Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

Organometallic B
12
-derivatives are rather resistant
against proteolytic cleavage of the (Co–C)-bond under
physiological conditions, crucial for the cofactor role of
the B
12
-coenzymes, but they are very sensitive to visible
light, which induces the homolysis of the (Co– C)-bond.
B
12
: Biosynthesis and
Total Synthesis
The natural corrinoids are made exclusively by microor-
ganisms, which are used for the industrial production of
vitamin B
12
(world output , 10 tons per year). A “last”
common intermediate in the biosynthesis of the corrins
and of other porphyrinoids is the tetra-pyrrole uropor-
phyrinogen III. The ‘corrin pathway’ separates off with
the sequential incorporation of ‘extra’ methyl groups into
the reduced ligand. A major difference of the two main
biosynthetic paths in aerobic and in anaerobic micro-
organisms concerns the timing of the cobalt insertion on
the way to cobalt-containing corrins. The nucleotide
portion of the “complete” corrinoids is then built-up in
the later phases of the B
12
-biosynthesis, beginning at the
stage of cobalt-adenosylated cobyric acid.
The total synthesis of vitamin B
12
was achieved in the
1970s in the laboratories of Eschenmoser and Wood-
ward. This conquest of the B
12
-structure by abiotic
synthesis led to the “incomplete” corrinoid cobyric acid
first, to which the nucleotide part was attached in a
specific way. The Co(II)-ion was incorporated easily into
the corrin ligand, but attempts to remove cobalt from
intact natural corrinoids have not been successful.
B
12
-Dependent Enzymatic
Reactions
B
12
-DEPENDENT METHYL
TRANSFERASES
The reactivity of the “supernucleophilic” Co(I)-corrins
and of methyl-Co(III)-corrins makes B
12
-derivatives
well-suited as cofactors in B
12
-dependent enzymatic
methyl group transfer reactions, which are widely
relevant in many organisms. Anaerobic acetogenesis,
methanogenesis and catabolism of acetic acid to
methane and carbon dioxide make use of B
12
-catalyzed
enzymatic methyl transfer reactions. Various substrates
act as sources of methyl groups, such as methanol,
aromatic methyl ethers, methyl amines or N
5
-methyl-
tetrahydropterins (such as N
5
-methyltetrahydrofolate).
Thiols are the methyl group acceptors in methanogenesis
and in methionine synthesis. In the anaerobic biosyn-
thesis of acetyl-coenzyme A from one-carbon precursors
the methyl group acceptor is suggested to be the nickel
center of an Fe/Ni-cluster.
B
12
-dependent methionine synthesis is one of the
two essential roles of B
12
in mammalian metabolism.
In methionine synthase of Escherichia coli (MetH)
B
12
-dependent methyl transfer involves a sequential
mechanism, in which tetrahydrofolate and methionine
are formed and homocysteine and N
5
-methyltetra-
hydrofolate act as methyl group acceptors and donors,
respectively (see Figure 3). MetH is a modular
protein, where the B
12
-binding domain is bound to
an N
5
-methyltetrahydrofolate-binding module, the
homo-cysteine-binding module and a reactivating
module (that binds S-adenosyl-methionine). During
turnover MetH catalyzes two methyl group transfer
reactions which occur with an overall retention of
configuration (consistent with two inversions). In a
formal sense, these methyl-transfer reactions are SN
2
reactions with heterolytic cleavage/formation of the
(Co–CH
3
)-bond.
X-ray crystal analysis of the B
12
-binding domain of
MetH provided the first insight into the structure of a
B
12
-binding protein. This work revealed the cobalt-
coordinating DMB-nucleotide tail of the bound cofactor
MeCbl to be displaced by a histidine and to be anchored
in a pocket of the protein. Accordingly, in MetH the
corrinoid cofactor is bound in a “base-off/His-on”
constitution. The crucial cobalt-ligating histidine residue
is part of a Gly-X-X-His-X-Asp-sequence, which is
conserved in a group of B
12
-binding proteins.
COENZYME B
12
-DEPENDENT ENZYMES
About ten coenzyme B
12
-dependent enzymes are now
known. These enzymes are four carbon skeleton
mutases, diol dehydratase, glycerol dehydratase,
ethanolamine ammonia lyase, two amino mutases, and
B
12
-dependent ribonucleotide reductase. Of these
AdoCbl-dependent enzymes, only methylmalonyl-CoA
mutase is indispensable in human metabolism.
The coenzyme B
12
-dependent enzymes perform
transformations, that are difficult to achieve by typical
“organic” reactions. With the exception of the enzy-
matic ribonucleotide reduction, the results of the
coenzyme B
12
-catalyzed enzymatic reactions formally
correspond to isomerizations with vicinal exchange of a
hydrogen atom and of a group with heavy atom centers.
Homolytic cleavage of the (Co–C)-bond of the protein-
bound AdoCbl to a 5
0
-deoxy-5
0
-adenosyl radical and
cob(II)-alamin (B
12r
) is the entry to H-abstraction
reactions induced by the 5
0
-deoxy-5
0
-adenosyl radical.
The AdoCbl-dependent enzymes rely upon the reactivity
of bound organic radicals, which are formed (directly or
indirectly) by a H-atom abstraction by the 5
0
-deoxy-5
0
-
adenosyl radical. The substrate radicals rearrange rapidly
to the product radicals in a (pseudo-)intramolecular
rearrangement and without noticeable participation
of the bound B
12r
(i.e., the corrinoid is a “spectator”).
VITAMIN B
12
AND B
12
-PROTEINS 363
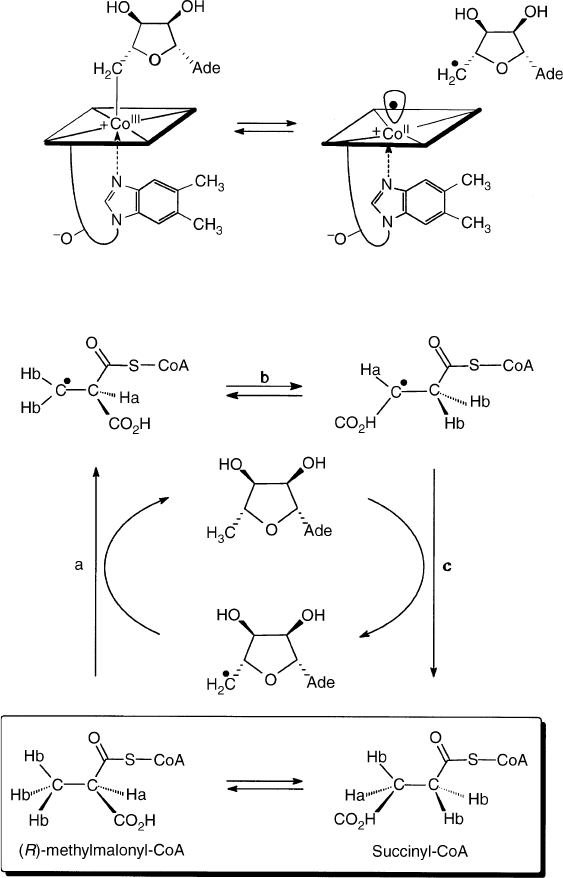
The major tasks of the enzymes thus concern the
enhancement of the critical radical reactions, the
reversible generation of the radical intermediates and
the protection of the proteins from nonspecific radical
chemistry.
Coenzyme B
12
-Dependent Carbon
Skeleton Mutases
Methylmalonyl-CoA mutase interconverts R-methyl-
malonyl-CoA and succinyl-CoA. Binding of the substrate
triggers the homolysis of the (Co–C)-bond of the bound
AdoCbl. The radical carbon skeleton rearrangement
reaction then proceeds, as outlined in Figure 4.
In all coenzyme B
12
-dependent carbon skeleton
mutases (methylmalonyl-CoA mutase (MMCM),
glutamate mutase (GM), methyleneglutarate mutase,
isobutyryl-CoA mutase) the B
12
-binding motif (Gly-X-
X-His-X-Asp) occurs. Consistent with this, the B
12
-
cofactor is bound “base-off/His-on” at the interface
between the B
12
-binding and the substrate-activating
domains. The B
12
-binding domain in MMCM, the
B
12
-binding subunit in GM and even the B
12
-binding
domain of MetH exhibit high sequence homology. Such a
homology does not extend to the substrate-binding
domains of these enzymes. The crystal structures of
MMCM (from Propionibacterium shermanii) and GM
(from Clostridium cochlearium) are available. The X-ray
FIGURE 4 Top: coenzyme B
12
(AdoCbl) is a reversible source of the 5
0
-deoxy-5
0
-adenosyl radical and cob(II)alamin (B
12r
). Bottom:
methylmalonyl-CoA mutase interconverts (R)-methylmalonyl-CoA and succinyl-CoA. This rearrangement is proposed to involve H-atom
abstraction (step a), radical rearrangement (step b) and back transfer of H-atom (step c). (Formation of the 5
0
-deoxy-5
0
-adenosyl radical from
protein bound AdoCbl is substrate triggered.)
364 VITAMIN B
12
AND B
12
-PROTEINS
analysis of MMCM provided the first crystal structure of
a coenzyme B
12
-dependent enzyme. Detailed crystallo-
graphic analysis of GM with bound AdoCbl revealed a
likely structural basis for the operation of the radical
mechanism in AdoCbl-dependent mutases: the ribose
part of the 5
0
-deoxyadenosyl moiety is present in
two conformations, related to each other by a pseudo-
rotation of the furanose ring. The solution structure
of the B
12
-binding subunit of GM from Clostridium
tetanomorphum (analyzed by heteronuclear NMR)
provided a first structure of a cofactor-free B
12
-binding
protein and indicated it to be largely preorganized
for B
12
-binding.
In contrast to MMCM and GM, the crystal structure
of the AdoCbl-dependent diol dehydratase from
Klebsiella oxytoca showed the corrinoid to be bound
“base-on” at the interface of the B
12
-binding and
of the substrate-binding subunits.
B
12
-Dependent Ribonucleotide Reductase
In all organisms, ribonucleotide reductases (RNRs) play
essential roles in the biosynthesis of DNA by catalyzing
the reduction of nucleoside di- or triphosphates to the
corresponding 2
0
-deoxynucleotides. The RNRs use
various metal cofactors to initiate the nucleotide
reduction by a radical reaction. The reductase from
Lactobacillus leichmanii (RNR-Ll) uses AdoCbl as
cofactor and nucleoside triphosphates as substrates,
while 2
0
-deoxynucleoside triphosphates are allosteric
effectors. In RNR-Ll the (Gly-X-X-His-X-Asp)-“B
12
-
binding motif” is absent and ESR-spectroscopy and
crystallography showed “base-on” binding of the B
12
-
cofactor. In RNR-Ll homolysis of the bound AdoCbl
generates a protein-centered thiyl-radical, which then
induces the radical reactions that lead to the reductive
removal of the 2
0
-OH group of the ribonucleotide.
Other B
12
-Dependent
Enzymatic Transformations
Methanogens and acetogens dechlorinate chloro-
methanes with reduced metal cofactors (corrinoids, in
particular). Environmentally relevant microbiological
dehalogenation reactions of chloroethenes were found
recently. The anaerobe Dehalospirillum multivorans
uses tetrachloroethene as the terminal electron acceptor,
which is dechlorinated by a B
12
-dependent tetrachloro-
ethene reductive dehalogenase to trichloroethene and
(then) cis-trichloroethene.
B
12
: Medical Aspects
B
12
-deficiency was found in the last century to be the
cause for “pernicious anemia.” Recently B
12
-deficiency
was recognized as a rather common condition
with older persons. In most cases B
12
-deficiency results
from impaired uptake from the ingested food. A dose
of about 3–4 mgperdayofB
12
are considered
necessary for sustained physical well-being. In the
human body B
12
is metabolically active as MeCbl
and as AdoCbl.
Three soluble B
12
-binding proteins are known to be
involved in the uptake and transport of cobalamins in
humans: intrinsic factor (IF), transcobalamin (TC) and
haptocorrin (HC). These three genetically related B
12
-
binders (apparent binding constants of . 10
12
l mol
21
)
ensure that the needed amounts of B
12
reach the two
enzymes, methionine synthase (in the cytosol) and
methylmalonyl-CoA mutase (in the mitochondria). IF is
synthesized in the stomach and binds cobalamins in the
small intestines. The IF-cobalamin complex is absorbed
by specific receptors on the brush borders of the
epithelial cells in the small intestines. Intracellular B
12
-
trafficking depends upon a complex interplay between
the B
12
-binders and cellular surface receptors that
recognize complexes between B
12
and the B
12
-binding
proteins IF, TC, and HC.
SEE ALSO THE FOLLOWING ARTICLES
Giant Mitochondria (Megamitochondria) † Inorganic
Biochemistry † Porphyrin Metabolism † Propionyl
CoA–Succinyl CoA Pathway
GLOSSARY
heteronuclear NMR Nuclear magnetic resonance (NMR) experi-
ments that correlate nuclei of different elements (e.g.,
1
H and
13
C)
to obtain information on their mutual positions in a molecule (or a
supramolecular complex).
organic radical Highly reactive organic molecules with unpaired
electrons.
pernicious anemia Microscopic abnormalities of blood and bone
marrow correlating with B
12
-deficiency.
FURTHER READING
Banerjee, R. (1999). Chemistry and Biochemistry of B
12
. Wiley,
New York.
Buckel, W., and Golding, B. T. (1996). Glutamate and 2-methylene-
glutarate mutase: From microbial curiosities to paradigms for
coenzyme B
12
-dependent enzymes. Chem. Soc. Rev. (London) 25,
329–338.
Eschenmoser, A. (1988). Vitamin B
12
: Experiments concerning the
origin of its molecular structure. Angew. Chem. Int. Ed. Engl. 27,
5–40.
Kratky, C., and Gruber, K. (2002). Coenzyme B
12
dependent glutamate
mutase. Curr. Opin. Chem. Biol. 6, 598–603.
Kra
¨
utler, B., and Ostermann, S. (2003). Structure, reactions and
functions of B
12
and B
12
-proteins. In The Porphyrin Handbook,
Vol 11, pp. 227–274.
VITAMIN B
12
AND B
12
-PROTEINS 365
Kra
¨
utler, B., Arigoni, D., and Golding, B. T. (eds.) (1998).
Vitamin B
12
and B
12
-Proteins. Wiley-VCH, Weinheim,
Germany.
Matthews, R. G. (2001). Cobalamin-dependent methyltransferases.
Acc. Chem. Res. 34, 681–689.
Stubbe, J. (2000). Ribonucleotide reductases: The link between an
RNA and a DNA World? Curr. Opin. Struct. Biol. 10,
731–736.
Toraya, T. (2003). Radical catalysis in coenzyme B
12
-
dependent isomerization (eliminating) reactions. Chem. Rev. 103,
2095–2127.
BIOGRAPHY
Bernhard Kra
¨
utler studied chemistry at the ETH (Zu
¨
rich), where he did
a dissertation with Prof. Albert Eschenmoser. He had postdoctoral
association with Prof. Allen Bard (Austin, Texas) and Prof. Nick Turro
(Columbia University). After return to the ETH he obtained his
habilitation in organic chemistry (1985). Since 1991 he is a Professor of
Organic Chemistry at the University of Innsbruck. His main research
interests include bioorganic chemistry, porphyrinoid compounds,
coenzyme B
12
, chlorophyll, fullerenes, structure/function of bioma-
cromolecules, and supramolecular chemistry.
366 VITAMIN B
12
AND B
12
-PROTEINS

Vitamin C
Robert B. Rucker and Francene Steinberg
University of California, Davis, California, USA
One of the most important redox cofactors in plant and animal
systems is ascorbic acid or vitamin C. Although most animals
make sufficient ascorbic acid, for some animals, ascorbic acid
is a true vitamin because of their inability to carry out
synthesis. For example, in humans, ascorbic acid deficiency can
resulst in the nutritional disease scurvy, which causes a range of
pathologic symptoms, because of defects in ascorbic-acid-
specific enzymatic steps and processes.
In most plants and animals, ascorbic acid is derived as a
product from the direct oxidation of glucose, galactose, and
mannose (in plants), e.g., glucose or galactose ! UDP-D-
glucuronic acid ! glucuronic acid/glucuronolactone !
gulono-1, 4-lactone ! ascorbic acid. In animals, a key enzyme
in this process is
L-gulonolactone oxidase (GLO, EC 1.1.3.8),
which catalyzes the terminal step in ascorbic acid synthesis,
i.e., gulono-1, 4-lactone is oxidized to ascorbic acid.
L-gulonolactone oxidase resides in the kidney of most birds
and reptiles, and during the course of evolution was transferred
to the liver of mammals. For reasons that are not clear, the
ability to express sufficient
L-gulonolactone oxidase activity
disappeared from the guinea pig, some fruit-eating bats, and
most primates, including humans. Accordingly, a dietary source
of ascorbic acid is needed in these animals. There is also a
possibility that minor alternative pathways exist in plants and
mammals for ascorbic acid synthesis.
Chemistry
The chemical designation for ascorbic acid is 2-oxo-L-
theo-hexono-4-lactone-2, 3-enediol. Ascorbic acid is a
near planar five-member ring with two chiral centers
that resolves into the four stereoisomers. The oxidized
form of ascorbic acid, dehydroascorbic acid, retains
vitamin C activity and can exist as a hydrated hemi-
ketal. Crystalline dehydro-
L-ascorbic acid can exist as a
dimer. The most important chemical property of
ascorbic acid is the reversible oxidation of ascorbic
acid to semidehydro-
L-ascorbic acid and to dehydroas-
corbic acid (Figure 1).
In addition to facilitating reduction–oxidation
reactions, ascorbic acid has the ability to form
relatively stable free radical intermediates. Ascorbic
acid can act as a free radical scavenger in reactions
involving reactive oxidant species (ROS). In this
regard, the rate constants for the generation of
ascorbate radicals vary considerably, but often dictate
rapid radical formation, e.g., 10
5
–10
10
k
obs
z
/M
21
s
21
.
Once formed, ascorbate (Asc) radicals decay relatively
slowly by a process of disproportionation (2 Asc
z2
þ
H
þ
! AscH
2
þ DHA). The ability to form free radical
intermediates can significantly delay or prevent free
radical-initiated oxidations. Ascorbic acid readily
scavenges reactive oxygen and nitrogen species, such
as superoxide, hydroperoxyl, peroxynitrite, and nitr-
oxide radicals. Asc[H
2z
] donates a hydrogen atom
(H
z
or H
þ
þ e
2
) to an oxidizing radical to produce
the resonance-stabilized tricarbonyl ascorbate free
radical. Asc[H
z
]hasapK
a
of 2 0.86; thus, it is not
protonated. Further, the unpaired electron of Asc [2 ]
resides in the
p
-system that includes the tricarbonyl
moiety of ascorbate. It is a weakly oxidizing and
reducing radical. Due to its
p
-character, Asc [2 ] does
not react with oxygen to form peroxyl radicals capable
of damaging oxidations. It is relatively unreactive with
a one-electron reduction potential of only þ 282 MV,
and as such is considered a terminal, small-molecule
antioxidant.
Ascorbic acid is often associated with the protection
of lipid, DNA, and proteins from oxidants. As examples,
when peroxyl radicals are generated in plasma, vitamin
C is consumed faster than other antioxidants, e.g., uric
acid, bilirubins, and vitamin E. Ascorbic acid is 10
3
times more reactive than a polyunsaturated fatty acid
in reacting with peroxyl radicals. In contrast, ascorbic
acid can be viewed as a pro-oxidant under aerobic
condition when metals capable of redox (Fe
þ2
$ Fe
þ3
;
Cu
þ1
$ Cu
þ2
) are also present. Metals, such as iron
and copper in their reduced states, are effective Fenton
catalysts.
With regard to other chemical properties, the acidity
of ascorbic acid is due to the low pK
a
of the proton on
oxygen-3. In addition, ascorbic acid is not very stable in
aqueous media, wherein it can decay within a few hours
or even minutes at high pH (. 10.0). In contrast,
ascorbic acid is relatively stable in blood (a day or
more), or if stored at acid pH (, 3.0) or below 2 208C
(often weeks to months).
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 367
Nutritional and Biochemical
Importance
Ascorbic acid functions in many mono- and dioxy-
genases to maintain metals in a reduced state. For
example, mono- and dioxygenases usually contain
copper or iron as redox cofactors, respectively. As an
additional characteristic, dioxygenases require
a
-keto-
glutarate and O
2
as cosubstrates in reactions, while
mono-oxygenases require only O
2
. Important reactions
and processes that require ascorbic acid include:
† Norepinephrine synthesis – dopamine-b-
hydroxylase, EC 1.14.17.1, is the final and rate-limiting
step in the synthesis of norepinephrine and requires
copper and ascorbic acid for activity.
† Hormone activation – posttranslational steps
involving
a
-amidations activate many hormones and
hormone-releasing factors. Examples of hormone acti-
vation include melanotropins, calcitonin, releasing
factors for growth hormone, corticotrophin and thyro-
tropin, pro-ACTH, vasopressin, oxytocin, cholecysto-
kinin, and gastrin. Petidylglycine
a
-amidating mono-
oxygenase (EC 1.14.17.3), the enzyme that carries out
a
-amidation is found in secretory granules of neuro-
endocrine cells.
† Carnitine Biosynthesis – ascorbate is a cofactor for
two of the hydroxylation steps in the pathway of
carnitine biosynthesis, gamma-butyrobetaine hydroxy-
lase and epsilon-N-trimethyllysine hydroxylase. Ascor-
bate deficiency results in as much as 50% decrease in
carnitine in heart and skeletal muscle in animals that
require ascorbic acid.
† Prolyl and lysyl hydroxylations – ascorbic acid is
an important cofactor in the hydroxylation of proline
and lysine amino acid residues. In scurvy, poor wound
healing, bruising, and osteopenic abnormalities can
result from the under-hydroxylation of collagens. The
various types of collagens are distinguished by their
relative amounts of hydroxyproline and hydroxylysine.
Hydroxylation of proline and lysine is important in
collagen assembly and maturation into fibers. Because
collagen is the principal connective tissue protein in the
body, it is essential to all phases of normal growth,
development, and repair. There are also other proteins
that are not defined as collagens, which contain
hydroxyproline, e.g., the subcomponent C1q of comp-
lement, acetylcholine esterase, pulmonary surfactant
proteins, and proteins that function as cell surface
receptors. In plants, prolyl hydroxylase hydroxylates
proline residues in cell wall hydroxyproline-rich glyco-
proteins required for cell division and expansion.
Approximately 50 hydroxyproline-containing proteins
have been identified in viruses and bacteria, wherein
ascorbic acid may also play a role as cofactor.
Along with glutathione, ascorbic acid is also an
important nonenzymatic antioxidant/reductant in
the water-soluble compartment of cells. Glutathione is
L-(-glutamyl-L-cysteine-glycine (GSH). The antioxidant
actions of glutathione and ascorbate are closely linked
and involve mechanisms in which decreased glutathione
may even stimulate ascorbic acid synthesis in those
animals that can produce it. During postnatal develop-
ment, a rapid change occurs as animals adapt, from a
relatively hypoxic to a relatively hyperoxic environment.
In this regard, one of glutathione’s many functions is to
keep ascorbic acid in a reduced form. In adult animals
that can make ascorbic acid, a reduction in glutathione
levels can lead to a rapid increase in liver dehydro-
ascorbic acid. An adequate ascorbic acid intake is
HO
HO
OH
OH
O
O
HO
O
.
OH
O
–
O
O
OH
HO
O
O
O
O
AscH
2
Asc[
.
-]
DHAsc
Chemical forms of ascorbic acid
and
major metabolites
and
degradation products
2,3-diketo-
L-gulonic acid
Oxalic acid
CO
2
CO
2
+
L-xylose + CO
2
L
-threonic acid
L-xylonic acid + L-lyxonic acid
+e
–
+e
–
+2H
+
–e
–
–2H
+
–e
–
FIGURE 1 The major redox forms of ascorbic acid are shown in addition to products that result from both the chemical and enzymatic
degradation of ascorbic acid. In alkaline solutions ascorbic acid is easily modified to 1 carbon (CO
2
) and various 5-carbon compounds (e.g., xylonic
acid, lyxonic acid) or the 2-carbon compound, oxalic acid and the 4-carbon compound, L-threonic acid. In addition, L-xylose and CO
2
may be
formed in vivo following decarboxylation at the C-1 position of ascorbic acid followed by reduction.
368 VITAMIN C
particularly important in newborns and neonates, who
do not yet have the potential to synthesize ascorbate. A
deficiency of both ascorbic acid and glutathione can
cause severe damage to liver and other organs.
With regard to plants, ascorbic acid reaches a
concentration of over 20 mM in chloroplasts and
occurs in all cell compartments, including the cell
wall. It has a major role in photosynthesis, acting in the
Mehler peroxidase reaction with ascorbate peroxidase
to regulate the redox state of photosynthetic electron
carriers and as a cofactor for violaxanthin de-epox-
idase, an enzyme involved in xanthophyll cycle-
mediated photoprotection. Ascorbic acid also serves
as a cofactor in metabolic pathways important to
ethylene, gibberellins, and anthocyanins. There is a
rapid response of ascorbate peroxidase expression to
(photo)-oxidative stress. Of interest, the ascorbate-
deficient vtc 1 mutant of Arabidopsis thaliana, is very
sensitive to ozone, ultraviolet-B radiation, and other
forms of oxidant stress.
Cellular Regulation
of Ascorbic Acid
Specific non-overlapping transport proteins mediate the
transport of ascorbic acid across biological mem-
branes. Dehydroascorbic acid uptake is via the
facilitated-diffusion glucose transporters, (GLUT 1, 3,
and 4), but under physiological conditions these
transporters do not appear to play a major role in
the uptake of dehydroascorbic acid due to the high
concentrations of glucose that effectively block influx.
L-ascorbic acid enters cells via Na
þ
-dependent systems.
Two isoforms of these transporters have recently been
cloned from human and rat sources, which differ in
their tissue distribution. Cell accumulation of ascorbic
acid occurs, because of cellular dehydroascorbate
reduction systems that are capable of rapidly generat-
ing ascorbic acid.
In addition to cell regulation, ascorbate reduction
systems are also important in reducing the ascorbic acid
radical. As indicated, the ascorbic acid radical is
relatively stable. An accessory enzymatic system is
needed to reduce the potential of transient accumulation
of the ascorbate radical. Excess ascorbate radicals may
initiate free radical cascade reaction or nonspecific
oxidations. In plants, NADH-monodehydroascorbate
reductase (EC 1.6.5.4) has evolved to maintain ascorbic
acid in its reduced form. NADH-monodehydroascor-
bate reductase plays a major role in stress-related
responses in plants. In animal tissues, glutathione
dehydroascorbate reductase (EC 1.8.5.1) serves this
purpose.
Digestion, Absorption, and
Nutritional Requirements
When ascorbic acid is consumed in the diet, the ileum
and jejunum are major sites of ascorbic acid absorption.
The bioavailability of vitamin C is dose dependent. In
humans, saturation of transport occurs with dosages of
200–400 mg per day. Approximately 70% of a 500 mg
dose is absorbed. However, much of the absorbed dose
(. 50%) is nonmetabolized and excreted in the urine.
With a dose of 1250 mg, only 50% of the dose absorbed
and most (. 85%) of the absorbed dose is excreted.
Vitamin C is not protein-bound and is eliminated with
an elimination half-life of 10–12 hours. In Western
populations plasma, vitamin C concentrations range
from 54 to 91 mmol l
21
. Tissue concentrations range
from mM to mM, i.e., high tissue levels of vitamin C are
well tolerated in mammalian systems and lead to the
conclusion that ascorbic acid is relatively nontoxic,
although there is also some evidence that accelerated
metabolism or disposal of ascorbic acid may occur after
prolonged supplementation of high doses.
Sources and Detection
A number of approaches are available for ascorbic acid
detection. Ascorbic acid absorbs strongly in UV light,
the basis for some spectrophotometric assays. The redox
properties of ascorbic acid allow for electrochemical
detection or detection from interacting with redox-
sensitive chromophores and dyes. Enzymatic methods
(using ascorbate oxidase) and conventional and isotope
ratio mass spectrometry are used for detection and
quantitation of ascorbic acid following separation by
means of ion exchange, absorption or partition chro-
matographic approaches. Typical food and tissue values
are given in Table I.
Defining Ascorbic Acid Status
The requirements for vitamin C in humans (given as the
range for female–male and expressed as the rec-
ommended dietary allowance, RDA or adequate uptake,
AI) are as follows: infants, 40 mg per day (AI based on
what is present in human milk); children (3–13 years),
15–45 mg per day (extrapolated from the RDA for
adults); adolescence, 45–65 mg per day; adults (. 19
years), 75–90 mg per day; lactating mothers, 115–
120 mg per day. In keeping with these estimates, more
recent clinical investigations have noted vitamin C
deficiency in 2–6% of individuals surveyed (often
defined as individuals meeting less than two-thirds of
the RDA). With regard to marginal vitamin C status
VITAMIN C 369

based on plasma ascorbic acid values, e.g., , 20 mg/dL,
the prevalence ranges from 17 to 24% US. In this regard,
smokers are more likely to have marginal vitamin C
status compared to nonsmoking adults. Several studies
suggest that smokers require over 200 mg vitamin C
daily to maintain plasma vitamin C concentrations at a
level equivalent to that of nonsmokers.
Summary
Ascorbic acid usually carries out redox reactions by
mechanisms dependent upon free-radical processes.
Ascorbate metabolism is linked to the metabolism of
glutathione. Ascorbic acid is also required in animals
that lack or have mutations in the gene for
L-
gulonolactone oxidase. Ascorbic deficiency results in
reduced mono- and dioxygenase activities. The con-
sequences of severe deficiency are profound, since
growth, extracellular matrix, and hormonal regulation
are impaired. Recent data suggest optimal intakes of
ascorbic acid, based on a range of criteria, should be as
high as 75–90 mg per day for adult humans.
SEE ALSO THE FOLLOWING ARTICLES
Carnitine and
b
-Oxidation † Peptide Amidation
GLOSSARY
adequate intake (AI) One of the four terms used to define dietary
reference intakes (DRIs), which are reference values that are
quantitative estimates of nutrient intakes to be used for planning
and assessing diets for healthy people. The AI is used when the
RDA for a nutrient is not available. It is an RDI that is based on
observed or experimentally determined approximations of nutrient
intake by a group of healthy people.
amidation A complex reaction, which in some hormones results in
a C-terminal protein modification. A C-terminus consensus
sequence is required. The consensus is glycine, followed by two
basic amino acids (Arg or Lys). The end product is an amide
of glycine ( –NH– CH
2
–CO–NH
2
) with loss of the basic amino
acid residues.
anthocyanins Naturally occurring compounds that impart color to
fruit, vegetables, and plants. They also have antioxidant and
insecticidal properties.
carnitine Essential component of fatty acid transport into and out of
mitochondria for their eventual oxidation. Carnitine is derived
from the amino acid lysine following extensive modification.
complement A term originally used to refer to the heat labile factor in
serum that causes immune cytolysis, the lysis of antibody-coated
cells. The term now refers to the entire functionally related system
comprising at least 20 distinct serum proteins that is the effector
immune cytolysis and other biologic functions immune system
related functions.
dioxygenase An oxidoreductase that incorporates two atoms of
oxygen (from one molecule of O
2
) into the (reduced) substrate.
Fenton reaction The formation of OH
z
,OH2 , and Fe
3þ
from the
nonenzymatic reaction of Fe
2þ
with H
2
O
2
.
TABLE I
Vitamin C in Selected Foods and Tissues
Sources of vitamin C
Mg of ascorbic acid per
100 g of wet weight or
edible portion
Animal products
Cow’s milk 0.5–2
Human milk 3–6
Beef, pork, veal 2–10
Liver, chicken 15–20
Kidney, chicken 6–8
Heart, chicken 5
Crab muscle, lobster 1–4
Shrimp 2–4
Fruits
Apple 3–30
Banana 8–16
Blackberry 8–10
Cherry 15–30
Currant, red 20–50
Currant, black 150–200
Grapefruit 30–70
Kiwi fruit 80–90
Lemon, orange 40–50
Melon 9–60
Strawberry 59–70
Pineapple 15–25
Rose hips 250–800
Vegetables
Beans, various 10–15
Brussels sprouts 100–120
Cabbage 30–70
Carrot 5–10
Cucumber 6–8
Cauliflower 50–70
Eggplant 15–20
Chive 40–50
Kale 70–100
Onion 10–15
Peas 8–12
Potato 4– 30
Pumpkin 15
Radishes 25
Spinach 35–40
Condiments
Chicory 30–40
Coriander (spice) 90
Garlic 15–25
Horseradish 50
Lettuce, various 10–30
Leek 15
Parsley 200–300
Pepper, various 150–200
370 VITAMIN C
gibberellins Plant hormones that stimulate growth in the stem and
leaves, and trigger the germination of the seed
Mehler reaction Describes the photoreduction of oxygen in chloro-
plasts by photosystem I in plants yielding O
2
.2
.
monooxygenase Oxidoreductases that induce the incorporation of
one atom of oxygen from O
2
into the substance being oxidized.
recommended dietary allowance (RDA) The average daily dietary
intake level that is sufficient to meet the nutrient requirement of
nearly all (97–98%) healthy individuals in a group.
redox oxidation–reduction reactionary reaction in which electrons
are removed from one molecule or atom and given to another
molecule or atom.
transporter A class of transmembrane protein that allows substances
to cross plasma membranes far faster than would be possible by
diffusion alone. A major class of transport proteins that expend
energy to move substances (called active transport) are the
transport ATPases.
violaxanthin Photopigment involved in photoprotection in plants.
When light energy absorbed by plants becomes excessive (relative
to the capacity of photosynthesis), the xanthophyll (a chemical
classification designation), violaxanthin is reversibly modified by
violaxanthin de-epoxidase as a protective function in plants.
FURTHER READING
Frei, B. (ed.) (1994). Natural Antioxidants in Human Health and
Disease. Academic Press, San Diego.
National Academy Press (2000). Dietary Reference Intakes for
Vitamin C, Vitamin E, Selenium, and Carotenoids. A report of
the Panel on Dietary Antioxidants and Related Compounds,
Subcommittees on Upper Reference Levels of Nutrients and
Interpretation and Uses of Dietary Reference Intakes, and the
Standing Committee on the Scientific Evaluation of Dietary
Reference Intakes, Food and Nutrition Board, Institute of
Medicine, National Academy Press, Washington, DC.
Packer, L., and Fuchs, J. (eds.) (1997). Vitamin C in Health and
Disease. Marcel Dekker, New York.
Simopoulos, A. P (Vol. Ed.) (1991). Selected vitamins, minerals, and
functional consequences of maternal malnutrition. In The Series
World Review of Nutrition and Dietetics,Vol. 64, Karger, Basel,
New York.
BIOGRAPHY
Robert Rucker is a Professor of Nutrition and Internal Medicine at
the University of California, Davis. Dr. Rucker’s research interests
are focused on extracellular matrix assembly, the role of micro-
nutrients in early growth and development, and the physiological
roles of quinone cofactors derived from tyrosine, such as pyrrolo-
quinoline quinone. He holds a Ph.D. from Purdue University. Honors
and appointments include serving as a past president of the
American Society for the Nutritional Sciences, serving as an
associate editor for the American Journal of Clinical Nutrition,
receiving the Borden Research Award from the American Society for
Nutritional Sciences, and recognition as a Fellow of the AAAS.
Francene Steinberg is an Associate Professor in the Department of
Nutrition at UC Davis and Director of the Didactic Program in
Dietetics. She received the Ph.D. in nutrition at UC Davis, received
additional postdoctoral training in the Department of Endocrinology
at the University of Washington. She is also a registered Dietitian. Dr.
Steinberg’s research area concerns the metabolism of lipids and
lipoproteins, with an emphasis on the effects of nutrition on
cardiovascular disease risk and the development of atherosclerosis.
Areas of particular interest include dietary soy phytoestrogens,
structured lipids, and antioxidant vitamins.
VITAMIN C 371

Vitamin D Receptor
Diane R. Dowd and Paul N. MacDonald
Case Western Reserve University, Cleveland, Ohio, USA
The vitamin D receptor is a member of the nuclear
receptor/steroid hormone receptor superfamily. These recep-
tors function as ligand-activated, transcriptional regulatory
proteins. Thus, the vitamin D receptor selectively binds the
1,25-dihydroxyvitamin D
3
[1,25(OH)
2
D
3
] hormone and con-
trols the expression of selected genes in target cells. The main
actions of 1,25(OH)
2
D
3
, VDR, and the vitamin D endocrine
system are to maintain overall calcium and mineral homeo-
stasis. A fundamental physiological role is to maintain
adequate absorption of dietary calcium at the level of the
small intestine. Dietary deficiency of vitamin D or mutations in
the vitamin D receptor result in human conditions of
hypocalcemia and secondary skeletal undermineralization.
The molecular details involved in vitamin D-regulated gene
expression by the VDR in key mineral regulating tissues such
as the intestine, kidney, and bone are revealing important
insights into the manner in which our bodies maintain the
structural integrity of skeletal tissue.
Introduction
Vitamin D
3
, or cholecalciferol, was discovered nearly a
century ago as a micronutrient that is essential for
normal skeletal development and for maintaining bone
integrity. 1,25(OH)
2
D
3
is the bioactive, hormonal form
of vitamin D. However, vitamin D
3
is more appro-
priately classified as a hormone since it is produced in
the body in response to serum calcium levels and it
functions through a mechanism that is analogous to
other steroid hormones. It is generated by two sequential
hydroxylations of vitamin D
3
in response to hypocalce-
mia and elevated parathyroid hormone. The predomi-
nant role of 1,25(OH)
2
D
3
is to enhance the intestinal
absorption of dietary calcium and phosphorus.
1,25(OH)
2
D
3
also acts on mineral-regulating target
tissues such as intestine, bone, kidney, and parathyroid
glands to maintain normal calcium and mineral homeo-
stasis. 1,25(OH)
2
D
3
also directly affects skeletal bone
remodeling by causing osteoblasts to terminally differ-
entiate into osteocytes and deposit calcified matrix.
1,25(OH)
2
D
3
also promotes the differentiation of pre-
cursor cells into mature osteoclasts which function to
resorb bone and maintain appropriate bone remodeling.
Thus, it is through an integrated series of diverse effects
that vitamin D is thought to preserve and maintain the
integrity of the bony tissues. To highlight this role,
vitamin D
3
deficiency leads to rickets in children and
osteomalacia in adults. The vitamin D endocrine system
is also involved in a number of other important
physiological processes including blood pressure regu-
lation, immune function, mammary gland development,
and hair follicle cycling.
The biological effects of 1,25(OH)
2
D
3
are mediated
through a soluble receptor protein termed the vitamin D
receptor (VDR), a member of the steroid receptor or
nuclear receptor (NR) superfamily. The VDR binds
1,25(OH)
2
D
3
with high affinity and high selectivity. In
the target cell, the interaction of the 1,25(OH)
2
D
3
hormone with VDR initiates a complex cascade of
molecular events culminating in alterations in the rate of
transcription of specific genes or gene networks
(Figure 1). Central to this mechanism, is the requisite
interaction of VDR with retinoid X receptor (RXR) to
form a heterodimeric complex that binds to specific
DNA sequence elements (VDREs) in vitamin D-respon-
sive target genes and ultimately influences the rate of
RNA polymerase II-mediated transcription. An emer-
ging concept in this mechanism is that protein–protein
interactions between the VDR–RXR heterodimer,
transcriptional comodulatory proteins and the transcrip-
tion machinery are essential for the mechanism of
vitamin D-mediated gene expression. This article
discusses the molecular biology of the VDR with a
focus on the macromolecular interactions that are
required for the transcriptional regulatory activity of
the VDR.
Functional Domains of the VDR
THE N-TERMINAL DNA-BINDING
DOMAIN (DBD)
Receptors in the nuclear receptor superfamily generally
have two transcriptional activation domains, termed
activation function (AF) 1 and 2, which are required
for the receptor to function as a ligand-activated
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 378
