Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

FURTHER READING
Arata, Y., Baleja, J. D., and Forgac, M. (2002). Localization of subunits
D, E and G in the yeast V-ATPase complex using cysteine-mediated
cross-linking to subunit B. Biochemistry 41, 11301– 11307.
Bowman, B. J., and Bowman, E. J. (2002). Mutations in subunit c
of the V-ATPase confer resistance to bafilomycin and identify
a conserved antibiotic binding site. J. Biol. Chem. 277,
3965–3972.
Nishi, T., and Forgac, M. (2002). The vacuolar (H
þ
)-ATPases:
Nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol.
3, 94–103.
Smardon, A. M., Tarsio, M., and Kane, P. M. (2002). The RAVE
complex is essential for stable assembly of the yeast V-ATPase.
J. Biol. Chem. 277, 13831–13839.
Toyomura, T., Oka, T., Yamaguchi, C., Wada, Y., and Futai, M.
(2000). Three subunit a isoforms of mouse vacuolar (H
þ
)-ATPase.
Preferential expression of the a3 isoform during osteoclast
differentiation. J. Biol. Chem. 275, 8760– 8765.
BIOGRAPHY
Michael Forgac is a Professor in the Department of Physiology at Tufts
University School of Medicine. His principal research interest is in the
structure, mechanism, and regulation of the V-ATPases. He received his
B.S. in biology and chemistry from Caltech in 1976 and his Ph.D. in
Biochemistry and Molecular Biology from Harvard in 1981. He is
author of over eighty publications and a recipient of a MERIT Award
from the National Institutes of Health.
V-ATPases 353

Vitamin A (Retinoids)
Joseph L. Napoli
University of California, Berkeley, CA, USA
Vertebrates require vitamin A, all-trans-retinol (atROH), for
vision, fertility, embryogenesis, growth, and optimum neuro
and immune function. atROH generates the visual pigment,
11-cis-retinal, and the humoral effector all-trans-retinoic
acid (atRA). atROH transport and metabolism relies on
serum and cellular chaperones (binding proteins) to maxi-
mize efficiency and impose specificity. Cleavage of caroten-
oids in the intestinal mucosa by carotenoid monooxygenase
produces all-trans-retinal. CRBP(II) binds all-trans-retinal
and dietary retinol, and allows only reduction and
esterificationintoall-trans-retinyl esters (atRE). atRE
storage occurs mostly in liver stellate cells. Liver only
releases atROH bound with serum retinol binding protein,
sRBP. In extra-intestinal tissues, cellular retinol binding-
protein (CRBP) binds atROH and allows re-esterification. In
the retinal pigment epithelium, the binding-protein RPE65
facilitates concerted atRE hydrolysis and conversion into
11-cis-retinol, catalyzed by an isomerohydrolase. Short-
chain dehydrogenase/reductases (SDR) convert 11-cis-retinol
into 11-cis-retinal. 11-Cis-retinal crosses the interphoto-
receptor matrix and enters the rod outer segments, where it
binds covalently with opsin to form rhodopsin. Light
isomerizes 11-cis-retinal into all-trans-retinal, changing the
conformation of rhodopsin and generating a nerve impulse.
The transporter ABCR aids leaching of all-trans-retinal
from rhodopsin. Other SDR reduce all-trans-retinal into
atROH. atROH crosses the interphotoreceptor space,
rebinds with CRBP in the retinal pigment epithelium, and
undergoes re-esterification. atRA biogeneration takes a
different path. atROH, from hydrolysis of atRE by retinyl
ester hydrolase or from serum, binds with CRBP and
undergoes dehydrogenation by microsomal SDR. The all-
trans-retinal produced undergoes dehydrogenation into
atRA, catalyzed by retinal dehydrogenases. CRABP(II)
delivers atRA to retinoic acid receptors, whereas the
complex CRABP-atRA has higher enzymatic efficiency for
atRA degradation than does free atRA. Cytochromes P-450
initiate atRA degradation through C4 and C18 hydroxy-
lation and 5,6-epoxydation. Primary regulators of atROH
homeostasis include apo-CRBP, which inhibits esterification
of atROH and accelerates hydrolysis of atRE, and atRA,
which induces its own catabolism.
Retinoids and their Functions
The term retinoid refers to compounds, both naturally
occurring and synthetic, with vitamin A activity: vitamin
A denotes the organic compound all-trans-retinol
(atROH). All vertebrates require vitamin A/atROH for
vision, fertility, embryogenesis, and growth. atROH does
not support the physiological functions attributed to
vitamin A; rather it acts as precursor for biosynthesis of
retinoids directly responsible for producing “vitamin A
activity”. These atROH metabolites include 11-cis-
retinal, the cofactor covalently bound with opsin to
form the visual pigment rhodopsin, and all-trans-retinoic
acid (atRA), the humoral effector of the non-visual,
systemic functions attributed to vitamin A. These sys-
temic functions include: controlling the differentiation
programs of all epithelia, and of stem cells in the skin,
nervous system, bone, immune system, hemopoietic
system; acting as a tumor suppressor; and regulating
apoptosis. Specific non-visual effects of retinoids include
memory formation, immune responsivity, stress adap-
tation, and cell fate determination. atRA activates three
nuclear receptors, RAR
a
,
b
and
g
, and thereby controls
the expression of hundreds of genes. atRA, or perhaps
other retinoids, may also function through non-genomic
mechanisms, but the research is in its infancy.
The Chemistry of atROH
Generation, Storage, and
Metabolic Activation
Carotenoids with at least one
b
-ionone ring, especially
b
-carotene, provide the major retinoid precursors in
most diets. Oxidative central cleavage by a plasma-
membrane associated, but soluble, carotene 15,15
0
-
monooxygenase (CMO) produces all-trans-retinal—a
transient intermediate that occurs in very low concen-
trations outside of the neural retinal. Microsomal
retinal reductases, RRD, members of the short-chain
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 354
dehydrogenase/reductase (SDR) gene family, catalyze
reduction of all-trans-retinal into atROH, which then
undergoes esterification into all-trans-retinyl esters
(atRE), predominantly palmitate, by microsomal
lecithin:retinol acyltransferase (LRAT). Chylomicrons
carry atRE into circulation and chylomicron remnants
deliver them to liver, where ultimately, most are stored
in stellate cells.
In the visual cycle, atRE undergo concerted
hydrolysis– isomerization into 11-cis-retinol by a micro-
somal isomerohydrolase (IMH), followed by dehydro-
genation into 11-cis-retinal (SDR). 11-Cis-retinal forms
a Schiff’s base with a lysine residue in the protein opsin
to form rhodopsin. When light strikes the neural retinal,
11-cis-retinal in rhodopsin undergoes cis to trans-
isomerization, causing a conformation change that
initiates nerve impulses and releases the newly formed
all-trans-retinal. Reduction of all-trans-retinal into
atROH (microsomal SDR) and re-esterification
(LRAT) into atRE completes the visual cycle.
Activation of atROH into atRA uses the same
intermediate used in the visual cycle, all-trans-retinal,
but relies on a metabolically distinct route. atROH,
either from blood or from hydrolysis of retinyl esters
by microsomal retinyl ester hydrolase (REH), undergoes
reversible dehydrogenation into all-trans-retinal, cata-
lyzed primarily by microsomal retinol dehydrogenases,
RDH, also members of the SDR gene family. In contrast
to the comparatively high concentrations of retinals that
allow the visual cycle to function, all-trans-retinal
concentrations during atRA biosynthesis are kept low
by reduction (back reaction of RDH and reduction
by microsomal and peroxisomal reductases), and by
irreversible dehydrogenation into atRA, catalyzed by
soluble, , 54 kDa, high V
max
retinal dehydrogenases
(RALDH), members of the aldehyde dehydrogenase
(ALDH) gene family. atRA isomers occur in vivo, such
as 13-cis-RA and 9,13-di-cis-RA, but their significance
and source(s) remain unclear.
These straightforward reactions offer complex oppor-
tunities for physiological regulation, owing to compart-
mentalization, distinct enzymes catalyzing each
direction of chemically reversible reactions (e.g. dehy-
drogenation/reduction of retinol/retinal; esterification/
hydrolysis of atROH/atRE), cell-distinct expression
patterns, and multiple homologs/paralogs that catalyze
several reactions. For example, at least four reductases
have been identified, which belong to the SDR gene
family, Rrd (peroxisomal) and RalR1 outside of the eye,
and retSDR and prRDH, in the neural retina. At least
three RDH, also SDR, have been identified in the rat:
Rodh1, Rodh2 and Rodh3. Four Raldh have been
identified in human, rat and mouse: Raldh1, 2, 3, 4 (aka
ALDH 1A1, 1A2, 1A6 and 8A1). These constitute a
complex enzyme system for absorbing and storing
vitamin A, maintaining atRA homeostasis, and recycling
vitamin A in the visual cycle.
Retinoid Binding-Proteins
and their Contributions to
Retinoid Homeostasis
Processing of dietary retinoids and retinoid precursors
and biogeneration of active retinoids relies on serum
and cellular chaperones for efficient and specific
retinoid use, as demonstrated by studies in vitro and
the consequences of gene knockouts and/or naturally
occurring mutations.
Liver and other tissues synthesize a retinol binding-
protein, sRBP, a member of the lipocalin gene family.
atROH egress from liver requires sRBP, and sRBP-
atROH represents the major form of vitamin A in serum.
sRBP circulates as a complex with a tetramer of
transthyretin, which protects the , 20 kDa sRBP from
kidney filtration. The mechanism of atROH delivery
from sRBP into cells has not been established. Some data
suggest a membrane receptor, other data indicate that
cellular retinol binding-protein(s) draw atROH from
sRBP through the membrane. A third hypothesis would
have a sRBP receptor mainly in eye, the major site of
vitamin A consumption. The sRBP null mouse seems
phenotypically normal, except for impaired vision after
weaning. Feeding a vitamin A-adequate diet for months
restores vision. Although the eye relies on sRBP for
efficient atROH uptake, atROH obtained from post-
prandial lipoprotein delivery can substitute, at least
under laboratory conditions. Interestingly, atRA serum
levels increase in the sRBP null mouse, indicating that
serum delivery of atRA to tissues helps compensate for
impaired atROH delivery.
Binding-proteins channel retinoid intermediates
through the series of reactions that constitute the visual
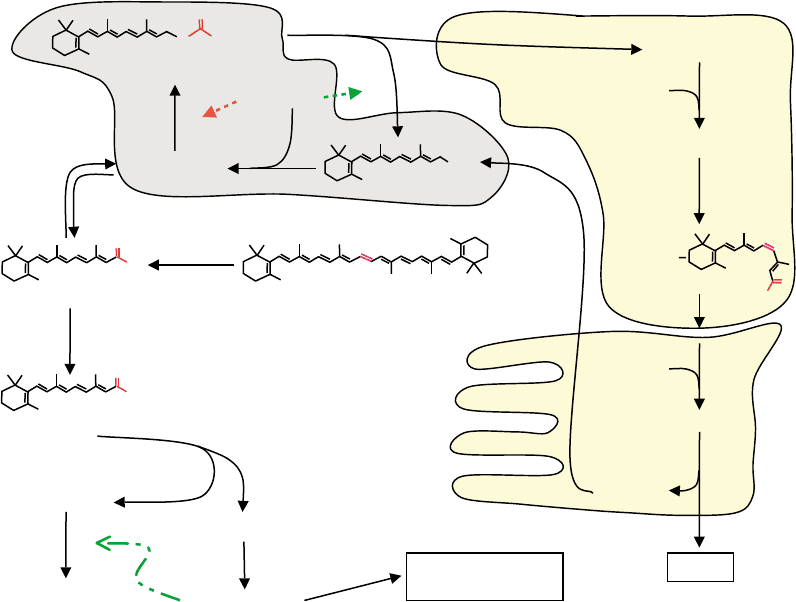
cycle (Figure 1). Mice null in the retinal pigment protein
(RPE) RPE65, for example, cannot produce 11-cis-
retinoids, consistent with an RPE65 function of binding
the hydrophobic atRE (K
d
value , 20 pM), and accel-
erating their mobilization and delivery to the next step,
acyl hydrolysis and C11 isomerization by the IMH.
RPE65 belongs to the same gene family as the
carotenoid-metabolizing enzyme CMO (the mouse
proteins have only 37% amino acid identity, however),
suggesting a gene family devoted to transport/meta-
bolism of hydrophobic substances. The IMH product,
11-cis-retinol, undergoes sequestration in the RPE by the
36 kDa cytosolic cellular retinal binding-protein
(CRALBP), a member of the gene family that includes
the
a
-tocopherol transfer protein, TTP. CRALBP facili-
tates dehydrogenation of 11-cis-retinol into 11-cis-
retinal, and drives forward the trans to cis isomerization.
VITAMIN A (RETINOIDS) 355
Mutations in human CRALBP cause night blindness and
photoreceptor degeneration. RDH4 and RDH5, respect-
ively, represent the murine and human 11-cis-retinol
dehydrogenase, which interacts with and/or accepts
substrate from CRALBP. Mutations in RDH5 are
associated with the rare, autosomal recessive disease
fundus albipunctatus, i.e. night blindness from delayed
photopigment regeneration. Lack of total blindness in
the case of the RDH5 mutation, indicates that SDR in
addition to RDH4/5 contribute to 11-cis-retinal biosyn-
thesis. 11-Cis-retinal tranverses the interphotoreceptor
matrix by an unknown mechanism and covalently binds
with opsin to form rhodopsin in the rod outer segment
(ROS). After the action of light, an ATP-dependent
transporter, ABCR, facilitates release of all-trans-retinal
from rhodopsin. prRDH then reduces the all-trans-
retinal into atROH, which then travels back through the
interphotoreceptor space to the RPE.
Several additional cellular retinoid binding-proteins
occur. The four understood to be the most important,
other than those mentioned above with respect to the
visual cycle, include CRBP, CRBP(II), CRABP, and
CRABP(II) (Table I). All vertebrates express all four,
with well-conserved amino acid sequences among
orthologs. All four are high-affinity, soluble, and specific
for their ligands. CRBP binds atROH, and closely
related compounds such as 3,4-didehydro-atROH, and
all-trans-retinal to a lesser extent, and discriminate
against cis-retinols and atRA. CRABP binds atRA,
metabolites such as 4-OH-atRA, and discriminates
against cis-RAs and atROH. These , 15 kDa globular
proteins belong to the intracellular lipid binding-protein
(iLBP) gene family, which includes the various fatty acid
binding-proteins.
The cellular retinol/retinal binding-proteins enclose
atROH and all-trans-retinal with the hydroxyl/aldehyde
function, crucial to metabolic activation, sheltered
inside. These two proteins apparently confer selective
advantage to vertebrates by enhancing efficiency of
sequestering, transporting and storing vitamin A, and
limiting its catabolism. Vitamin A absorption and
biosynthesis in the intestine relies on CRBP(II). CRBP(II)
systemic, humoral
vitamin A actions
atRA
CRBP-atROH
LRAT
(–)
O
(CH
2
)
n
CH
3
O
all-
trans
-retinyl ester
OH
atROH
CMO
b-carotene
15
15′
RRD RDH1
4
18
9
13
O
OH
all-
trans
-retinal
RALDH
O
H
RXR-RAR
CRABP(II)
CRABP-atRA
catabolites
CYP
(+)
CRABP(II)-atRA
RXR-RAR-atRA
CRABP
IMH
CRALBP
RPE65
RPE65-atRE
CRALBP-11-
cis
-ROH
RDH5
11-
cis
-retinal
O
H
11
CRALBP
vision
opsin
rhodopsin
atRCHO
ABCR
prRDH
apo-CRBP
(+)
REH
ROS
RPE
FIGURE 1 Major paths of retinoid metabolism. The white background designates reactions of retinol homeostasis and activation into atRA in
the liver and extra-hepatic vitamin A target-tissues. The light gray background depicts reactions that occur both in extra-ocular tissues and in the
retinal pigment epithelium (RPE) of the neural retina. The light yellow background designates reactions that occur in the RPE and in the rod outer
segments (ROS). Numbers indicate the positions of trans to cis isomerization (C11), the positions of hydroxylations that occur during catabolism of
atRA (C4 and C18), and the positions of atRA isomers of physiological interest (C9, C13). The dotted lines indicate the direct actions of apo-CRBP
in regulating atRE hydrolysis and atROH esterification. The dashed-dotted line indicates transcriptional induction of cytochromes P-450 (CYP) by
atRA. For simplicity, CRBP(II) is not shown.
356 VITAMIN A (RETINOIDS)

null mice pups die within 24 hours after birth, when
delivered by dams fed a diet marginal in vitamin A
content. CRBP(II) contributes , 1% of the soluble
protein to the intestinal enterocyte—an indication of a
mass-action function to sequester newly synthesized
(from carotenoids) all-trans-retinal or newly absorbed
dietary atROH. In vitro, all-trans-retinal bound with
CRBP(II) undergoes reduction readily, but neither it nor
bound atROH undergoes dehydrogenation. This would
limit production of atRA, and other metabolism, from
the bolus of all-trans-retinal produced during carotenoid
metabolism. In the intestine, the esterifying enzyme
LRAT recognizes atROH bound with CRBP(II) to
produce atRE for incorporation into chylomicrons.
Thus, CRBP(II) likely aids atROH uptake and chaper-
ones the products of carotenoid metabolism down the
pathway to atRE to enhance efficiency of retinoid
recovery from the diet.
Extra-intestinal vitamin A uptake and storage relies
on CRBP. CRBP-null mice seem morphologically nor-
mal, but eliminate atRE 6-fold faster than wild-type
mice, and may sequester/esterify atROH less efficiently.
Clearly, efficient use of atROH in vivo depends on the
chaperone. Strikingly, CRBP has a K
d
value for atROH
far lower than the atROH concentration in tissues
(1–30 mM), and CRBP concentrations exceed atROH
concentrations, where measured. The law of mass action
predicts from these data that non-esterified atROH
would occur nearly exclusively in the CRBP-bound
state, assuming no alternative high-affinity or very high-
capacity acceptors. Alternatives to CRBP exert limited
influence in vivo, because isolation of CRBP from
animal tissues by traditional (time-consuming and
containing membranes, lipids and lipid vesicles) bio-
chemical techniques (tissue homogenization, centrifu-
gation and several types of column chromatography)
produces largely holo-protein. Evidently, the capacity of
membranes and other potential acceptors to sequester
atROH does not overwhelm the ability of CRBP to
sequester atROH. Like CRBP(II), LRAT can access
atROH bound with CRBP to produce atRE. Ultimately,
liver stellate cells accumulate most of the atRE.
CRBP seems necessary for retinoid transfer from
hepatocytes to stellate cells, because the CRBP null
mouse does not accumulate atRE in stellate cells.
atROH sequestering within CRBP, the need to store
retinol as esters, and the need for atRA biosynthesis
during specific times at specific loci, suggest that transfer
of atROH for metabolism might depend on relation-
ships between metabolizing enzymes and CRBP. Like
CRBP(II), CRBP allows esterification of bound atROH,
but in contrast to CRBP(II), CRBP also allows dehy-
drogenation of atROH. The CRBP–atROH complex
shows Michaelis–Menton relationships with atRE
formation by LRAT and atROH dehydrogenation by
RDH. This relationship with the microsomal RDH is
maintained even with changes in the CRBP/atROH ratio
(provided the CRBP concentration exceeds the atROH
concentration). This eliminates a “free diffusion”
mechanism of transfer from the complex to select
enzymes. Specific crosslinking of holo-CRBP with both
RDH and LRAT confirms close proximity of CRBP and
these two enzymes. Additionally, a single mutation in an
exterior residue of CRBP (L35A) reduces the Vm of
atROH dehydrogenation by microsomes, but does not
alter the K
m
, or the K
d
of atROH binding to CRBP,
consistent with conservation of exterior residues that aid
transfer of atROH from CRBP to enzymes. Obviously,
atRE and atRA biosynthesis in vivo occurs in the
absence of CRBP, as indicated by the lack of morpho-
logical pathology in the CRBP null mouse, and their
ability to sequester esters. This was predicted by the
experiments in vitro, which showed that neither RDH
nor LRAT require presentation of atROH by CRBP. Not
surprisingly, the enzymes’ active sites recognize their
substrates in the absence of CRBP. CRBP operates as a
chaperone, which restricts atROH metabolism to select
enzymes, and seems required only for efficient atROH
use in vivo. The ability of LRAT and RDH to access
retinol from CRBP addresses the issue of how atROH
would undergo efficient metabolism in the face of
limited diffusion from the binding protein.
RALDH catalyze the irreversible conversion of
atRCHO into atRA in the presence of CRBP, and also
TABLE I
Major Extra-ocular Retinoid Binding-Proteins
Retinoid binding-protein Ligand(s) K
d
(nM) Post-embryonic distribution
CRBP (cellular retinol binding-protein, type I) atROH , 0.1 Nearly ubiquitous (low in intestine)
All-trans-retinal 10–50
CRBP(II) (cellular retinol binding-protein, type II) atROH 10–50 Intestine, neonatal liver
All-trans-retinal 10–50
CRABP (cellular retinoic acid binding-protein, type I) atRA 0.4 Widespread
CRABP(II) (cellular retinoic acid binding-protein, type II) atRA 2 Limited (inducible?) (skin, uterus, ovary)
VITAMIN A (RETINOIDS) 357
can use atRCHO generated in situ from CRBP-atROH
and RDH, or in cells presented with atROH and
transfected with RDH and RALDH. In the rat,
RALDH1 and RALDH2 have differing but overlaping
expression patterns, and respond differently to changes
in atROH status. This suggests a purpose for more than
one—precise control over atROH use and atRA
generation. The RALDH1 null mouse remains fertile
and healthy, but may have decreased ability to produce
atRA in the liver. The RALDH2 null mouse dies in utero
at midgestation, demonstrating its unique contribution
to atRA biosynthesis during embryogenesis. The situ-
ation may differ in the adult, as testes express RALDH2
strongly, but RALDH1 prevails outside of the testis. The
RALDH3 null mouse dies during suckling from an
obstruction in the nose. Apparently, RALDH can
compensate for each other after critical developmental
milestones.
A CRBP(III) has been detected in mouse heart and
skeletal muscle, which express little or no CRBP or
CRBP(II), but has not been detected in other retinoid
target tissues, such as liver, kidney, brain, etc. CRBP(III)
seems to bind about equally well with atROH, 9-cis-
retinol and 13-cis-retinol, but with much lower affinity
that either CRBP or CRBP(II) (K
d
, 80–110 nM).
Humans express yet another CRBP, originally referred
to as CRBP(III), but distinct from mouse CRBP(III), and
therefore CRBP(IV). CRBP(IV) mRNA is much more
abundant in human liver and intestine than CRBP
mRNA, but the mouse does not encode a complete
CRBP(IV) gene. CRBP(IV) binds atROH with a K
d
value
of , 60 nM, and does not bind cis-isomers. The precise
functions of CRBP(III) and CRBP(IV) have not been
clarified, and unlike CRBP and CRABP, their endogen-
ous ligands have not been established.
The atRA binding-proteins, CRABP and CRABP(II),
do not have well-defined functions. Doubly null mice
have a , 4-fold higher rate of death from unknown
causes by 6 weeks after birth than wild-type, but the
survivors appear normal, with one exception. The
doubly null mouse, as well as the CRABP(II)-only null
mouse, show 83% and 45%, respectively, incidence of
a small outgrowth anomaly on the post-axial side of
digit five, predominantly in the forelimbs. Mice doubly
null in CRABP and CRABP(II) do not exhibit enhanced
sensitivity to exogenous atRA, suggesting that the
binding-proteins do not protect against atRA toxicity.
In contrast to CRBP, both CRABP and CRABP(II)
allow projection of the
b
-ionone ring of their ligand.
Significantly, the first reactions of atRA degradation
occur at these comparatively accessible sites, i.e. C4
and C18. Presenting atRA to microsomes bound
with CRABP enhances kinetic efficiency (K
cat
/K
m
)of
catabolism 7-fold. There seems to be little doubt
that CRABP sequesters atRA: delivering the seques-
tered atRA for efficient catabolism seems a logical
mechanism to discharge the ligand without releasing it
back into the cell. Unfortunately, this insight doesn’t
reveal the primary purpose for CRABP impounding
atRA in the first place, although CRABP tends to
express in cells different from those that express CRBP
and CRABP(II). CRABP(II), but not CRABP, seems to
deliver atRA to RAR, via a transfer that does not
require free diffusion. This would complete the
chaperoning of atROH on its journey from atRE
through atRA biogenesis to nuclear localization.
Many other enzymes, including medium-change
alcohol dehydrogenases, and aldo-keto reductases,
metabolize retinoids in vitro, which seems to confirm
the need for evolution of CRBP to protect the sparse and
valuable vitamin A from clearance as a “xenobiotic”.
These enzymes do not access atROH bound with CRBP.
Neither the ADHI-null deermouse, a natural mutant,
nor mice made null in ADHI, ADHIV, or ADHIII or
doubly null in ADHI and ADHIV, present with vitamin
A deficiency symptoms, revealing no inability to activate
atROH. ADHI-null mice do show decreased ability to
convert a very large dose (50 mg kg
21
) of atROH into
atRA. Such a dose has no natural equivalent: the results
indicate only that determination can overwhelm physio-
logical chaperones.
Other Naturally
Occurring Retinoids
Discrete loci, such as skin and the chick limb bud,
synthesize 3,4-didehydro-atRA. 3,4-Didehydro-atRA
binds retinoic acid receptors with affinity similar to that
of atRA. The purpose has not been clarified of creating a
signaling molecule that functions as atRA in specialized
loci that also biosynthesize atRA. Although 9-cis-RA
was reported as an activated retinoid, it is virtually
undetectable in vivo: its putative function as a physio-
logical ligand that controls RXR remains uncertain.
Control of Vitamin A Homeostasis
The primary regulators of retinoid homeostasis appear
to be apo-CRBP and atRA. apo-CRBP inhibits LRAT
and stimulates retinyl ester hydrolysis. Thus, the
direction of flux into or out of atRE would reflect
the ratio apo-CRBP/holo-CRBP, which would reflect the
atROH status of the cell. atRA may serve as a signal to
liver to release atROH into the serum. Little has been
revealed about humoral regulation of atRA biosynthesis,
but estrogen and PGE, increase and decrease, respect-
ively, atRA biosynthesis in cultured cells. The meta-
bolism of atRA limits its activity; conversely, inhibitors
of atRA metabolism enhance atRA potency. atRA
358
VITAMIN A (RETINOIDS)
induces its own oxidative metabolism into 5,6-epoxy-
atRA, 18-hydroxy-atRA, and 4-hydroxy-atRA, through
inducing cytochrome P-450 s (CYP). CYP26A1 and
CYP2C39 appear to have major functions in the
catalysis of atRA catabolism. CYP26A1 null mice die
in mid to late gestation with serious morphological
defects. Two other CYP, 26B1 and 26C1, also catabolize
atRA, but their precise function has not been clarified.
Many other CYP reportedly catabolize atRA (e.g.
CYP1A1/2, CYP2A6, CYP2C8/9, CYP2E1,
CYPP3A4/5), but atRA does not induce these isoforms
and most have inefficient kinetics with atRA in vitro.
PPAR
b
induces transcription of CMO, CRBP,
CRBP(II), LRAT, and RAR
b
, suggesting correlation
between vitamin A homeostasis and general lipid
metabolism, whereas LRAT and CRBP are among the
numerous genes induced by atRA. This action of atRA
may represent a “housekeeping” function, rather than
acute control over retinoid homeostasis.
Several xenobiotics, including ethanol and poly-
chlorinated aromatic hydrocarbons reduce atRE stores,
possibly through enhancing atRA catabolism by indu-
cing CYP: the polychlorinated hydrocarbons, such as
dioxin, function through the AH receptor to decrease
atRE stores.
Clinical Uses of Retinoids
Numerous studies have correlated vitamin A insuffi-
ciency in laboratory animals with increased incidence
of spontaneous and carcinogen-induced cancer. Chemo-
preventive trials in humans show some promise for
retinoids in actinic keratoses, oral premalignant lesions,
laryngeal leukoplakia, and cervical dysplasia. The FDA
has approved retinoids for acute promyelocytic
leukemia and in non-life-threatening diseases such
as cystic acne and psoriasis. Retinoids also provide
the active ingredients in agents to treat sun-/age-
damaged skin.
The WHO recognizes vitamin A-deficiency as a
mortality factor for childhood measles. Two large
doses (60,000 REQ each) of a water-soluble vitamin A
formulation given to children on each of two days
decreases the risk of death from measles 81% in areas of
prevalent vitamin A-deficiency.
SEE ALSO THE FOLLOWING ARTICLES
Retinoic Acid Receptors † Steroid/Thyroid Hormone
Receptors
GLOSSARY
K
cat
/K
m
A measure of the efficiency of an enzyme for its substrate
from dividing the rate of turnover of the substrate by the enzyme by
the Michaelis constant.
K
d
(equilibrium dissociation constant) A measure of the affinity of a
protein for its ligand. Lower numbers indicate higher affinity.
retinoid binding-protein Proteins that bind specific retinoids with
high affinity, several with K
d
values , 10 nM.
short-chain dehydrogenase/reductase (SDR) A gene family consisting
of , 50 mammalian members in the range of 25–35 kDa that uses
pyridine nucleotide cofactors to dehydrogenate or reduce steroids,
retinoids, prostanoids, and intermediates in lipid metabolism.
FURTHER READING
Blomhoff, R., Green, M. H., Green, J. B., Berg, T., and Norum, K. R.
(1991). Vitamin A metabolism: new perspectives on absorption,
transport, and storage. Physiological Reviews 71, 951– 990.
Harrison, E. H. (1998). Lipases and carboxyesterases: possible roles in
the hepatic metabolism of retinol. Annual Reviews of Nutrition 18,
259–276.
Maden, M. (2001). Role of retinoic acid in embryonic and post-
embryonic development. Proceedings of the Nutrition Society
59, 65–73.
Napoli, J. L. (2000). Retinoic acid: its biosynthesis and metabolism.
Progress in Nucleic Acids Research 63, 139–188.
Newcomer, M. E. (1995). Retinoid-binding proteins: structural
determinants important for function. FASEB Journal 9, 229–239.
Saari, J. C. (2000). Biochemistry of visual pigment regeneration: the
Friedenwald lecture. Investigative Ophthalmology and Visual
Science 41, 337–348.
Stephensen, C. B. (2001). Vitamin A, infection, and immune function.
Annual Reviews of Nutrition 21, 167–192.
Sun, S. Y., and Lotan, R. (2002). Retinoids and their receptors in cancer
development and chemoprevention. Critical Reviews in Oncology
and Hematology 41, 41–55.
Wolf, G. (1984). Multiple functions of vitamin A. Physiological
Reviews 64, 873–937.
BIOGRAPHY
Joseph L. Napoli is a Professor in the Department of Nutrition Sciences
and Toxicology and a biochemist in the experimental station at UC-
Berkeley. His main research interests are in the metabolism and
functions of retinoids. He received his Ph.D. from the University of
Michigan-Ann Arbor in medicinal chemistry and was a post-doctoral
fellow in biochemistry at the University of Wisconsin-Madison.
VITAMIN A (RETINOIDS) 359

Vitamin B
12
and B
12
-Proteins
Bernhard Kra
¨
utler
University of Innsbruck, Innsbruck, Austria
Vitamin B
12
(CNCbl), the antipernicious anemia factor, is
required for human and animal metabolism and was dis-
covered in the late 1940s. The B
12
-derivatives belong to the
tetrapyrrolic natural compounds and are cobalt complexes of
the unique and remarkably complex corrin ligand. The B
12
-
coenzymes are the cofactors in important organometallic
enzymatic reactions and are particularly relevant in the
metabolism of anaerobes. Indeed, the microorganisms are the
only natural sources of the B
12
-derivatives, whereas most
living organisms (except for the higher plants) depend on these
cobalt corrinoids. Vitamin B
12
and its derivatives thus hold an
important position in the life sciences and have attracted strong
interest from medicine, biology, chemistry, and physics.
B
12
: Structure and Reactivity
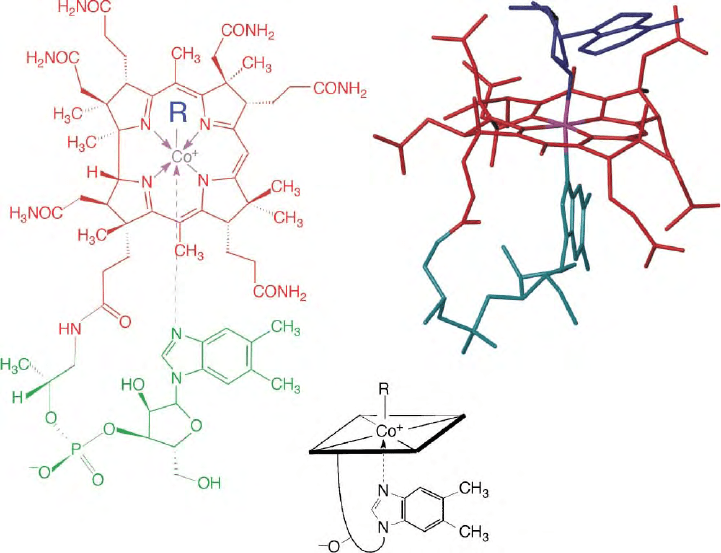
The red, cyanide-containing cobalt-complex vitamin B
12
(cyanocobalamin, CNCbl) is a relatively inert and
physiologically rather inactive Co(III)-corrin. The bio-
logically relevant B
12
-derivatives are the light sensitive
and chemically more labile organometallic coenzyme
forms, coenzyme B
12
(5
0
-deoxy-5
0
-adenosylcobalamin,
AdoCbl), and methylcobalamin (MeCbl, see Figure 1).
STRUCTURE OF VITAMIN
B
12
-DERIVATIVES
The structures of vitamin B
12
(CNCbl) and of coenzyme
B
12
(AdoCbl) were established by X-ray crystallographic
studies from the laboratory of D. C. Hodgkin. This work
helped clarify the nature of the corrin ligand and to
discover the organometallic nature of the coenzyme
AdoCbl (see Figure 1). CNCbl and other cobalamins, in
which the cyanide ligand is replaced by another “upper”
b
-ligand (see Figure 1), represent the most common of
the nucleotide containing (i.e., “complete”) B
12
-deriva-
tives. The crystal structures of various (organometallic)
Co(III)-corrins were analyzed, including MeCbl, to
study the axial bonding at the corrin-bound cobalt
center and the inherent “non-planar” nature of the
corrin ligand (as opposed to the porphyrin ligand), as
well as possible implications of this for B
12
-catalyzed
enzymatic reactions. An interesting structure is that of
the oxygen-sensitive Co(II)-corrin cob(II)alamin (B
12r
),
the corrinoid moiety resulting from (Co–C)-bond
homolysis of AdoCbl during the catalytic cycle of
coenzyme B
12
-dependent enzymes.
UV/vis- and circular dichroism (CD)-spectroscopy
have been used to study the colored and chiral B
12
-
derivatives in solution. Nuclear magnetic resonance
(NMR) spectroscopy and mass spectrometry helped to
identify the corrinoids from anaerobes (methanogens,
sulfur metabolizing, and acetogenic bacteria) and to
characterize their solution structures. The Co(II)-forms,
in turn, have been investigated by electron spin
resonance (ESR) spectroscopy, a technique used increas-
ingly to analyze for paramagnetic intermediates in
B
12
-catalyzed enzymatic reactions.
The natural B
12
-derivatives are either “complete” or
“incomplete” corrinoids (which lack the nucleotide
function, see Figure 1). The natural “complete” corri-
noids carry different functional
b
-axial ligands. In
addition they may vary by the constitution of the
“nucleotide base,” a 5,6-dimethylbenzimidazole (DMB)
in the cobalamins (such as CNCbl), but an adenine in
pseudovitamin B
12
. The “complete” corrinoids are also
unique due to the unusual
a
-configuration of their
(pseudo-)nucleotide appendage. The specific build-up of
this function enables the heterocyclic base to bind in an
intramolecular fashion to the “lower”
a
-axial coordi-
nation site of the corrin-bound cobalt center. In this way,
the nucleotide function steers the organometallic reac-
tivity at the cobalt center of “complete” B
12
-derivatives
and is also relevant for recognition and tight binding by
the B
12
-binding proteins.
B
12
-DERIVATIVES IN ELECTRON
TRANSFER REACTIONS
Oxidation– reduction processes are of key importance
for the chemistry and biology of B
12
. Under physiologi-
cal conditions B
12
-derivatives may exist in three
different oxidation states (Co(III), Co(II), or Co(I)),
each possessing different coordination properties and
reactivities: Axial coordination to the corrin-bound
cobalt center depends primarily on the formal oxidation
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 360
state of the cobalt ion. As a rule, the number of axial
ligands decreases in parallel with the cobalt oxidation
state: In the thermodynamically predominating forms of
cobalt corrins, two axial ligands are bound to the
diamagnetic Co(III)-center, one axial ligand is bound to
the paramagnetic Co(II)-center and axial ligands are
assumed to be absent at the diamagnetic Co(I)-center.
Electron transfer reactions involving B
12
-derivatives,
therefore, are accompanied by changes of the number of
axial ligands and depend upon the nature of axial
ligands. Axial coordination of the nucleotide base and of
strongly coordinating ligands stabilizes the cobalt center
against reduction and the reduction of alkyl-Co(III)-
corrins typically occurs at potentials more negative than
that of the Co(II)/Co(I)-redox-pair B
12r
/B
12s
.
ORGANOMETALLIC REACTIONS
OF
B
12
-DERIVATIVES
The reactivity of B
12
-derivatives in organometallic
reactions holds the key to much of the biological activity
of the B
12
-dependent enzymes: formation and cleavage
of the (Co–C)-bond in the B
12
-cofactors are essential
steps of the reactions catalyzed by B
12
-dependent
enzymes and are of particular interest.
In solution formation and cleavage of the (Co–C)-
bond in organometallic B
12
-derivatives were observed to
occur on all oxidation levels of the cobalt center. Two of
these reaction modes were also found to be relevant for
B
12
-dependent enzymatic reactions:
1. the homolytic mode of formation/cleavage of the
organometallic axial bond at the cobalt center (formally
a one-electron reduction/oxidation of the metal center,
see Figure 2), is of particular importance for the role of
AdoCbl as a cofactor AdoCbl is considered a “reversible
carrier of an alkyl radical” (or a reversibly functioning
“radical source”). The (Co–C)-bond of AdoCbl has
been determined to be , 30 kcal mol
21
strong and to be
affected only slightly by the nucleotide (in the “base-on”
form). The (reverse) reactions of B
12r
with alkyl
radicals (such as the 5
0
-deoxy-5
0
-adenosyl radical) are
remarkably fast. Indeed, the radicaloid B
12r
is a highly
efficient “radical trap” and its reactions with radicals
can occur with minimal restructuring of the cobalt-
corrin moiety.
2. the heterolytic, nucleophile induced (SN
2
) mode of
formation/cleavage of the (Co–C)-bond at the cobalt
center (formally a two-electron oxidation/reduction of
the metal ion) is accompanied by formation/cleavage of
a second axial bond (Figure 2): Heterolytic formation/
cleavage of the (Co–C)-bond is particularly impor-
tant in enzyme-catalyzed methyl-transfer reactions
(Figure 3). This mode is represented by the reaction of
FIGURE 1 Left: structural formulas of vitamin B
12
(R ¼ CN, CNCbl), of coenzyme B
12
(R ¼ 5
0
-deoxy-5
0
-adenosyl, AdoCbl), methylcobalamin
(R ¼ methyl, MeCbl), cob(II)alamin (R ¼ e
2
,B
12r
). Right/top: structure of coenzyme B
12
(AdoCbl). Bottom: symbols used for vitamin B
12
(R ¼ CN: CNCbl) and other cobalamins.
VITAMIN B
12
AND B
12
-PROTEINS 361
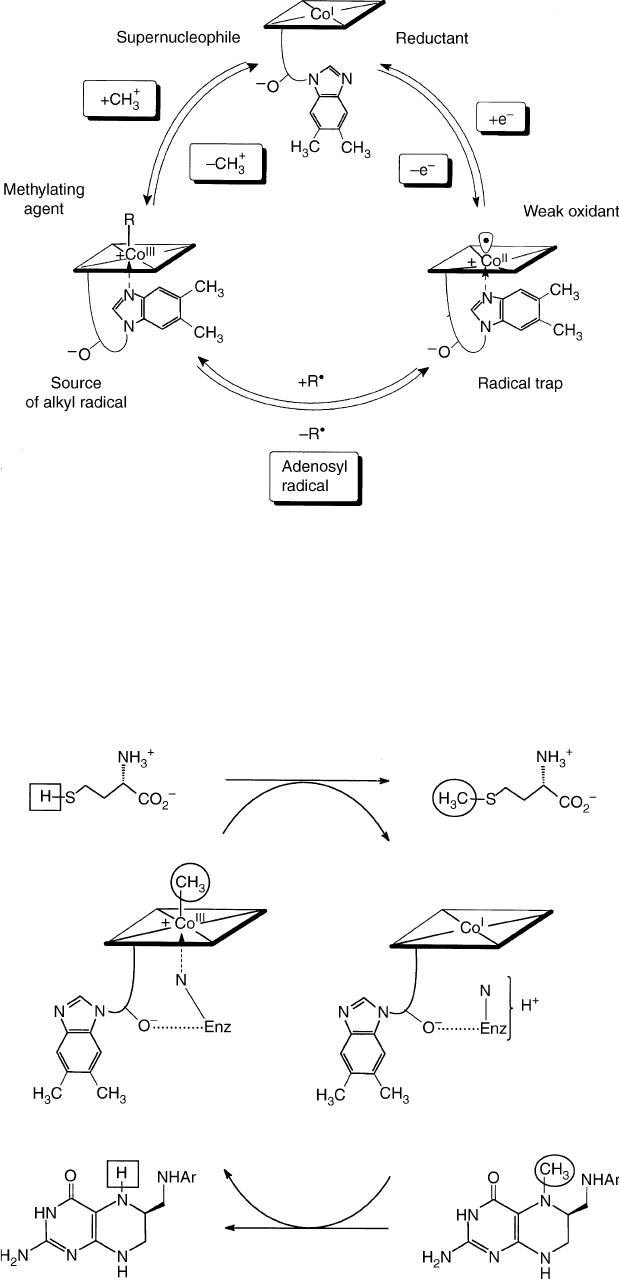
(“supernucleophilic”) Co(I)-corrins with alkylating
agents and by the nucleophile-induced demethylation
of methyl-Co(III)-corrins. Alkylation at the Co(I)-center
usually occurs via “classical” bimolecular nucleophilic
substitution (SN
2
). The intramolecular coordination of
the DMB-base in MeCbl has a notable thermodynamic
effect on this type of reaction: e.g., it stabilizes “base-
on” MeCbl by , 4 kcal mol
21
.
FIGURE 2 Elementary formal reaction steps of “complete” corrinoids characterizing their patterns of reactivity relevant for their cofactor
function in B
12
-dependent enzymes.
FIGURE 3 Biosynthesis of methionine by methylation of homocysteine is catalyzed by methionine synthase (MetH, Enz signifies the MetH-
apoenzyme), where the bound corrinoid shuttles between MeCbl, in a “base-off/His-on” form, and cob(I)alamin.
362 VITAMIN B
12
AND B
12
-PROTEINS
