Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.


Vasopressin/Oxytocin
Receptor Family
Michael J. Brownstein
National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland, USA
Large neurons at the base of the brain (the hypothalamus) of
humans and other mammals send their axons through the
median eminence to terminate in the posterior lobe (neurohy-
pophysis) of the pituitary. Each of these “magnocellular” nerve
cells typically synthesizes one of two neurohypophyseal
hormones – vasopressin (VP) or oxytocin (OT). In fact, all
vertebrates higher than jawless fish make vasopressin- and
oxytocin-like hormones. Jawless fish and invertebrates, on the
other hand, appear to produce only one VP/OT-like peptide
per species.
The Structures of “Pressins”
and “Tocins”
For the last billion years, the peptides in VP/OT family
have retained certain structural features. With the
exception of the hydrins – partially processed relatives
of vasotocin, which are physiologically active in some
amphibia – they all have nine amino acids and a
C-terminal amide. Cysteine (C) is invariably present in
positions 1 and 6, and the two cysteine residues form a
cystine bridge, creating conformationally constrained,
cyclic peptides. Except for seritocin (serine5, isoleu-
cine8-oxytocin), which was reported in a single species,
Bufo regularis, all family members have asparagines (N)
in position 5, and proline (P) and glycine (G) in positions
7 and 9, respectively. VP-like peptides (“pressins”) are
characterized by the presence of arginine (R) or lysine
(K) in position 8, while OT-like molecules (“tocins”)
have leucine (L), isoleucine (I), glutamine (Q), valine
(V), or threonine (T) there. All of the invertebrate
peptides except for the locust diuretic hormone, which
has not been detected in other insect species, have an R
instead of Q, N, or S in position 4. Thus, the primordial
peptide that gave rise to the molecules we know today
should have looked rather similar to one of the
conopressins or to vasotocin, the likely ancestor of all
of the vertebrate hormones. (The molecules detected
in Hydra with antivasopressin antibodies share the
C-terminal PRG-amide motif with VP, but are
otherwise unrelated.)
In addition to the peptides shown in Table I and
Figure 1, it is worth noting that the major metabolite of
VP (pGlu4, Cystine6-AVP) mobilizes calcium in some
cells in the central nervous system, and has been
suggested to have a unique, but as yet unidentified,
receptor of its own.
Peptide Biosynthesis
The peptides in the VP/OT family are synthesized as
parts of precursor proteins. The vasopressin precursor
has the following structural features:
signal peptide-CYFNQNCPRGG-K
p
R
p
neurophysin-copeptin
The signal peptide is removed cotranslationally in the
endoplasmic reticulum, liberating the N terminus of the
peptide. Then, after the precursor is packaged into
vesicles by the Golgi apparatus, it is cleaved at the
asterisked sites by a Lys-Arg calcium-dependent endo-
protease. The remaining basic amino acids are
removed by a carboxypeptidase B-like enzyme, leaving
a glycine (G) on the carboxy terminus. This glycine
serves as the donor of the amide group, which is
generated by the sequential actions of a peptidyl-glycine
monooxygenase and a peptidyl-hydroxyglycine lyase.
Neurophysin is thought to serve as an internal chapar-
one, promoting proper folding and cystine-bridge
formation. Copeptin, a glycosylated species, is part of
the vasopressin, but not the oxytocin precursor. Its
function is unknown. The genes encoding the VP and
OT precursors are adjacent to one another on the same
chromosome. In mammals, they are oriented tail-to-tail,
and share regulatory elements.
The Functions of Vasopressin
and Oxytocin
VP/OT-like peptides play different roles in the
various species where they are found. They are very
commonly involved in water homeostasis and/or
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 343
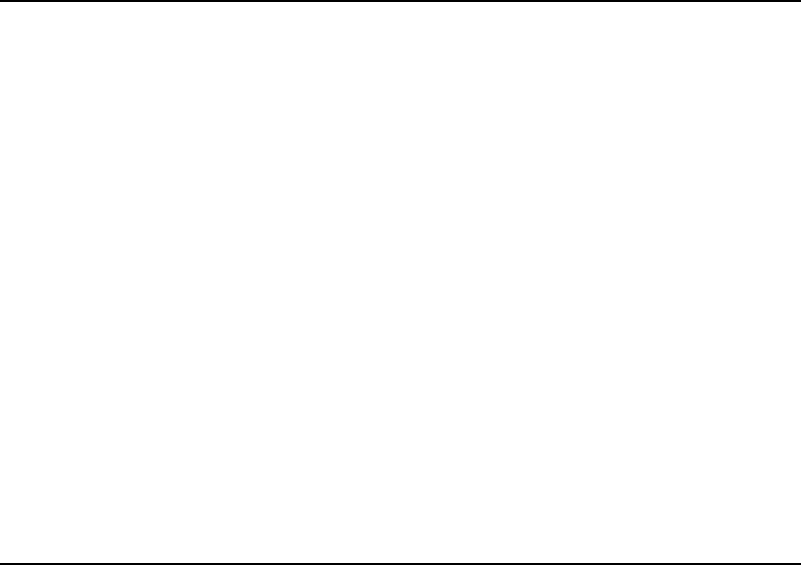
reproductive function. In humans vasopressin is an
antidiuretic hormone. It is released from the posterior
pituitary into the blood stream when the osmolarity
(solute content) of the blood increases, and it acts on V2
receptors in the kidney to cause water retention. V2
receptors are Gs-coupled, and increase intracellular
cyclic AMP. This, in turn, causes preformed aquaporin
(AQP) 2 channels to be inserted into the luminal surfaces
of cells in the collecting duct of the kidney. Water enters
these channels, traverses the cells, exits through AQP 3
and 4 channels on their interstitial faces, and enters
the bloodstream. Mutations in the genes encoding
vasopressin, the V2 receptor, or AQP2 cause diabetes
insipidus – an inability to concentrate urine, resulting in
high urine volumes and a need to consume large amounts
of fluid each day. Since the V2 receptor gene is on the X
chromosome, female carriers pass X-linked nephrogenic
diabetes insipidus along to their sons, but not their
daughters.
Nonosmotic as well as osmotic stimuli can trigger
vasopressin secretion. Osmoreceptors on cells in the
anterior hypothalamus, and baroreceptors in the left
ventricle of the heart, aortic arch, and carotid
sinus monitor osmolarity and blood pressure, respect-
ively, and participate in controlling the firing of
VP-producing cells.
In addition to V2 receptors, vasopressin acts on V1a
and V1b receptors. The former are found on blood
vessels and are responsible for VP’s pressor (blood
pressure increasing) activity. The physiological impor-
tance of this is moot, but it is clear that certain
vascular beds are especially sensitive to VP, including
those in the skin and uterus. In fact, it has been
suggested that V1a receptor antagonists might be
useful for treating Raynaud’s disease (excessive constric-
tion of digital arteries) and dysmenorrhea (menstrual
cramps which may be caused by dilation of vessels in
the uterus).
V1b receptors are found on corticotrophs (adreno-
corticotropic hormone or ACTH-producing cells) in
the anterior pituitary. In addition to being made by
magnocellular neurons, VP is also synthesized by small
cells in the paraventricular nucleus of the hypothala-
mus. These same cells also make corticotropin releas-
ing hormone (CRH), and their axons terminate on
portal vessels in the external zone of the median
eminence. VP and CRH are released into these vessels
and transported to the anterior pituitary, where they
act in concert to stimulate ACTH secretion. ACTH, in
turn, causes the adrenal to release glucocorticoids.
Thus, VP is important in mediating the body’s
response to stress.
TABLE I
Vasopressin, Oxytocin, and Related Peptides
Oxytocin-like peptides
(Tocins)
Oxytocin (OT) C YIQNCP LG-amide Mammals, Pacific ratfish
Mesotocin (MT) CYIQNCPIG-amide Nonmammalian tetrapods, marsupials, lungfish
[Phe2]Mesotocin (FMT) CFIQNCPIG-amide Australian lungfish
Seritocin (ST) C YIQSCP IG-amide Dryness-resistant African toad
Isotocin (IT) C YISNCP IG-amide Bony fish
Aspargtocin (AspT) C YINNCP LG-amide Spiny dogfish
Asvatocin (AsvT) C YINNCPVG-amide Spotted dogfish
Glumitocin (GT) C YISNCPQG-amide Rays
Phasvatocin (PhT) C YFNNCPVG-amide Spotted dogfish
Valitocin (ValT) C YIQNCPVG-amide Spiny dogfish
Annetocin (AnT) C FVRNCPTG-amide Earthworm
Cephalotocin (CT) C YFRNCP IG-amide Octopus
Vasopressin-like peptides
(Pressins)
A-Vasopressin (AVP) C YFQNCPRG-amide Most mammals
L-Vasopressin (LVP) C YFQNCPKG-amide Pig, marsupials
Phenypressin (PP) C YFQNCPRG-amide Marsupials
Vasotocin (VT) C YIQNCPRG-amide Non-mammalian vertebrates
Hydrin 2 (H2) CYIQNCP RGG Frogs, toads
A-conopressin (ACP) C IIRNCP RG-amide Snail
L-conopressin (LCP) C FIRNCP KG-amide Snail, sea hare, leech
Locust diuretic C LITNCP RG-amide Locust (but not fruitfly)
hormone (LDH)
Putative Ancestor C(F/Y)I(Q/R)NCP(R/K)G-amide
Abbreviations: C (cysteine), F (phenylalanine), G (glycine), I (isoleucine), K (lysine), N (asparagine), P (proline),
Q (glutamine), R (arginine), S (serine), T (threonine).
344 VASOPRESSIN/OXYTOCIN RECEPTOR FAMILY
V1a and V1b, but not V2, receptors are found in
the brain. The central effects of vasopressin agonists
and antagonists must be mediated by these receptors.
These effects vary from species to species, and they
are unknown in humans. It appears, however, that VP
may mediate behavioral reactions to stress, aggressive
and affiliative behavior, juvenile recognition, and
parenting in rodents. In addition, V1a antagonists
appear to block the vomiting associated with
motion sickness in pigs and ferrets. This appears to
be a central effect of the drugs, but could have a
peripheral component.
Oxytocin-producing mammals make a single recep-
tor for this peptide. Like the V1a and V1b receptors, it
is Gq-coupled, and activates phospholipase C, increas-
ing intracellular calcium. Oxytocin receptors increase
dramatically in the pregnant uterus as term approaches.
Despite the fact that it has been used for decades to
induce uterine contractions, oxytocin is not essential for
this process, and oxytocin receptor antagonists have not
Jawless fish
Cartilaginous fish
Bony fish
Amphibians
Lizards, birds
Egg-laying mammals
Marsupials
Placental mammals
Pig
Rat, mouse
Man
1000
560
530
450
360
310
(>170?)
170
65
40
1 peptide
per species
2–3 peptides
per species
Arthropods, nematodes, molluscs(?)
Pressins Tocins
ACP, LCP
LDH
AnT, CT
VT
VT
AspT, AsvT, GT
PhT, ValT, OT
VT IT, MT, FMT
VT, H2 MT, (ST)
VT MT
AVP OT
LVP OT
AVP OT
AVP OT
AVP, LVP
PP
MT
}
}
FIGURE 1 Evolution of vasopressin-related (pressins) and oxytocin-related (tocins) peptides. The timescale (millions of years ago) was taken
from Hedges (2002). Note that invertebrates and jawless fish make a single pressin or tocin per species, and that vertebrates make two or, in the case
of marsupials, three such peptides – one or two pressins and one tocin. It appears that vasotocin was the ancestor of the vertebrate hormones, and it
continued to be synthesized by all vertebrates except mammals, in which it was replaced by arginine or lysine vasopressin. Tocins began to be
produced in fish. It is not clear why cartilaginous fish make so many of these; lungfish make MTor FMT, and all of the remaining bony fish make IT.
Based on the structures of the peptide precursors, it has been argued that lungfish are related to tetrapods (e.g., toads) and that bony fish form a
separate lineage. Amphibians, lizards, birds, and marsupials make MT; egg-laying and placental mammals differ in the hormones they produce.
Marsupials appear to have branched away as suggested by other analyses. The abbreviations used in this figure are defined in Table I.
VASOPRESSIN/OXYTOCIN RECEPTOR FAMILY 345
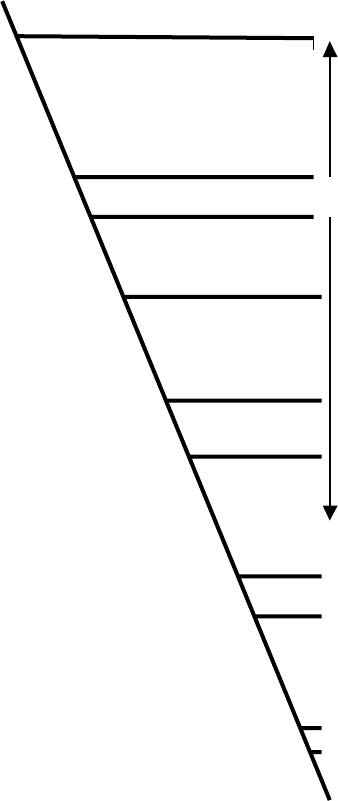
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
GRDEE LAKV E I GVL A TV LV LATGGNL AVL L T LGQLGRK - - RSRMHL FV LHL A L TDL AVAL
KRNEEIAKVEVTVLALILFLALAGNICVLLGIYINRHK- -HSRMYFFMKHLSIADLVVAV
RRNEALARVEVAVLCLI LLLAL SGNACVLLALRTTRQK - -HSRLFFFMKHLS IADLVVAV
VRNEELAKLEIAVLAVTFAVAVLGNSSVLLALHRTPRK- - TSRMHLFI RHLSLADLAVAF
GRNEEVAKMEITVLSVTFFVAVIGNLSVLLAMHNTKKK- - SSRMHLFIKHLSLADMVVAF
GRNEEVAQI EIMVLSI TFVVAVI GNVSVLLAMYNTKKK- -MSRMHL FIKHLSLADLVVAF
ERDEQLAQVEIAVLGVI FLTASVGNF I L I LVLWRRRKK- - LSRMYVFMLHLS IADLVVAF
TRDPLLARAELALLSIVFVAVALSNGLVLAALARRGRRGHWAPIHVFIGHLCLADLAVAL
GVDEDLLKIEIAVQATILYMTLFGNGIVLLVLRLRRQK- -LTRMQWFIAHLAFADIFVGF
GRDESLAR I EI LVQSI I LALA I I GNSCVLTALARRGKA - - ASRMHL F I FHLS I ADLLVAV
TM1
TM2
R1
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
FQVLPQLLWDI TYRFQGPDLLCRAVKYLQVLSMFASTYMLLAMTLDRYLAVCHPLRSLQQ
FQVLPQL IWDITFRFYAPDFVCRLVTYLQVVGMFASTYMLL LMSLDRCLA ICQPLRSLHR
FQVLPQLLWDI TFRFYGPDL LCRLVKYLQVVGMFASTYLLLLMSLDRCLA ICQPLRSLRR
FQVLPQMCWDI TYRFRGPDWLCRVVKHLQVFGMFASAYMLVVMTADRYI AVCHPLKTLQQ
FQVLPQLCWEITFRFYGPDFLCRIVKHLQVLGMFASTYMMVMMTLDRYIAICHPLKTLQQ
FQVLPQLCWE ITYRFFGPDFLCRI VKHLQVTGMFASTYMMVMMTLDRY IA I CHPLKTLQQ
FQVLPQL IWDITDVFI GPDFLCRI IKYLQL LGMFASTYMI VVMTVDRYQAVCYPMVTFQK
FQVLPQLAWKATDRFRGPDALCRAVKYLQMVGMYASSYMI LAMTLDRHRAICRPMLAYRH
FNI LPQL I SDVTI VFHGDDFTCRF IKYFQV I AMYASSYVLVMAAI DRYL SICHPLTSQTL
FNI LPQL IWDITERFYGGDL LCRY I KFMQVYVMYLSTYMLVMTAVDRYRAVCHPLSAFNT
TM3
Q1 Q2
Q3
C1 R2
V
K
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
PG-QSTYLL I AAPWLLAA I FSL PQVFIFSLREV IQGSGVLDCWADFGFPWGPRAYLTWTT
- - -RSDCVYVLFTWI LSFLLS I PQTA I FSLT EVENG- -VYDCRADF I L PWGPKAY I TWI T
- - -RTDRLAVLATWLGCLVASAPQVHIFSLREVADG- -VFDCWAVFIQPWGPKAYI TWIT
PA-RRSRLMIAAAWVLSFVLSTPQYFVFSMIEVNNVTKARDCWATFIQPWGSRAYVTWMT
PT-QRAYIMIGSTWLCSLLLSTPQYFIFSLSEIQNGSYVYDCWGHFIEPWGIRAYITWIT
PT-QRSYIMIVSTWMCSLVFSTPQYFIFSLSEVKNGSTVKDCWAHFIEPWGARAYITWIT
KR-ALWNI PICTSWSI SL I LSL PQVFIFSKI EI SPG- - I FECWAEFIQPWGPRAYVTWIL
GSGAHWNRPV LVAWAFSL LLSL PQL F I FAQRNVEGGSGVTDCWACFAEPWGRRTYVTWI A
SP-KRVHLMIALAWLI SFLCALPQVFIFSLQAVGPD- -QYDCLATFEPDWGMQAYITWVF
STRTPLYCMIVSAYV I SGVLSL PQPI IFKYREKSHGSGDYECWGRFEPPWTLNLY ITSFT
Q4
C2
TM4
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
LAI FVLPVTMLTACYSL ICHEICKNLKVKTQAWRVGGGG- - - - - - - - - - - - - - - -WRTWD
LSVYIIPVLILSICYGLISYKIWQNIRLKTVCESN-----------------------LR
LAVYIVPVIVLATCYGLISFKIWQNLRLKTAAAAA-----------------------AE
GGIFVAPVVILGTCYGFICYNIWCNVRGKTASRQSKGAE----------------QAGVA
VGIFLIPVIILMICYGFICHSIWKNIKCKTMRGTR------------------------N
GGI FLVPVVI LVMCYGF ICHTIWKNIKYKKRKTI PG- - - - - - - - - - - - - - - - - - - - - -AA
VVI FF I PSTIL I TCQVK ICKI IKRNIYVKKQNEYQ- - - - - - - - - - - - - - - - - - - - - - - -V
LMVFVAPTLGIAACQVLIFREIHASLVPGPSERPG------------------------G
VANYV I PFLLLAFCYGRI CHVVWMSVAAKESAAYSSMRNGCTESSRP- - - - - IKMRI SFH
FAVYI VPLA IL I FAYVSI CCTIWRKYKSAENERKHMLNGSDSSLGNRNI YSNHVTHSALF
TM5
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
RPSPSTLAAT--------TRGLPSR----VSSINTISRAKIRTVKMTFVIVLAYIACWAP
LSTSRRAT--------------LSR----VSSVRLISKAKIRTVKMTFIIVLAYIVCWTP
APEGAAAGDG--------GRVALAR----VSSVKLISKAKIRTVKMTFIIVLAFIVCWTP
FQKGFLLA--------------PC-----VSSVKSISRAKIRTVKMTFVIVTAYIVCWAP
TKDGMIGK--------------VS-----VSSVTIISRAKLRTVKMTLVIVLAYIVCWAP
SKNGLIGK--------------NS-----VSSVTTISRAKLRTVKMTFVIVLAYIICWAP
TNQKQVLP---------------SR----ASSVNCISKAMIKTVKMTIVTVVAYVLCWSP
RRRGRRTG---------------S-----PGEGAHVSAAVAKTVRMTLVIVVVYVLCWAP
RRRDNTNATLTSLDRHDASAVTSSDSKKPRGHQRGVSKSKMKTI KL TLTVVLCYL FCWAP
RHRGV I ERRRNLVQRCRPAPMAAPR----AHSLRGFSRAKLKTVKLTFVVIVAYVVCWSP
TM6
Human V1B
Tree frog MT
Human OT
Human V1A
White sucker IT
Flounder AVT
Chicken VT1
Human V2
Snail CP1
Snail CP2
FFSVQMWSVWDKNAPDEDSTNVAFTISMLLGNLNSCCNPWI YMGFNSHL
FFFVQMWSVWDPNPPKE- - -ASLFI IAMLLGSLNSCCNPWIYMLFTGHL
FFFVQMWSVWDANAPKE- - -ASAFI IVMLLASLNSCCNPWIYMLFTGHL
FF I I QMWSVWDPMSVWTESENP T I T I TA LL GSL NSCCNPWI YMF FSGHL
FF I VQMWSVWDENFSWDDSENAAVTLSALLASLNSCCNPWI YML FSGHL
FFTVQMWSVWDENFQYADSENTAVTISALLASLNSCCNPWIYMI FSGHL
FF I AQLWSVWF PSG I TEG- - - SAF T I IML L GNLNSCTNPWI YMYFCGH I
FFLVQLWAAWDPEAPLEG- - -APFVLLMLLASLNSCTNPWI YASFSSSV
FFVVQMWSAFDDDSGI EH- - - PVTVI CMLLASLNSCTNPWIYLAFSGRT
FFL SQLWWLYDEQQEHN- - - -HAVVIMVLLASLNSCCNPWI YLAFSGNL
TM7
Q5
C3
346 VASOPRESSIN/OXYTOCIN RECEPTOR FAMILY

proven useful for treating preterm labor. There is no
doubt, however, that the pulsatile release of OT from the
pituitary in response to cervical dilation and vaginal
stimulation, facilitates the expulsion of the fetus.
Oxytocin is required for milk ejection. Mechanical
stimulation of pressure sensitive receptors in the nipple
of the breast by the nursing infant results in activation of
magnocellular neurons in the hypothalamus and release
of pulses of OT into the bloodstream. The hormone
causes breast myoepithelial cells to contract, increasing
intramammary pressure and forcing milk into the ducts.
In the absence of OT, milk cannot be let down, and the
infant will starve if it is not provided an alternative
source of food.
In addition to its roles in parturition and lactation,
oxytocin appears to affect maternal and social beha-
viors, stimulate lipogenesis to compensate for lipid loss
in the milk (via an action on insulin secretion), and
possibly participate in regulating salt and water balance.
While OT causes natriuresis in rats, it is not clear that
this is the case in humans.
Vasopressin and
Oxytocin Receptors
As expected from the fact that their ligands are similar,
VP and OT receptors are structurally related. They are
members of the rhodopsin superfamily, and have seven
a-helical membrane-spanning domains connected to one
another by intracellular and extracellular loops. The N
terminus of each receptor faces the outside of the cell;
the C terminus is cytoplasmic. The intracellular loops
and C-terminal tail of the receptors interact with G
proteins, coupling agonist binding to activation of
second messenger systems. More than 40 VP/OT
receptors found in species ranging from snails to humans
have been cloned and sequenced. The primary sequences
of some of these are shown in Figure 2. It is remarkable
that from the beginning of its first transmembrane
domain (TM1) to the end of its seventh one (TM7),
the snail conopressin receptor 2 is 43% identical in
amino acid sequence to the human V1a, V1b, and OT
receptors and the white sucker fish vasotocin recep-
tor. Unlike the vertebrate proteins, however, the
conopressin receptor responds equally well to lysine8-
and isoleucine8-conopressin (an OT-like synthetic
analogue of lysine-conopressin). Duplication of a rela-
tively promiscuous receptor of this sort might
have permitted trial-and-error evolution of functionally
distinct pressors and tocins in vertebrates.
A number of amino acids are conserved among all of
the receptors in Figure 2. Some of these residues are
found in most G-protein-coupled receptors. Among
them, the arginine (R2) in the DRY motif, found just
beneath TM3, is thought to dwell in a pocket formed by
polar residues in TMs 1, 2, and 7 when the receptor is in
its inactive state. Hormone binding dislodges this
arginine from its polar pocket, exposing G-protein
docking sites on the cytoplasmic loops.
The cysteines in extracellular loops 1 and 2 (C1 and
C2, respectively) are also highly conserved among
rhodopsin-like receptors. They form a cystine bridge
that links these loops, stabilizing the conformation of
the receptors. The pair of cysteines (C3 and its neighbor)
located 15 aa’s below TM7 in the cytoplasmic tails of
most VP/OT receptors are likely to be palmitoylated and
are thought to anchor their C termini to the plasma
membrane. Like other members of the rhodopsin
superfamily, VP and OT receptors appear to be
glycosylated on their N termini, and regulated by
phosphorylation of their intracellular domains.
A number of attempts have been made to model
the binding of VP, OT, and vasotocin to their
receptors. The models are fundamentally similar in
the sense that they all predict that the peptide
hormones fill a cleft located in the upper third of
the barrel formed by the seven membrane-spanning
a
-helices. The hydrophobic amino acids that comprise
the cyclic portion of the peptides (cysteine1, tyro-
sine2, isoleucine or phenylalanine3, and cystein6)
appear to reside in a hydrophobic pocket formed by
aromatic residues on helices 5 and 6 (and adjacent
helices). The more polar amino acids (asparagine4,
glutamine5), and the amidated C terminus of the
hormones must occupy a hydrophilic region formed
by residues on helices 2, 3, and 4. More specifically,
residues that are conserved in the N-terminal domain
and TMs 2, 3, 4, and 6 in most, if not all, of the
VP/OT receptors (labeled R1, Q1, Q2, K, Q3, Q4,
and Q5 in Figure 2) have been shown to be important
for high-affinity binding, even though their predicted
interactions with specific amino acids in the peptide
hormones vary from model to model. Parsimony
dictates that residues conserved among the various
FIGURE 2 Alignment of vasopressin and oxytocin receptors and selected relatives. To save space, the extracellular N termini and intracellular C
termini have been removed. They are quite divergent, but it is remarkable that the transmembrane domains (TMs), the first two extracellular loops
(linking TMs 2 and 3, and TMs 4 and 5), and portions of intracellular loops 2 and 3 (linking TMs 3 and 4, and TMs 5 and 6), have remained so
similar throughout the course of their evolution. Specific residues in these domains are responsible for ligand binding and selectivity (see text), and
other motifs are important for signal transduction. The variable intracellular portions of the receptors allow them to interact with specific G
proteins.
VASOPRESSIN/OXYTOCIN RECEPTOR FAMILY 347
VP/OT peptides should interact with conserved
domains in the receptors, but perhaps things are not
this simple.
One amino acid appears to be crucial for peptide
agonist selectivity. This residue (marked with a V in
Figure 2) is found in the first extracellular loop, and it
interacts with the eighth amino acid of the peptide
hormones (arginine and leucine in AVP and OT,
respectively). The aspartic acid or tyrosine residues
found at V in the human V2 and V1a/V1b receptors,
respectively, are responsible for their marked preferences
for AVP versus OT. The phenylalanine in this position in
the OT receptor accounts for its modest preference for
OT over AVP.
VP and OT antagonist-binding sites appear to be
different from the ones where the peptide hor-
mones bind.
SEE ALSO THE FOLLOWING ARTICLES
Amino Acid Metabolism † Phospholipase C
GLOSSARY
baroreceptor Receptor in the walls of the heart or blood vessels that
is stimulated by alterations in pressure.
diuresis Increased urine excretion.
diuretic An agent that increases urine excretion.
hypothalamus The part of the brain that regulates the endocrine and
autonomic and autonomic nervous systems, controlling water
balance, blood pressure, body temperature, growth, and sexual
function.
lipogenesis Formation of body fat.
magnocellular neurons Large neurons in the hypothalamus that
manufacture vasopressin or oxytocin.
natriutesis Sodium excretion in the urine.
pressin An agent that increases blood pressure. In the context of this
review, “pressins” are vasopressin-like peptides with nine
amino acids, having a basic residue (arginine or lysine in the
eighth position).
tocin An agent that promotes childbirth by causing uterine contrac-
tions; an oxytocin-like peptide lacking arginine or lysine in the
eighth position.
FURTHER READING
Acher, R., and Chauvet, J. (1995). The neurohypophsial endocrine
regulatory cascade: Precursors, mediators, receptors, and effectors.
Front. Neuroendocrinol. 16, 237 –289.
Chini, B., and Fanelli, F. (2000). Molecular basis of ligand binding and
receptor activation in the oxytocin and vasopressin receptor family.
Exp. Physiol. 85S, 59S– 66S.
Hedges, B. S. (2002). The origin and evolution of model organisms.
Nature Rev. Genet. 3, 838–849.
Ivell, R., and Russell, J. A. (1995). Oxytocin: Cellular and Molecular
Approaches in Medicine and Research. Kluwer Academic/Plenum,
New York.
Zingg, H. H., Bourque, C. W., and Bichet, D. G. (1998). Vasopressin
and Oxytocin: Molecular Cellular, and Clinical Advances. Kluwer
Academic/Plenum, New York.
BIOGRAPHY
Michael J. Brownstein is Chief of the Laboratory of Genetics,
National Institute of Mental Health, NIH, Bethesda, MD.
He received his Ph.D. and M.D. degrees from the University
of Chicago and did postdoctoral training with Julius Axelrod.
His research has been in the areas of neurobiology, endocrinology,
genetics, and genomics. He and his co-workers are known for their
studies of vasopressin and oxytocin biosynthesis, and for cloning the
vasopressin and oxytocin receptors.
348 VASOPRESSIN/OXYTOCIN RECEPTOR FAMILY

V-ATPases
Michael Forgac
Tufts University School of Medicine, Boston, Massachusetts, USA
The V-ATPases (or vacuolar (H
1
)-ATPases) are ATP-driven
proton pumps whose primary function is to acidify intracellu-
lar compartments in eukaryotic cells, although they have
also been identified in the plasma membrane of certain cells.
V-ATPases have been shown to play a crucial role in a variety
of normal cellular processes as well as a number of human
diseases. The structure, mechanism, and regulation of these
proton pumps have been the topics of intense study.
V-ATPase Function
FUNCTION OF INTRACELLULAR
V-ATPASES
V-ATPases have been identified in many intracellular
compartments, including endosomes, lysosomes, Golgi-
derived vesicles and secretory vesicles. V-ATPases within
endosomal compartments are important for the process
of receptor-mediated endocytosis (Figure 1). During
receptor-mediated endocytosis, cells take up ligands
(such as the cholesterol carrying complex low density
lipoprotein, or LDL) from their environment by binding
them to receptors on the cell surface and clustering these
receptors in specialized regions of the plasma membrane
which then invaginate into the cell. Following this
internalization, the ligand-receptor complexes are
exposed to a low pH within the endosome that causes
the internalized ligand to dissociate from its receptor.
This dissociation allows the receptor to recycle to the
plasma membrane (where it is reutilized) and the
ligand to proceed to the lysosome, where it is degraded.
The low pH within the endosome is generated by the
V-ATPase.
Acidification of endosomes is also important in the
formation of carrier vesicles that carry the released
ligands from early to late endosomal compartments, and
in the delivery of newly synthesized lysosomal enzymes
from the Golgi to lysosomes. The latter process involves
the binding of these enzymes to mannose-6-phosphate
receptors in the trans-Golgi followed by their delivery to
an endosomal compartment. Within this compartment,
the low pH created by the V-ATPases causes dissociation
of the lysosomal enzymes from their receptors, allowing
delivery of the enzymes to the lysosome and recycling of
the receptors to the trans-Golgi. Finally, endosomal
acidification is involved in the entry of certain envelope
viruses (such as influenza virus) into cells. These viruses
bind to the surface of cells and are internalized by the
process of endocytosis. Upon exposure to a low pH, the
virus coat fuses with the endosomal membrane, releas-
ing the viral nucleic acid into the cytoplasm of the host
cell. Endosomal acidification is therefore essential in the
process by which these viruses infect cells.
Lysosomes are the major compartment in which
degradation of proteins and other macromolecules
occurs in cells. The lysosomal enzymes responsible
for this degradation all require an acidic environment
to be active. This acidic environment is created by the
V-ATPases. Secretory vesicles, such as synaptic vesicles,
are also acidic compartments. Synaptic vesicles are
located at the synaptic terminal of nerve cells and release
neurotransmitters (that chemically trigger the next nerve
cell) by fusion with the plasma membrane. Neurotrans-
mitters become concentrated within synaptic vesicles by
transport proteins within the synaptic vesicle membrane
that utilize either the proton gradient or the membrane
potential generated by the V-ATPases to drive uptake of
the transmitter.
FUNCTION OF PLASMA
MEMBRANE V-ATPASES
Plasma membrane V-ATPases play an important role in
a number of normal and disease processes. In alpha-
intercalated cells in the kidney, V-ATPases are located in
the apical membrane where they pump protons into the
urine, thus helping to control the pH of the blood. A
genetic defect in this pump leads to a disease called renal
tubule acidosis, in which the kidney is unable to secrete
sufficient acid. V-ATPases are also present in the plasma
membrane of osteoclasts, which are cells that function in
degradation of bone. These cells are essential during
development to facilitate bone remodeling. Plasma
membrane V-ATPases in osteoclasts create an acidic
extracellular environment that is necessary for bone
degradation to occur. A genetic defect in the V-ATPase in
osteoclasts leads to the human disease autosomal
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 349
recessive osteopetrosis, in which the inability to degrade
bone leads to severe skeletal defects and death.
Plasma membrane V-ATPases in macrophages and
neutrophils have been shown to help maintain a neutral
internal pH under conditions of severe acid load. In the
vas deferens, V-ATPases create a low pH environment
necessary for sperm development. V-ATPases in the
plasma membrane of tumor cells have also been
proposed to function in tumor invasion by providing
an acidic extracellular environment necessary for
secreted lysosomal enzymes to degrade extracellular
matrix. Finally, V-ATPases in intestinal cells in insects
create a membrane potential across the apical membrane
that is used to drive potassium transport into the gut.
V-ATPase Structure
The V-ATPase is a large complex composed of 13
different subunits. These subunits are arranged into two
separate domains termed V
1
and V
0
(Figure 2). The V
1
domain is made up entirely of subunits that are
peripheral to the membrane (i.e., not membrane-
embedded). This domain has a molecular mass of
, 640 kDa and contains eight different subunits (sub-
units A–H) of molecular mass 70– 13 kDa (Table I).
The V
1
domain is responsible for hydolysis of ATP,
which occurs on catalytic sites located on the three
copies of subunit A. There are therefore three catalytic
nucleotide binding sites per complex. The B subunits
(which are also present in three copies per complex) can
also bind nucleotides, but these sites are referred to as
“non-catalytic” sites, since they do not actually hydro-
lyze ATP. The function of these sites is not known,
but they may play a role in controlling the activity of the
V-ATPase. The A and B subunits are arranged in a
hexamer, like the segments of an orange, with alterna-
ting A and B subunits. ATP is hydrolyzed sequentially at
each of the three catalytic sites. The other subunits in the
V
1
domain (subunits C– H) function to connect the V
1
domain to the V
0
domain and are discussed here.
The V
0
domain is composed of five different subunits
(subunits a, d, c, c
0
,andc
00
)ofmolecularmass
100–17 kDa. All of the subunits in the V
0
domain
except subunit d are embedded in the membrane, and
thus require detergent for solubilization. The V
0
com-
plex has a molecular mass of 260 kDa and is responsible
for transport of protons across the membrane.
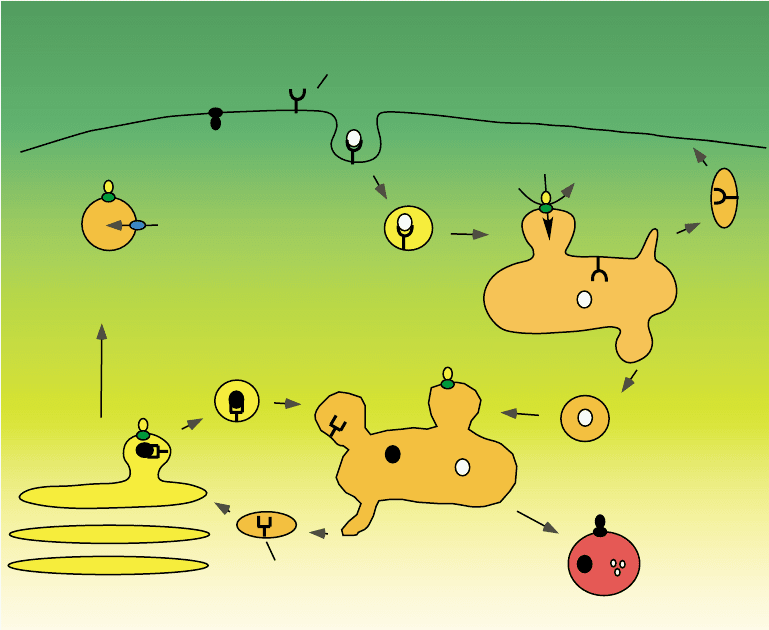
Receptor
Clathrin
coated
vesicle
Neuro-transmitter
Secretory
vesicle
Early
endosome
Recycling
vesicle
ATP
2H
+
Late
endosome
Endosomal
carrier vesicle
Lysosome
M6P
receptor
Golgi
FIGURE 1 Function of intracellular V-ATPases. Acidification of early endosomes by the V-ATPase facilitates receptor recycling following
endocytic uptake and formation of endosomal carrier vesicles. Recycling M6P receptors to the trans-Golgi is also low pH dependent. V-ATPase
activity is also required to drive neurotransmitter uptake and facilitates protein degradation in lysosomes.
350 V-ATPases
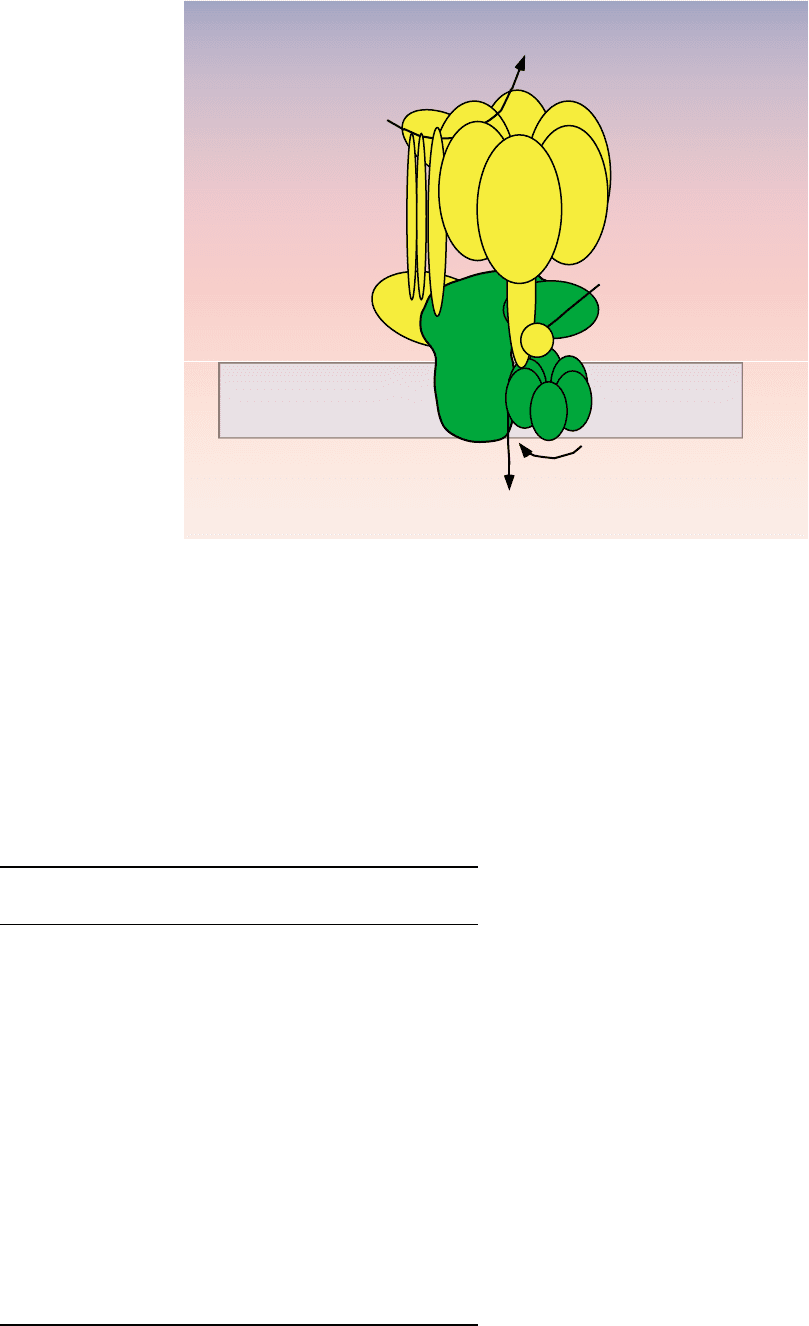
This proton transport only occurs when the V
1
domain
is attached to V
0
and is driven by the hydrolysis of ATP
in V
1
. Three of the subunits in the V
0
domain are called
proteolipid subunits (c, c
0
, and c
00
) because they are so
hydrophobic that they can be extracted from the
membrane using organic solvent mixtures, such as
chloroform:methanol. These subunits are almost com-
pletely embedded in the membrane and the polypeptide
chain of each one crosses the membrane four times
(these are called transmembrane segments). Buried in the
middle of one of the transmembrane segments of each of
the proteolipid subunits is a single essential glutamic
acid residue which is reversibly protonated and depro-
tonated during proton transport by the V-ATPases. Like
the A and B subunits in the V
1
domain, the proteolipid
subunits in the V
0
domain form a ring, with four copies
of subunit c and one copy each of subunit c
0
and c
00
. The
specific V-ATPase inhibitor bafilomycin has been shown
to bind to the proteolipid subunits of the V
0
domain.
In addition to the proteolipid subunits, the other V
0
subunit that is embedded in the membrane is the
100-kDa subunit a. This subunit is made up of two
domains. The carboxyl-terminal half of the molecule
contains nine transmembrane segments while the amino-
terminal half is a hydrophilic domain that is present on
the cytoplasmic side of the membrane. Like the proteo-
lipid subunits, the subunit a also contains amino acid
residues that are essential for proton transport. In par-
ticular, there is a positively charged arginine residue near
the middle of the seventh transmembrane segment of
subunit a that is absolutely required for proton transport
Lumen
MembraneV
0
domain
V
1
domain
ATP
G
2
Cytoplasm
2H
+
ADP + P
i
AB
A
C
B
A
B
E
D
F
a
c
c'
c
c''
c
H
FIGURE 2 Structure of the V-ATPases. The V-ATPases contain two domains. The V
1
domain is responsible for ATP hydrolysis and the V
0
domain
carries out proton transport across the membrane. Like the F-ATPases, the V-ATPases operate by a rotary mechanism in which ATP hydrolysis in
V
1
drives rotation of a central stalk which is connected to a ring of proteolipid subunits in V
0
. It is movement of the proteolipid ring relative to
subunit a that drives proton transport (see text).
TABLE I
Subunit Composition of the V-ATPase
Domain Subunit
Gene
(yeast)
M
r
(kDa)
Function/
location
V
1
A VMA1 69 Catalytic ATP binding site
B VMA2 58 Noncatalytic ATP
binding site
C VMA5 44 Peripheral stalk
D VMA8 29 Central stalk
E VMA4 26 Peripheral stalk
F VMA7 14 Central stalk
G VMA10 13 Peripheral stalk
H VMA13 54 Peripheral stalk
V
0
a VPH1/STV1 100 Proton translocation,
targeting
d VMA6 40 Cytoplasmic side
c VMA3 17 Proton translocation,
bafilomycin-binding site
c
0
VMA11 17 Proton translocation
c
00
VMA16 23 Proton translocation
V-ATPases 351
by the V-ATPases. The function of the remaining V
0
subunit d, which is tightly bound to the V
0
domain but is
not embedded in the membrane, is not known.
The V
1
and V
0
domains are connected by two stalks.
The central stalk is composed of the subunits D and F
whereas the peripheral stalk is composed of subunits C,
E, G, H, and the soluble domain of subunit a. The
function of these stalks is described below.
Mechanism of ATP-Driven Proton
Transport by the V-ATPases
The V-ATPases are believed to operate by a rotary
mechanism, similar to that demonstrated for the
F-ATPases (or ATP synthases), which are enzyme
complexes present in mitochondria, chloroplasts, and
bacteria that function in the reverse direction (that is in
proton-driven ATP synthesis). For the V-ATPases, ATP
hydrolysis in the V
1
domain drives rotation of the
central stalk (containing subunits D and F), which in
turn drives rotation of the ring of proteolipid subunits
relative to subunit a in the V
0
domain. Subunit a is held
fixed relative to the A
3
B
3
hexamer of V
1
by the
peripheral stalk (or stator), composed of subunits C,
E, G, H, and the soluble domain of subunit a. It is
rotation of the ring of proteolipid subunits relative to
subunit a that drives active transport of protons from
the cytoplasmic to the lumenal side of the membrane. A
proton enters from the cytoplasmic side of the mem-
brane via a cytoplasmic access channel in subunit a and
protonates a buried carboxyl group on one of the
proteolipid subunits. ATP hydrolysis in V
1
forces
rotation of the proteolipid ring in the plane of the
membrane such that the protonated carboxyl group on
the proteolipid subunit reaches a second access channel
in subunit a that leads to the lumenal side of the
membrane. Interaction between this carboxyl group on
the proteolipid subunit and the buried arginine residue
of subunit a (which is positively charged) forces the
proton off of the proteolipid subunit into the lumenal
access channel, where it can be released to the lumenal
side of the membrane, thus completing the transport
cycle. In this way, the rotary motion driven by hydrolysis
of ATP is converted into unidirectional transport of
protons across the membrane.
Regulation of V-ATPase
Activity In Vivo
The activity of V-ATPases in different membranes in
the cell is known to be regulated such that the pH of
different intracellular compartments and the degree
of proton transport across the plasma membrane is
carefully controlled, but the mechanisms employed
in regulating V-ATPase activity in cells are still being
elucidated. One important mechanism of regulation
involves reversible dissociation of the V-ATPase com-
plex into its component V
1
and V
0
domains. In yeast,
dissociation occurs in response to removal of glucose
from the media, probably as a way to preserve
cellular energy stores. Dissociation has also been
demonstrated to occur in insects and in mammalian
cells. A second proposed regulatory mechanism
involves the formation of a disulfide bond between
two conserved cysteine residues located at the catalytic
site on the subunit A.
Differential targeting of V-ATPases to different
cellular membranes has also been proposed as a
means of controlling proton transport. This has been
shown to occur in intercalated cells in the kidney, where
exposure to a low pH causes the fusion of intracellular
vesicles containing the V-ATPase with the plasma
membrane, thus increasing proton transport out of
the cell into the renal fluid. Differential targeting of
V-ATPases appears to be controlled by different iso-
forms of the subunit a. Thus the a3 isoform is able to
target the V-ATPase to the plasma membrane in
osteoclasts whereas the a4 isoform targets the V-ATPase
to the intercalated cell plasma membrane. It is
mutations in these isoforms that lead to the human
diseases osteopetrosis and renal tubule acidosis men-
tioned earlier. Isoforms have now been identified in
many of the V-ATPase subunits in mammalian cells,
and these have been shown to be expressed in both
tissue- and organelle-specific manner. This has led to
the expectation that specific inhibitors can be identified
that are selective in their ability to inhibit particular
V-ATPase complexes, which may in turn lead to cures
for diseases such as osteoporosis.
SEE ALSO THE FOLLOWING ARTICLE
Lipid Rafts
GLOSSARY
access channel An aqueous channel that allows protons to reach
buried carboxyl groups in the center of the membrane from one side
of the membrane or the other.
osteopetrosis A genetic disease in humans associated with the
inability to degrade bone, one cause of which is a defect in the
V-ATPase of osteoclasts.
receptor-mediated endocytosis The process by which cells take up
specific ligands from their environment (such as low density
lipoprotein) via cell surface receptors.
V-ATPase Vacuolar proton translocating ATPase, which carries
out active proton transport from the cytoplasmic to the
non-cytoplasmic side of the membrane driven by energy released
upon hydrolysis of ATP.
352 V-ATPases
