this model is high-affinity binding of the 1,25(OH)
2
D
3
ligand to the VDR. Ligand binding induces VDR/RXR
heterodimerization and the heterodimer specifically
binds VDREs in the promoter regions of vitamin D
responsive genes. The heterodimer then recruits coacti-
vator molecules that acetylate core histones to make the
DNA more accessible to the transcriptional machinery.
Meanwhile, other coactivators and the PIC are recruited
to the site and activated transcription proceeds. Under-
standing the complex interplay that occurs between
these various factors is crucial to unraveling the
complexities of activated or repressed transcription
mediated by vitamin D and the VDR.
SEE ALSO THE FOLLOWING ARTICLES
Calcium Buffering Proteins: Calbindin † Vitamin D †
Zinc Fingers
GLOSSARY
comodulatory proteins Proteins that interact directly with the
nuclear receptor and alter the transcriptional regulatory activity
of the receptor. Coactivators enhance the activity of the receptor,
and corepressors attenuate the transcriptional regulatory activity of
the receptor.
1,25-dihydroxyvitamin D
3
(1,25(OH)
2
D
3
) Theactiveformof
vitamin D in mammals.
histone acetyltransferase (HAT) Enzymes that add acetyl groups to
the positively charged lysine residues on core histones, effectively
negating the positive charge. Histone hyperacetylation is correlated
with areas of active transcription, while hypoacetylation is
correlated with nontranscribed promoters.
preinitiation complex (PIC) The complex of basic transcription
factors that are necessary and sufficient for the initiation of
transcription to occur. In addition to RNA Polymerase II, the PIC
consists of transcription factor IIA (TFIIA), TFIIB, TFIIE, TFIIF,
and TATA-binding protein or TBP.
retinoid X receptor (RXR) A member of the nuclear receptor
superfamily of transcription factors that serves as a common
heterodimeric partner for many of the class II nuclear receptors
including retinoic acid receptor, vitamin D receptor, thyroid
hormone receptor, and peroxisome proliferator-activating receptor.
vitamin D responsive elements (VDRE) Specific sequences of DNA
that the VDR–RXR heterodimer selectively recognize and bind.
This binding event is one of the initial steps in the mechanism
through which 1,25-dihydroxyvitamin D
3
and the VDR regulate
gene transcription.
FURTHER READING
Feldman, D., Glorieux, F. H., and Pike, J. W. (eds.) (1997). Vitamin D.
Academic Press, San Diego.
Haussler, M. R., Whitfield, G. K., Haussler, C. A., Hsieh, J. C.,
Thompson, P. D., Selznick, S. H., Dominguez, C. E., and
Jurutka, P. W. (1998). The nuclear vitamin D receptor: Biological
and molecular regulatory properties revealed. J. Bone Miner. Res.
13, 325–349.
Jones, G., Strugnell, S. A., and DeLuca, H. F. (1998). Current
understanding of the molecular actions of vitamin D. Physiol.
Rev. 78, 1193–1231.
Jurutka, P. W, Whitfield, G. K., Hsieh, J. C., Thompson, P. D.,
Haussler, C. A., and Haussler, M. R. (2001). Molecular nature of
the vitamin D receptor and its role in regulation of gene expression.
Rev Endocr. Metab. Disord. 2, 203–216.
Malloy, P. J., Pike, J. W., and Feldman, D. (1999). The vitamin D
receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-
resistant rickets. Endocr. Rev. 20, 156–188.
McKenna, N. J., Lanz, R. B., and O’Malley, B. W. (1999). Nuclear
receptor coregulators: Cellular and molecular biology. Endocr. Rev.
20, 321–344.
Norman, A. W., Mizwicki, M. T., and Okamura, W. H. (2003). Ligand
structure–function relationships in the vitamin D endocrine system
from the perspective of drug development (including cancer
treatment). Recent Results Cancer Res. 164, 55–82.
Rachez, C., and Freedman, L. P. (2001). Mediator complexes and
transcription. Curr. Opin. Cell Biol. 13, 274–280.
VDR
VDR
AF-2
1,25 D
3
SRC
L
X
X
L
L
FIGURE 4 The vitamin D receptor undergoes a conformational
change upon binding hormone. The VDR binds ligand and this induces
a conformational change in the AF-2 domain to trap the ligand in the
binding pocket. This change also creates a hydrophobic cleft or surface
on VDR that LXXLL motifs in coactivator proteins use for docking to
the VDR.
382 VITAMIN D RECEPTOR
Sutton, A. L., and MacDonald, P. N. (2003). Vitamin D: More than a
“bone-a-fide” hormone. Mol. Endocrinol. 17, 777–791.
BIOGRAPHY
Paul MacDonald and Diane Dowd are Faculty Members in the
Department of Pharmacology at Case Western Reserve University.
They are actively involved in basic research on the molecular, cellular,
and physiological roles of the vitamin D receptor in normal and
pathological states. Dr. MacDonald holds a Ph.D. in biochemistry
from Vanderbilt University and received his postdoctoral training
at the University of Arizona. Dr. Dowd received her Ph.D. in
biochemistry from Vanderbilt University and her postdoctoral training
at the Arizona Cancer Center and University of Arizona.
VITAMIN D RECEPTOR 383

Vitamin D
Hector F. DeLuca and Margaret Clagett-Dame
University of Wisconsin, Madison, Wisconsin, USA
This article discusses the chemical identity and production of
vitamin D. Its endocrine system, the nuclear receptor, and its
mechanism of action are treated, along with the degradation of
vitamin D hormone and its roles.
Chemical Identity, Natural
Occurrence, and Production
The vitamin Ds are secosterols in which the B-ring of the
steroid nucleus is replaced by a diene bridge. The two
most common forms are vitamin D
2
(ergocalciferol) and
vitamin D
3
(cholecalciferol). The structures of these two
nutritionally important compounds are illustrated in
Figure 1.
Although it is classified as a vitamin because of the
nature of the discovery process, vitamin D in actual fact
should not be considered a true vitamin for the following
reasons. First and foremost, vitamin D utilized by higher
organisms is manufactured in the epidermis of skin by
photolysis of 7-dehydrocholesterol, a side reaction
product of cholesterol synthesis. Secondly, vitamin D is
nearly absent from the food supply for the most part.
Vitamin D is found in fish liver oils and in egg yolk but is
not found in virtually all plant materials, in skeletal
meats, seeds, fruits, and vegetables. In fact, very little is
found in an expected source, milk. We consider milk an
important supply of vitamin D in many countries,
primarily because it is fortified by the addition of
vitamin D made artificially by man.
The chemical process whereby 7-dehydrocholesterol
is converted to vitamin D
3
is well known. The 5,7-diene
portion of either ergocalciferol or 7-dehydrocholesterol
will absorb 280– 310 nm ultraviolet light. This causes an
isomerization to form a compound, previtamin D
3
,
which in itself is biologically inactive and remains in
skin. Previtamin D is in equilibrium with vitamin D
3
. The
plant sterol, ergosterol, is converted to vitamin D
2
by an
identical process. For many years, vitamin D
2
was the
major synthetic form of vitamin D used in vitamin
capsules, animal feeds, and in the fortification of foods.
Because of improved synthetic methods, vitamin D
3
has
become the major form used for both fortification and
nutrition of domestic animals. The photolysis of
7-dehydrocholesterol is quite efficient in skin, and
summer sun on hands and face and will in 10 minutes
produce the daily requirement of vitamin D (currently
believed to be 10–20 mg per day). Winter sun is unable to
induce production of vitamin D because of the angle of
the sunlight and the filtration of the critical wavelengths
that cause vitamin D production. It is generally accepted
that skin production of vitamin D
3
is quantitatively the
most important source of the vitamin for human health.
Conversion of Vitamin D
to its Hormonal Form
The vitamin D
3
that is produced in skin or that is
absorbed from the intestine from vitamin pills or
fortified foods is biologically inactive as such. The
body must process vitamin D to form a final vitamin D
hormone that is believed to carry out all of the known
functions of vitamin D. Vitamin D
3
is first hydroxylated
in the liver to form the circulating form of vitamin D
3
,
25-hydroxyvitamin D
3
(25-OH-D
3
). Interestingly, this
compound is also biologically inactive and must be
further modified before function. The second step occurs
in the proximal convoluted tubule cells of the kidney
where a 1
a
-hydroxyl group is added to the molecule to
produce the final vitamin D hormone, 1
a
,25-dihydroxy-
vitamin D
3
(1,25-(OH)
2
D
3
). The reaction steps that
result in this transformation are shown in Figure 2.
The first step in conversion of vitamin D to the
circulating form occurs in the hepatocytes of liver but
not exclusively so. At least two enzymes are known to
make this conversion, a mitochondrial enzyme and a
microsomal enzyme, both of which have been cloned.
No natural human mutation blocking this initial step has
yet been discovered. However, the second step occurring
in the proximal convoluted tubule cells of the kidney is a
highly regulated one. This 1
a
-hydroxylase, also known
as CYP27B1, has also been cloned and natural
human mutants of this enzyme are now well known.
These mutations result in a disease called vitamin
D-dependency rickets type I in which children present
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 372

vitamin D-deficiency diseases, i.e., rickets, hypocalce-
mia, and hypophosphatemia, even though they are
naturally irradiated with sunlight or are provided
vitamin D as a supplement. These children are cured by
the administration of physiologic amounts of the natural
vitamin D hormone, 1,25-(OH)
2
D
3
, establishing clearly
the two-step activation process of vitamin D for man.
The Role of 1,25-(OH)
2
D
3
in Calcium, Phosphorus,
and Bone Metabolism
The basis for discovery of vitamin D in the first place is
the disease rickets in children. This disease is also
accompanied by hypocalcemia (low blood calcium)
causing convulsive tetany. In adults, the deficiency of
vitamin D causes the disease, osteomalacia. The
diseases, rickets and osteomalacia, result from a failure
to mineralize the organic matrix of newly synthesized
bone. Early investigations to understand how vitamin D
functions were, of course, directed toward the formation
of mineralized bone. However, it is now abundantly
clear that 1,25-(OH)
2
D
3
does not act directly on the
mineralization process in the healing of rickets or
osteomalacia but rather acts on intestine, kidney, and
bone to provide calcium and phosphorus in the plasma
required for the mineralization of skeleton and preven-
tion of the convulsive disease, hypocalcemic tetany.
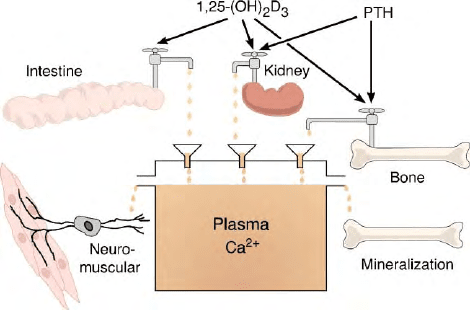
The essence of vitamin D action, therefore, in curing
rickets and osteomalacia is the elevation of plasma
calcium and phosphorus to supersaturating levels that
are required for normal mineralization. The vitamin D
hormone acts to provide this calcium by stimulating the
enterocyte of the small intestine to absorb calcium as
well as phosphate by an independent mechanism both of
which require active metabolic energy.
Because of the constant need for calcium to support
normal neuromuscular function, calcium must be
available in the plasma even when it is not available
in the diet. Thus, the vitamin D hormone, i.e., 1,25-
(OH)
2
D
3
acting together with parathyroid hormone will
cause the mobilization of calcium from the skeletal fluid
compartment into the plasma compartment. Thus, the
skeleton serves not only a structural function but also as
a source of calcium and phosphorus. A final point is
that 1,25-(OH)
2
D
3
works in concert with the parathyr-
oid hormone to induce the distal nephron of the kidney
to reabsorb the last 1% of the filtered load of calcium
into the plasma compartment. These then work to
maintain plasma calcium and phosphorus at levels that
support both bone formation and prevent hypocalcemic
tetany. A diagram representing these functions is
shown in Figure 3.
The Vitamin D Endocrine System
One of the most elegantly regulated substances in the
body is the plasma calcium level. The parathyroid glands
continuously monitor serum calcium levels and a
calcium receptor therein responds when calcium falls
even slightly below the normal level. By a G protein
FIGURE 1 The nutritionally important forms of vitamin
D. Vitamin D
3
is the form manufactured in skin and vitamin D
2
is
the form produced by the irradiation of ergosterol. In mammals, both
are equally active and both appear to be metabolized by almost
identical routes.
FIGURE 2 The required metabolism of vitamin D for function. Shown here is the metabolism of vitamin D
3
to the major blood form of vitamin
D, 25-hydroxyvitamin D3 and its subsequent metabolism in the kidney to form the final vitamin D hormone, 1
a
,25-dihydroxyvitamin D
3
. It is this
form of vitamin D
3
that carries out all of the known functions of the vitamin. Vitamin D
2
not shown here is metabolized in an almost identical
fashion to its active form, 1
a
,25-dihydroxyvitamin D
2
.
VITAMIN D 373
mechanism, the calcium receptor stimulates the
secretion of parathyroid hormone, a peptide hormone,
that is the body’s signal requesting calcium. It binds to
the entire nephron of the kidney and to the osteoblasts of
bone. Among its many functions in the kidney is to
stimulate the 1
a
-hydroxylase (CYP27B1) to produce
the vitamin D hormone in substantial quantities.
The vitamin D hormone then acts on the bone, kidney,
and intestine as described earlier to mobilize calcium as
needed to raise blood calcium into the normal range,
clearing the set-point of the parathyroids shutting down
parathyroid secretion. Overshoot of calcium results in
the C-cells of the thyroid secreting calcitonin, a 32
amino acid peptide hormone that blocks calcium
mobilization from the skeleton. Calcitonin also has a
stimulatory effect on the 1
a
-hydroxylase to produce the
vitamin D hormone in small amounts to support its
noncalcemic activities.
The Vitamin D Receptor
1,25-(OH)
2
D
3
is a true steroid hormone that acts
through a nuclear receptor called the vitamin D receptor
(VDR). The human receptor is a 427 amino acid protein
that serves as a ligand-dependent transcription factor.
It is a member of the nuclear receptor superfamily of
receptors. Other members of this superfamily include
the retinoic acid, thyroid, estrogen, testosterone, gluco-
corticoid, and other steroid hormone receptors. VDR is
the smallest of the steroid hormone receptors and largely
acts in a fashion quite similar to other steroid hormone
receptors. Unlike other nuclear receptors, there is only a
single VDR in all tissues. No subtypes are known and it
is this receptor through which all vitamin D actions
occur. Natural mutants of the human VDR are known
and produce a disease called vitamin D-dependency
rickets type II. In this disease, high blood levels of
1,25-(OH)
2
D
3
occur in the face of severe rickets and
severe hypocalcemia and hypophosphatemia. VDR null
mutant mice have been produced and are available as
experimental tools. The vitamin D-dependency rickets
type II patients present a variety of diseases depending
upon where the mutation has occurred. If there is a
complete absence of a functional receptor, then very
brittle vitamin D-resistant rickets occurs and can only be
treated by the infusion of calcium and phosphorus into
the blood stream to mineralize the skeleton but many of
the other functions of vitamin D go unmet. The fact that
VDR null mutant mice are normal at birth shows that
vitamin D is not an embryonic developmental factor but
functions primarily after parturition.
There are reports of nongenomic functions of
vitamin D. These have been largely cellular-based
observations, which have yet to be shown to occur
in vivo. In fact, the administration of large amounts of
1,25-(OH)
2
D
3
to receptor null mutant mice produces no
significant phenotype, suggesting that the only mechan-
ism of action of vitamin D is through its receptor under
physiologic circumstances.
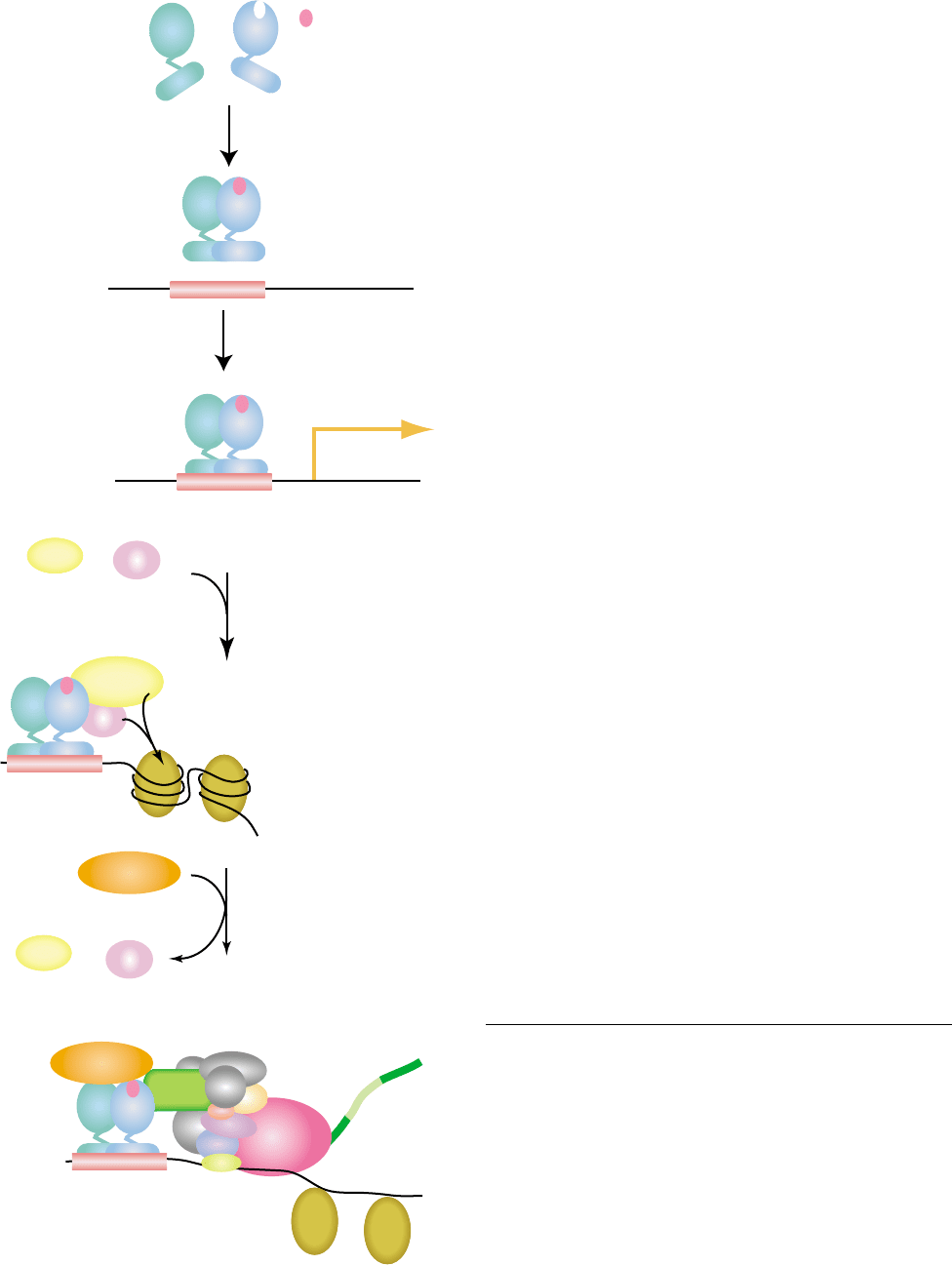
Molecular Mechanism
Vitamin D-response elements (VDE) are found in the
promoter region of target genes. These are hexameric
repeats separated by three nonspecified bases. There can
be as many as three repeat elements in a responsive
element system. The VDR must heterodimerize with
another transcription factor, the retinoid X receptor
(RXR), in order to bind to the responsive elements. The
RXR binds to the 5
0
repeat and the VDR to the 3
0
repeat.
Figure 4 provides a cartoon to illustrate what is currently
known about the mechanism of action of the vitamin D
hormone working through its receptor to regulate
expression or suppression of target genes. As with
other steroid hormones, it is anticipated that the
unliganded receptor may be retained in an inactive
state by being bound to a corepressor. This has not been
specifically shown for the VDR but is known for other
class II nuclear receptors. There is increasing evidence
that such a corepressor might be identified for the VDR.
In this model, the corepressor that interacts directly with
the VDR would be released upon binding of the ligand
to the receptor probably because of a change in VDR
conformation. The receptor would then be free to
interact with a coactivator protein that is believed to
be involved in chromatin remodeling and to link the
complex bound at the hormone response element to the
basal transcription machinery. Among the known
coactivators believed to interact directly with the VDR
FIGURE 3 A diagrammatic representation of how the vitamin D
hormone 1
a
,25-(OH)
2
D
3
) functions to maintain plasma calcium at
supersaturating levels which are required for prevention of hypocal-
cemic tetany (neuromuscular) and for the prevention of rickets and
osteomalacia (mineralization of bone).
374 VITAMIN D
are SRC 1, 2, 3, and DRIP 205. Likely other participat-
ing coactivators will be discovered. A phosphorylation
does occur on serine 205 of the VDR but whether this is
of functional importance remains to be determined.
Metabolism and Degradation
The vitamin D hormone is degraded specifically by
an enzyme it induces. That enzyme is the CYP24
(24-hydroxylase), which carries out a series of reactions
on the side chain of the vitamin D hormone and its
precursor, 25-OH-D
3
. It first causes hydroxylation in the
24R-position followed by oxidation of that same
hydroxyl to a ketone. The next step again carried out
by the same enzyme is a 23-hydroxylation followed by
cleavage and oxidation giving rise to the biliary
excretion product called calcitroic acid. Calcitroic acid
is biologically inactive. Thus, the vitamin D hormone,
an extremely potent biological substance, ensures
its limited activity by inducing its own destruction.
Other metabolism of vitamin D is known including the
formation of the 26,23-lactone derivative, 26-hydroxy-
lation, but quantitatively the most important reaction
regulating the level of vitamin D hormone is the CYP24.
The CYP24 is regulated in a negative fashion by the
parathyroid hormone causing an instability of the
mRNA encoding for that enzyme. Thus, the parathyroid
hormone not only stimulates the 1
a
-hydroxylase but
also stimulates the destruction of the enzyme that
destroys the hormone, thereby ensuring that large
amounts of the vitamin D hormone are available to
carry out its calcium functions.
New and Nonclassical Roles
of the Vitamin D Hormone
With the discovery of the VDR came the observation
that it is found in tissues not previously appreciated as
target tissues of vitamin D action. The discovery of
FIGURE 4 A cartoon representing the molecular mechanism of action of the vitamin D hormone (1,25-(OH)
2
D
3
) in regulating transcription of
target genes in the target tissues. Binding of the ligand, i.e., 1,25-(OH)
2
D
3
to the VDR causes a conformational change and a rejection of the
corepresssor. It also allows binding of a coactivator and formation of a heterodimer with RXR on the vitamin D responsive elements. Several
proteins are required to form the transcription complex, all of which have not yet been identified. There is a bending of the DNA and a
phosphorylation on serine 205 but their exact role in either suppression or activation of transcription remains unknown.
VITAMIN D 375
the VDR in the intestine, kidney tubules, and osteoblasts
was not unexpected. However, its presence in the
parathyroid gland, the islet cells of the pancreas, the
immune cells such as T-cells, macrophages, and kerati-
nocytes made it possible that the vitamin D hormone
had important functions besides raising blood calcium
and phosphorus and mineralizing the skeleton. It is now
clear that an important function of the vitamin D
hormone is to suppress the growth of parathyroid glands
and to suppress production of the parathyroid hormone.
This basic function has been utilized by physicians using
1,25-(OH)
2
D
3
and its analogues to treat secondary
hyperparathyroidism in kidney failure patients.
Another important VDR site are the cells of the
immune system and in the promyelocytes which are the
precursors of the monocyte. The vitamin D hormone
stimulates the formation of the monocyte by causing
the production of RANKL by osteoblasts and stromal
cells. This results in a coalescence of the monocytes to
form giant osteoclasts which are further activated by
RANKL. Thus, the formation of the giant osteoclast
that causes bone turnover is a vitamin D function.
Furthermore, vitamin D also functions to cause the
formation of new bone, thus playing an important role
in the bone remodeling and modeling systems.
The role of the vitamin D hormone in the keratinocyte
has also become of importance therapeutically. The
vitamin D hormone added to the keratinocytes in cultures
or added topically will cause a cessation of proliferation
of the keratinocyte and will cause its differentiation.
Thus, 1,25-(OH)
2
D
3
and analogues have been success-
fully used in the topical treatment of psoriasis.
Finally, attention must be focused on the immune
system where the vitamin D hormone is now known to
cause important immuno-modulatory effects. The vita-
min D hormone can be used to block certain animal
models of autoimmune disease. For example, it can be
used to prevent experimental autoimmune encephalo-
myelitis (EAE), a model of multiple sclerosis in mice.
However, in so doing, it does induce an abnormal rise in
serum calcium. 1,25-(OH)
2
D
3
can be used to prevent
diabetes in the nonobese diabetic mouse model of type 1
diabetes. It can be used to prevent rheumatoid arthritis,
systemic lupus, and inflammatory bowel disease.
Thus, the immuno-modulatory role of the vitamin D
hormone under normal circumstances is only now
becoming realized.
Therapeutic Uses of 1
a
,25-(OH)
2
D
3
and its Analogues
As discussed earlier, besides the treatment of vitamin
D-dependency rickets and vitamin D-resistant rickets of
many types, the vitamin D hormone and its analogues
are certainly central actors in the treatment of bone
disease secondary to kidney failure. This is primarily
by suppression of parathyroid proliferation and by
suppression of parathyroid hormone production.
1,25-(OH)
2
D
3
and its analogue, 1
a
-OH-D
3
has been
successfully used in certain parts of the world to treat
postmenopausal and age-related osteoporosis, giving a
modest rise in bone mass along with a clear reduction in
fracture rate. The danger of hypercalcemia has limited
its use in the treatment of this disease. However, newer
vitamin D analogues with greater bone anabolic activity
than 1,25-(OH)
2
D
3
are currently under development
and may make it possible to safely treat osteoporosis in
the future.
An analogue of 1,25-(OH)
2
D
3
called Dovonex is
currently marketed for the topical treatment of the
hyperproliferative skin disorder, psoriasis. Improved
analogues will likely appear for treatment of psoriasis.
There are potential new uses for the vitamin D
analogues in the autoimmune diseases as described
above or in the treatment of malignant cancer growth.
The vitamin D hormone has been found to suppress
growth of malignant cells in vitro and to cause their
differentiation into functional cells. Thus, the use of
vitamin D compounds as a potential treatment of
metastatic neoplastic disease has been exciting but it
has not yet yielded a therapeutic agent. The major
problem with the use of 1,25-(OH)
2
D
3
and many of its
analogues for treatment of disease is that its primary
purpose is to raise blood calcium and phosphorus
which means that is a major side effect that must be
dealt with before it can be used to treat noncalcemic
diseases. One approach to this is the synthesis of
analogues that retain the ability to suppress cancerous
growth but do not raise blood calcium. New analogues
with these desirable characteristics are currently
under basic study, but are not yet available for
pharmaceutical use.
SEE ALSO THE FOLLOWING ARTICLES
Calcium Buffering Proteins: Calbindin † Cholesterol
Synthesis † Cytochrome P-450 † Lipid Rafts † Para-
thyroid Hormone/Parathyroid Hormone-Related Pro-
tein Receptor † Vitamin D Receptor
GLOSSARY
coactivator Protein(s) that enhance receptor activity in transcription.
convulsive tetany A disease precipitated by either low blood
magnesium or low blood calcium resulting in a severe convulsive
state. It is lethal unless immediately treated with calcium and/or
magnesium.
corepressor A protein that silences transcription factors.
7-dehydrocholesterol The sterol precursor of vitamin D
3
.
376 VITAMIN D
