Latchman. Eukariotic Transciption Factors
Подождите немного. Документ загружается.

continuous elevation of these proteins, suc h as would occur when cells
become infected with a retrovi rus expressing one of them, would result in
cells which exhibited continuous uncontrolled growth and were not subject to
normal growth regulatory signals. Since such uncontrolled growth is one of
the characteristics of cancer cells it is relatively easy to link the rol e of Fos and
Jun in inducing genes required for growth with their ability to cause cancer.
Normally, however, the transformation of a cell to a transformed cancerous
phenotype requires more than simply its conversion to a continuously grow-
ing immortal cell (for review see Land et al., 1983). Since repeated treatments
with phorbol esters can promote tumour formation in immortalized cells, the
prolonged induction of phorbol ester responsive genes by elevated levels of
Fos and Jun may therefore result in the conversion of already continuously
growing cells into the tumorigenic phenotype characteristic of cancer cells
(Fig. 9.13).
Hence the ability of Fos and Jun to cause cancer represents an aspect of
their ability to induce transcription of specific cellular genes. In agreement
with this idea, mutations in Fos which abolish its ability to dimerize with Jun
and hence prevent it from binding to AP1 sites also abolish its ability to
transform cells to a cancerous phenotype. It should be noted, however, that
302 EUKARYOTIC TRANSCRIPTION FACTORS
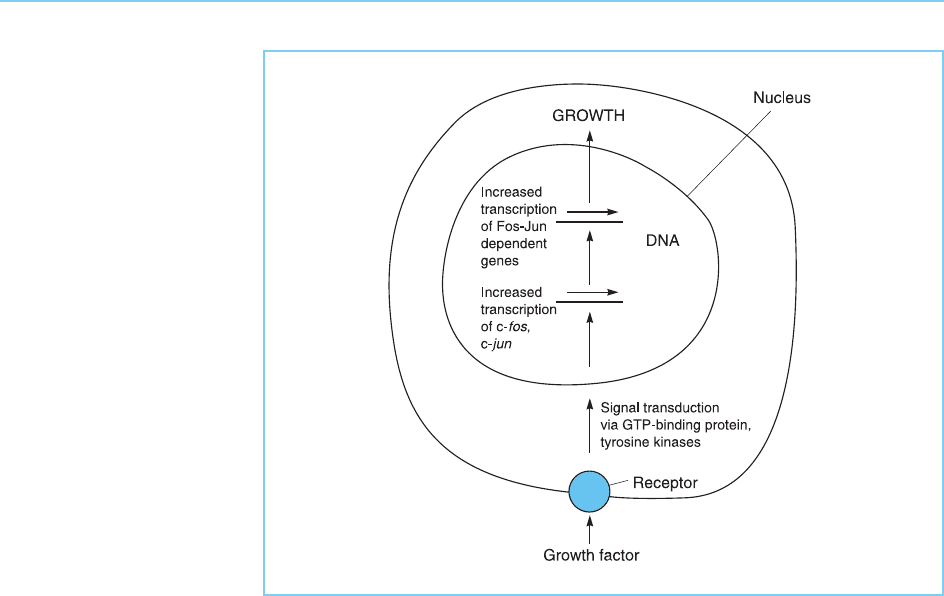
Figure 9.12
Growth factor stimulation
of cells results in
increased transcription of
the c-fos and c-jun
genes which in turn
stimulates transcription of
genes which are activated
by the Fos-Jun complex.
in addition to their over-expression within a retrovirus, there is also some
evidence that mutational changes render the viral proteins more potent tran-
scriptional activators than the equivalent cellular proteins. Thus the v-Jun
protein appears to activate transcri ption more efficiently than c-Jun due to
a deletion in a region which is involved in targeting the c-Jun protein for
degradation (Treier et al., 1994) and which also mediates its interaction
with a negatively acting cellular factor (Baichwal and Tjian, 1990).
Interestingly, in addition to its central role in the growth response, the Fos,
Jun, AP1 system also appears to represent a target for other oncogenes. Thus,
for example, the ets oncogene which, like fos and jun encodes a cellular tran-
scription factor, acts via a DNA binding site known as PEA3 which is located
adjacent to the AP1 site in a number of TPA-responsive genes such as col-
lagenase and stromelysin. Moreover, the Ets protein cooperates with Fos and
Jun to produce high level activation of these promoters (Wasylyk et al., 1990).
In addition to interacting positively with other factors, the Fos/Jun com-
plex can also inhibit the action of other transcription factors. Thus, as
described in Chapter 6 (section 6.5), the Fos/Jun complex requires the CBP
co-activator in order to activate transcription. It therefore competes with the
TRANSCRIPTION FACTORS AND HUMAN DISEASE 303
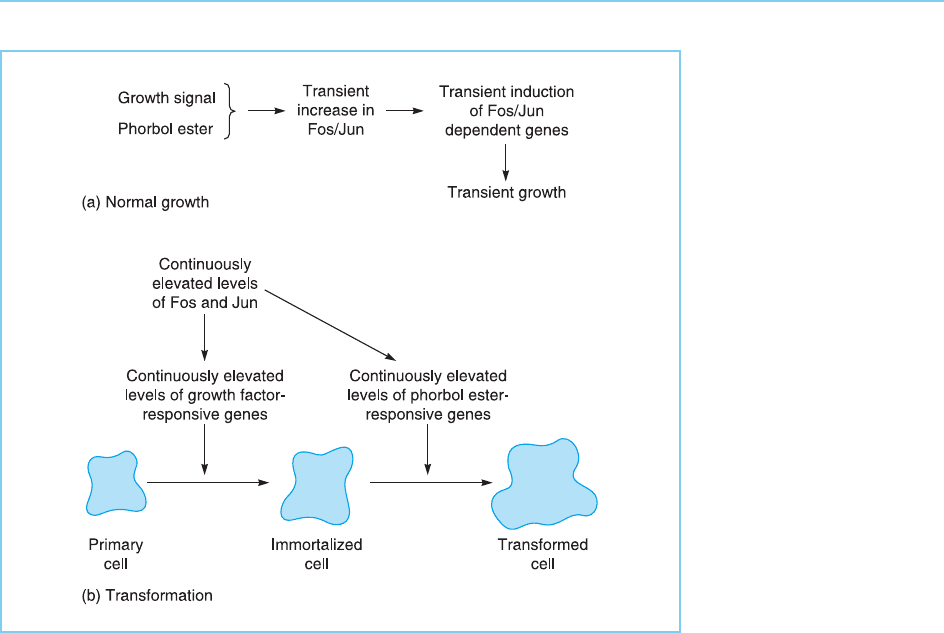
Figure 9.13
Effects of Fos and Jun
on cellular growth. In
normal cells (a) a brief
exposure to a growth
signal or phorbol ester
will lead to a brief period
of growth via the
transient induction of Fos
and Jun and hence of
Fos/Jun-dependent
genes. In contrast the
continuous elevation of
Fos and Jun produced
for example by infection
with a retrovirus
expressing Fos or Jun
results in continuous
unlimited growth and
cellular transformation
(b).
activated glucocorticoid receptor for CBP hence preventing the receptor from
activating transcription. Similarly both Fos and Jun can inhibit the activation
of muscle specific promoters by the MyoD transcription factor (see Chap ter 7,
section 7.2.1) thereby preventing cells from differentiating into non-dividing
muscle cells and allowing cellular proliferation to continue (Li et al., 1992).
Hence the Fos and Jun oncogene products play a critical role in the regula-
tion of specific cellular genes in normal cells, interacting with the products of
other transcription factors to produce the controlled activity of their target
genes necessary for normal controlled growth.
9.3.2 v-erbA AND THE THYROID HORMONE RECEPTOR
The v-erbA oncogene is one of two oncogenes carried by avian erythroblastosis
virus (AEV). The cellular equivalent of this oncogene c-erbA, has been shown
to encode the cellular receptor for thyroid hormone (Sap et al., 1986;
Weinberger et al., 1986) which is a member of the steroid/thyroid hormone
receptor super family discussed in Chapter 4 (section 4.4). Following the
binding of thyroid hormone, the receptor/hormone complex binds to its
appropriate recognition site in the DNA of thyroid hormone responsive
genes and activates their transcription (Fig. 9.14).
304 EUKARYOTIC TRANSCRIPTION FACTORS
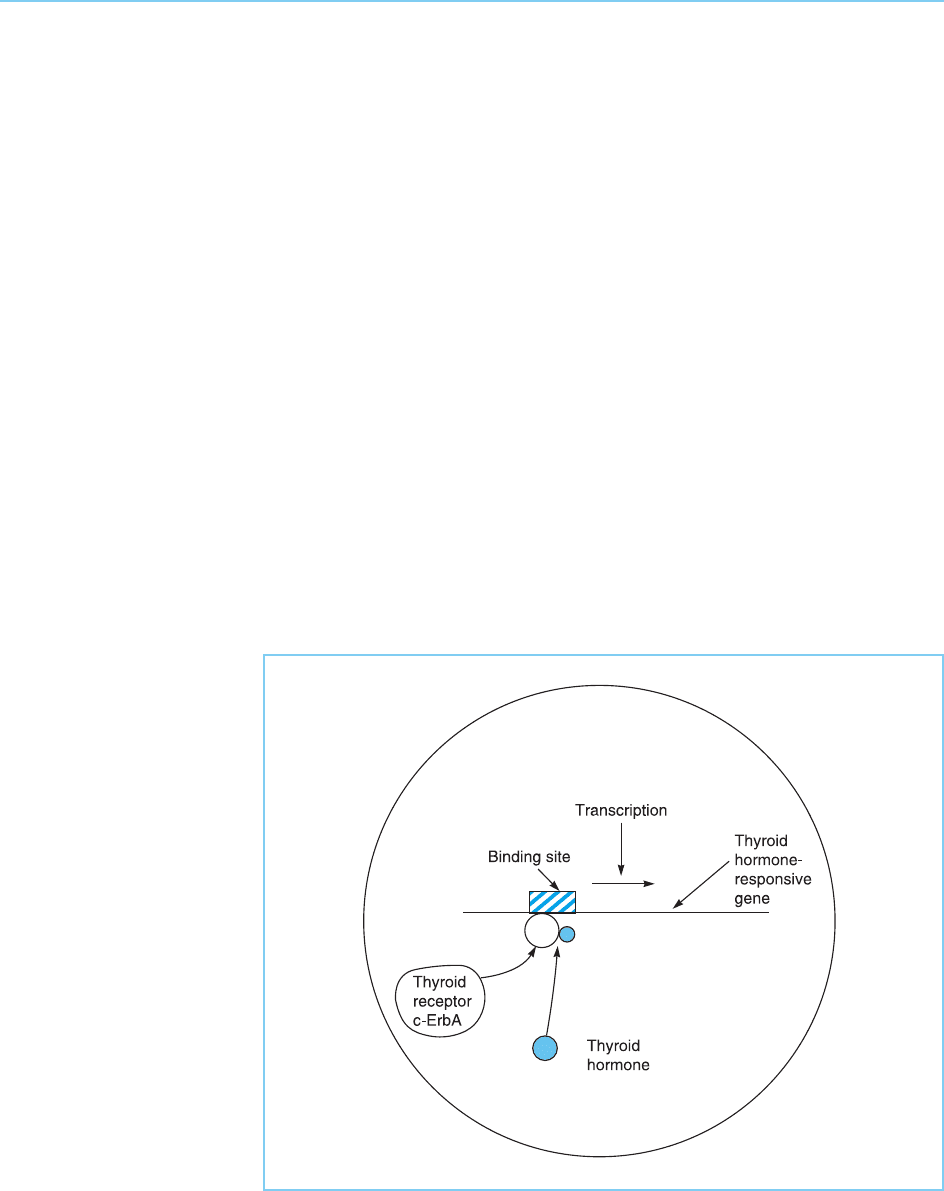
Figure 9.14
The c-erbA gene
encodes the thyroid
hormone receptor and
activates transcription in
response to thyroid
hormone.
Hence the protein encoded by the c-erbA gene represents a bona fide cellular
transcription factor involved in the acti vation of thyroid hormone responsive
genes. Unlike the case of the fos and jun gene products, which regulate genes
involved in growth, it is not immediately obvi ous how the form of thyroid
hormone receptor encoded by the viral v-erbA gene can transform cells to a
cancerous phenotype.
The solution to this problem is provided by a comparison of the cellular
ErbA protein, which is a functio nal thyroid hormone receptor, and the viral
ErbA protein encoded by AEV. Thus, in addition to being fused to the retro-
viral gag protein at its N terminus, the viral ErbA protein contains several
mutations in the reg ions of the receptor responsible for binding to DNA and
for binding thyroid hormone as well as a small deletion in the hormone
binding domain (Fig. 9.15).
Interestingly, although these changes do not abolish the ability of the viral
ErbA protein to bind to DNA, they do prevent it from binding thyroid hor-
mone and thereby becoming converted to a form which can activate transcrip-
tion (Sap et al., 1986, 1989). However, these changes do not affect the
inhibitory domain which, as discussed in Chapter 6 (section 6.3.2), allows
the thyroid hormone receptor to repress transcription. Hence, the viral v-
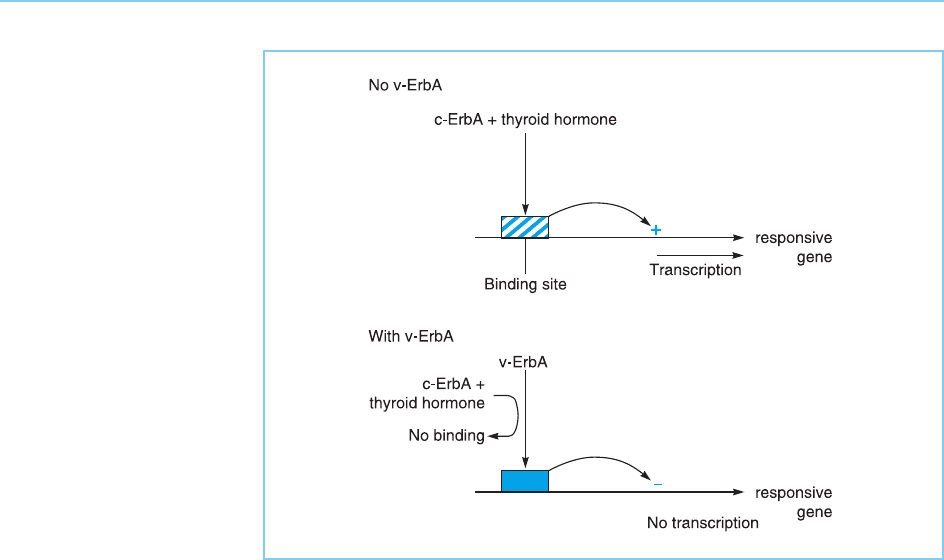
ErbA protein can inhibit the induction of thyroid hormone responsive
genes when cells are treated with thyroid hormone by binding to the thyroid
hormone response elements in their promoters and dominantly repressing
their transcription, as well as preventing binding of the activating complex of
thyroid horm one and the cellular ErbA protein (Fig. 9.16). In agreement with
this critical role for repression in producing transformation by v-ErbA, a
mutation in v-ErbA which abolishes its ability to repress transcription by pre-
venting it binding its co-repressor (see Chapter 6, section 6.3.2) also abolishes
its ability to transform cells (Perlmann and Vennstrom, 1995).
Hence the viral ErbA protein acts as a dominant repressor of thyroid
hormone responsive genes being both incapable of activating transcription
itself and able to prevent activation by intact receptor. This mechanism of
TRANSCRIPTION FACTORS AND HUMAN DISEASE 305
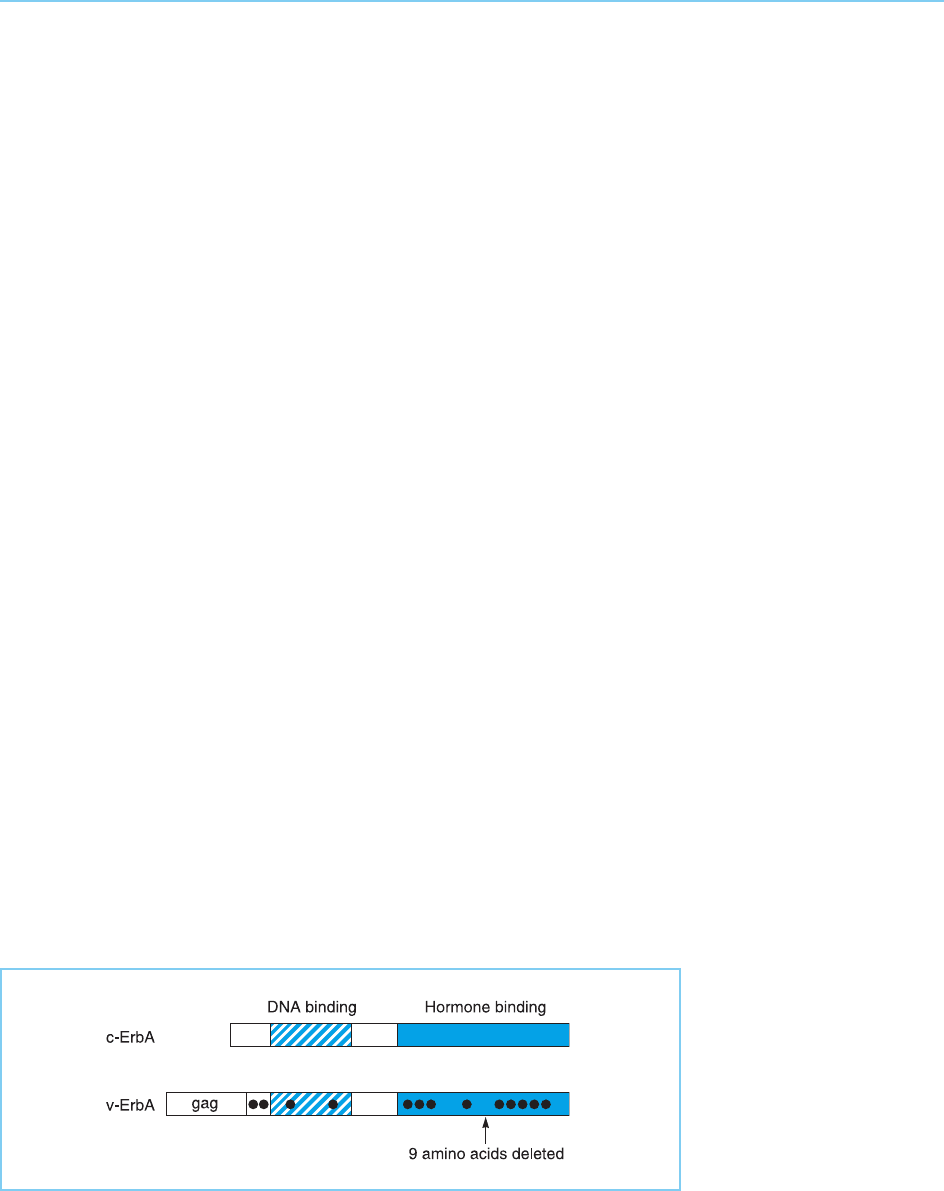
Figure 9.15
Relationship of the
cellular ErbA protein and
the viral protein. The
black dots indicate single
amino acid differences
between the two proteins
while the arrow indicates
the region where nine
amino acids are deleted
in the viral protein.
action is clearly similar to the repression of thyroid hormone responsive genes
by the naturally occurring alternatively spliced form of the thyroid hormone
receptor which, as discussed in Chapter 6 (section 6.3.2), lacks the hormone
binding domain and therefore cannot bind hormone. Thus the same mechan-
ism of gene repression by a non-hormone binding receptor is used naturally
in the cell and by an oncogenic virus.
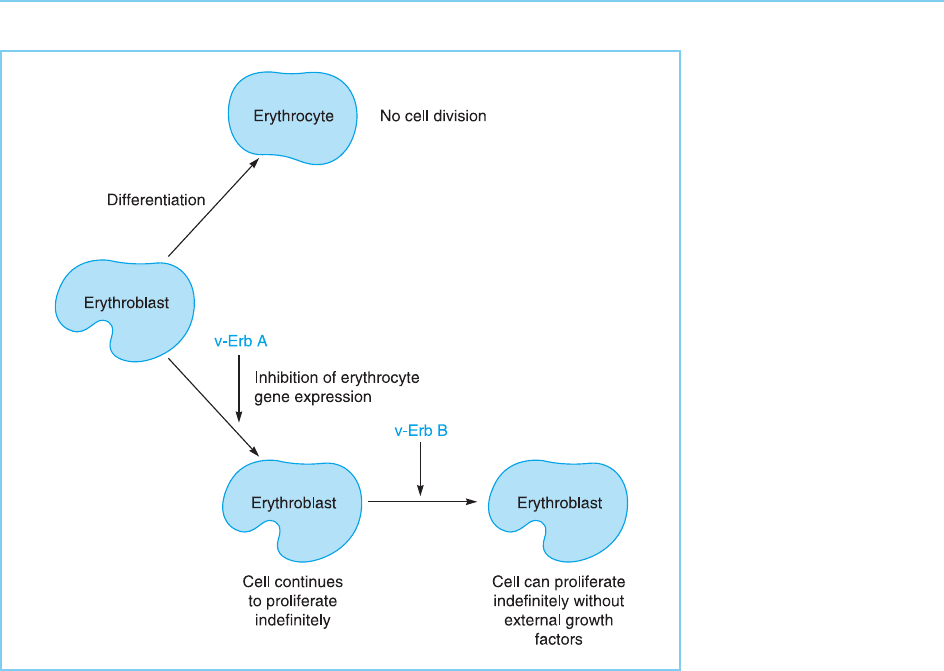
One of the targets for repression by the viral ErbA protein is the erythro-
cyte anion transporter gene (Zenke et al., 1988), which is one of the genes
normally induced when avian erythroblasts differentiate into erythrocytes.
This differentiation process has been known for some time to be inhibited
by the ErbA protein and it is now clear that it achieves this effect by blocking
the induction of the genes needed for differenti ation. In turn such inhibition
will allow continued prolifera tion of thes e cells rendering them susceptible to
transformation into a tumour cell type by the product of the other AEV
oncogene v-erbB which encodes a truncated form of the epidermal growth
factor receptor (Downward et al., 1984) and therefore renders cell growth
independent of external growth factors (Fig. 9.17).
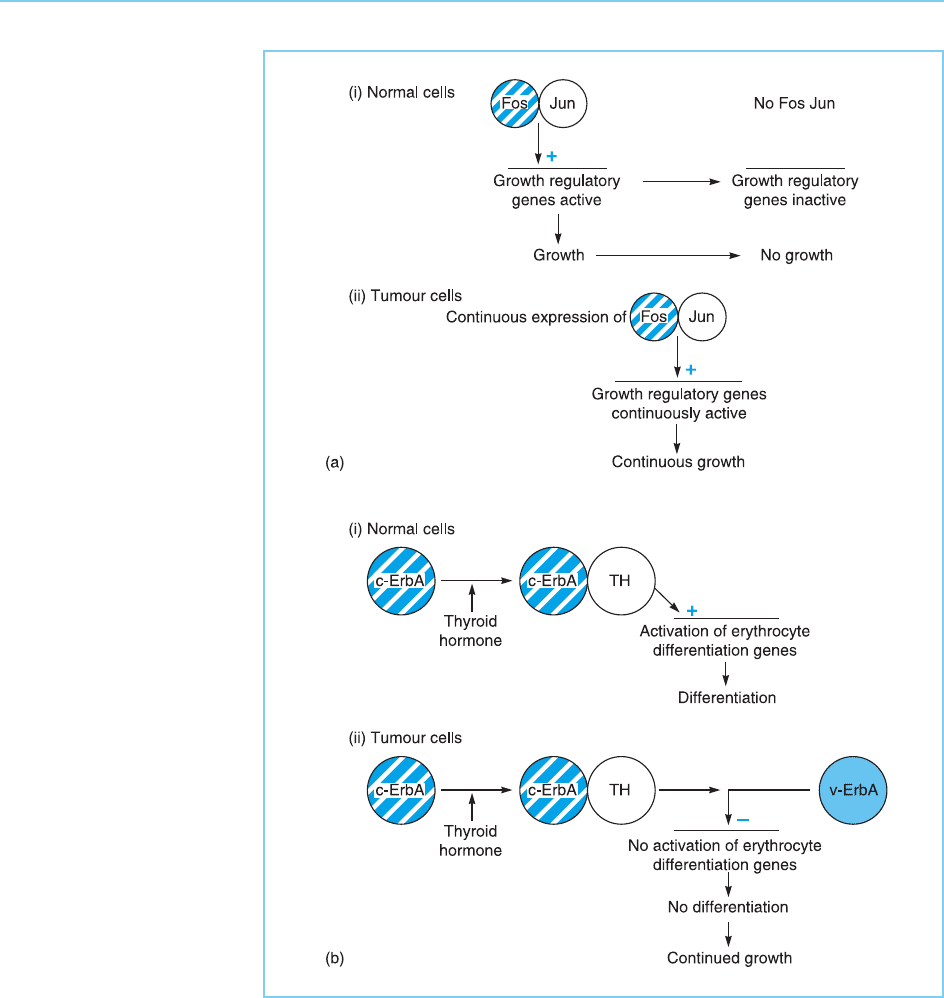
The two cases of Fos/Jun and ErbA therefore represent contrasting exam-
ples of the involvement of transcription factors in oncogenesis both in terms
of the mechanism of transformation and the manner in which the cellular
form of the oncogene becomes an active transforming gene. Thus, in the case
306 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.16
Inhibitory effect of the
viral ErbA protein on gene
activation by the cellular
protein, in response to
thyroid hormone. The viral
protein both inhibits
binding by the activated
c-ErbA protein and also
dominantly represses
transcription by means of
its inhibitory domain.
Note the similarity to the
action of the alpha-2
form of the c-ErbA
protein, illustrated in
Figure 6.14.
of Fos and Jun, transformation is achieved by the continuous activation of
genes necessary for growth in normal cell types. Moreover, it occurs, at least
in part, via the natural activity of the cellular oncogene in inducing these
genes being enhanced by their over-expression such that it occurs at an inap-
propriate time or place (Fig. 9.18a). In contrast in the ErbA case transforma-
tion is achieved by inhibiting the expression of genes whose products are
required for the differentiati on of a particular cell type therefore allowing
growth to continue. Moreover, this occurs via the activity of a mutated form
of the transcription factor which, rather than carrying out its normal function
more efficiently, actually interferes with the normal role of the thyroid hor-
mone receptor in inducing thyroid hormone responsive genes required for
differentiation (Fig. 9.18b).
9.3.3 THE myc ONCOGENE
Interestingly, for a considerable period, the techniques of molecular biology
failed in the case of the c-myc oncogene, which was one of the earliest cellular
TRANSCRIPTION FACTORS AND HUMAN DISEASE 307
Figure 9.17
Inhibition of erythrocyte-
specific gene expression
by the v-ErbA protein
prevents erythrocyte
differentiation and allows
transformation by the
v-ErbB protein.
oncogenes to be identified, with its expression being dramatically increased in
a wide variety of transformed cells (for review see Grandori et al., 2000;
Eisenman, 2001). Thus the Myc protein has a number of properties suggesting
that it is a transcription factor, notably nuclear localization, the possess ion of
several motifs characteristic of transcription factors such as the helix-loop-
308 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.18
Transformation
mechanisms of Fos/Jun
(panel a) and ErbA (panel
b). Note that Fos/Jun-
induced transformation
occurs because the
proteins induce the
continual activation of
growth regulatory genes
which are normally
expressed only transiently
while v-ErbA-induced
transformation occurs
because the protein
interferes with the action
of its cellular homologue
and hence inhibits the
induction of genes
involved in erythrocyte
differentiation.

helix and leucine zipper elements (see Chapter 4, section 4.5) and the ability
to activate target promoters in co-transfection assays. Despite exhaustive
efforts, however, no DNA sequence to which the Myc protein binds could
be defined, rendering its mechanism of action uncertain.
The solution to this problem was provided by the work of Blackwood and
Eisenman (1991) who identified a novel protein, Max, which can form hetero-
dimers with the Myc protein via the helix-loop-helix motif present in both
proteins. Myc/Max heterodimers can bind to DNA and regulate transcription,
whereas Myc/Myc homodimers cannot do so (for reviews see Grandori et al.,
2000; Baudino and Cleveland, 2001) (Fig. 9.19). This effect evidently parallels
the requirement of the Fos protein for dimerization with Jun in order to bind
with high affinity to AP1 sites (see section 9.3.1).
The Max protein therefore plays a critical role in allowing the DNA binding
of Myc and the structure of a Myc/Max heterodim er bound to DNA has
recently been defined (Nair and Burley, 2003). Moreover, the ability to inter-
act with Max, bind to DNA and modulate gene expression is critical for the
ability of the Myc protein to transform since mutations in Myc which abolish
its ability to heterodimerize with Max also abolish its transf orming ability.
Hence, as was previously speculated, the Myc protein is a transcription factor
whose over-expression causes transformation, presumably via the activation of
genes whose protein products are required for cellular growth (for reviews see
Zornig and Evan, 1996; Grandori and Eisenman, 1997; Levens, 2002).
Interestingly, the Max protein does not appear to represent a passive part-
ner whi ch merely serves to del iver Myc to the DNA of target genes. Rather it
plays a key role in regulating the activity of target gen es containing the appro-
priate bin ding site. Thus, it has been shown that, whereas Myc/Max hetero-
dimers can activate transcription, Max/Max homodimers can bind to the
same site and weakly repress transcription. Moreover, Max can also hetero-
dimerize with another member of the helix-loop-helix family, known as Mad,
to form a strong repressor of transcription (for review see Bernards, 1995)
(Fig. 9.20).
TRANSCRIPTION FACTORS AND HUMAN DISEASE 309
Figure 9.19
Both Myc/Max
heterodimers and Max/
Max homodimers can
bind to DNA whereas
Myc/Myc homodimers
cannot.
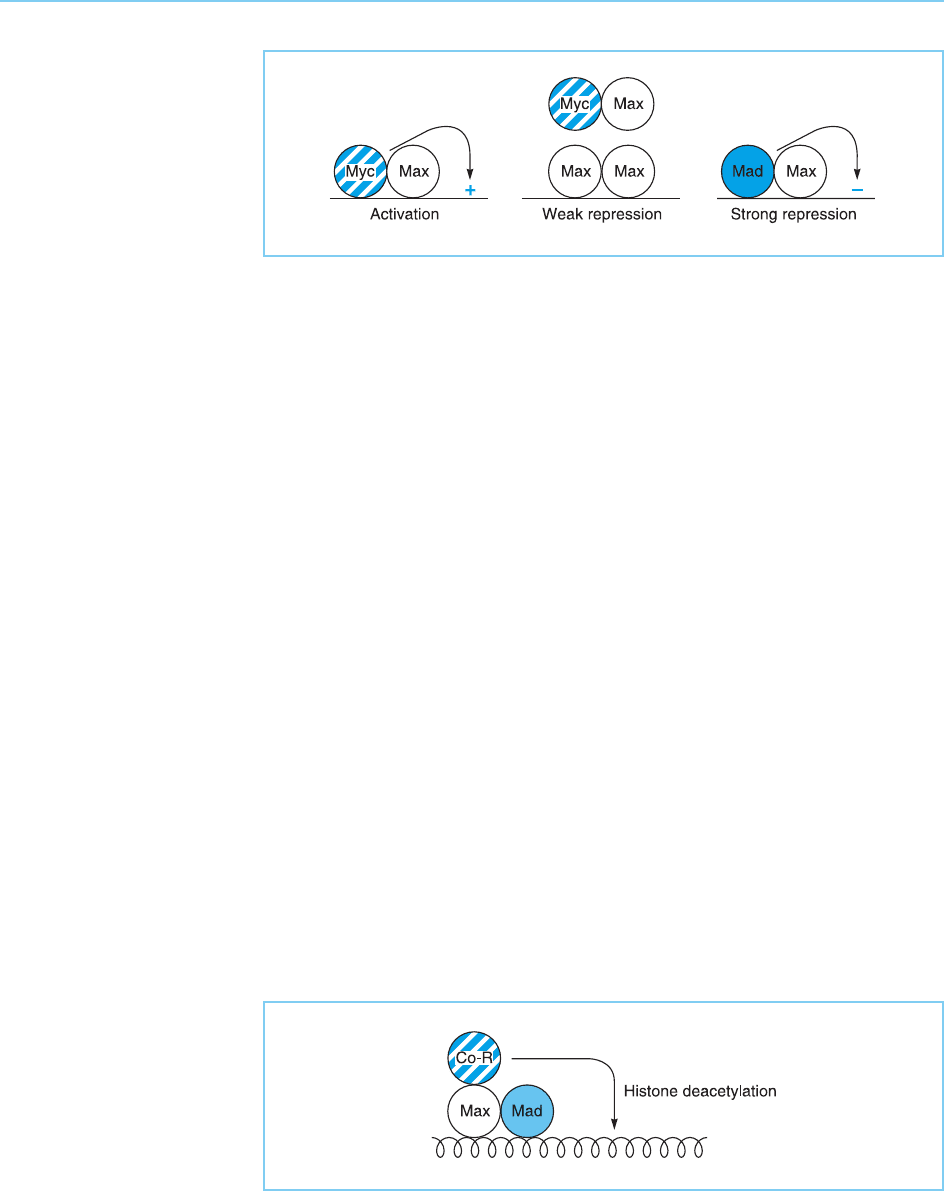
The Max/Max homodimer appears to act as a weak repressor simply by
preventing the Myc/Max activator from binding to its appropriate binding
sites and thereby preventing it from activating transcription. In contrast, the
Mad/Max heterodimer appears to act as an active repressor which is capable
of reducing transcription below that which would be observed in the absence
of any activator binding (see Chapter 6, for discussion of the mechanisms of
transcriptional repression). Thus, it has been shown that the Mad protein can
bind the same complex of N-CoR, mSIN-3 and mRPD3, which mediates active
repression by nuclear receptors suc h as the thyroid hormone receptor in the
absence of hormone (see Chapter 6, section 6.3.2) (for review see Wolffe,
1997). As this complex includes the mRPD3 protein, which has histone de-
acetylase activity, it is possible that the Mad/Max heterodimer may repress
transcription, at least in part, by recruiting a complex which deacetylates
histones thereby organizing a more tightly packed chromatin structure
(Fig. 9.21).
In the case of the nuclear receptors, the switch from the repressed state of
target genes to their activation is mediated by the addition of hormone. In
contrast, however, in the case of the My c family it is mediated by signals which
produce a rise in Myc expression and a corre sponding fall in the expression of
Mad. Thus Myc is expressed at very low levels in resting cells and its expres-
sion is induced when cells begin to grow, whereas Max is expressed at similar
high levels in both resting and proliferating cells and Mad is expressed at high
levels only in resting cells and not in proliferating cells. Hence in resting cells
310 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.20
Functional effects of
Max/Max homodimers
and of Myc/Max or Mad/
Max heterodimers. Note
that Max/Max
homodimers repress
transcription only weakly
by passively blocking
activator binding, whereas
Mad/Max heterodimers
actively repress
transcription and
therefore have a much
stronger effect.
Figure 9.21
The Max/Mad
heterodimer can recruit a
co-repressor complex
(Co-R) with histone
deacetylase activity which
can produce a more
tightly packed chromatin
structure (compare with
Fig. 6.26).
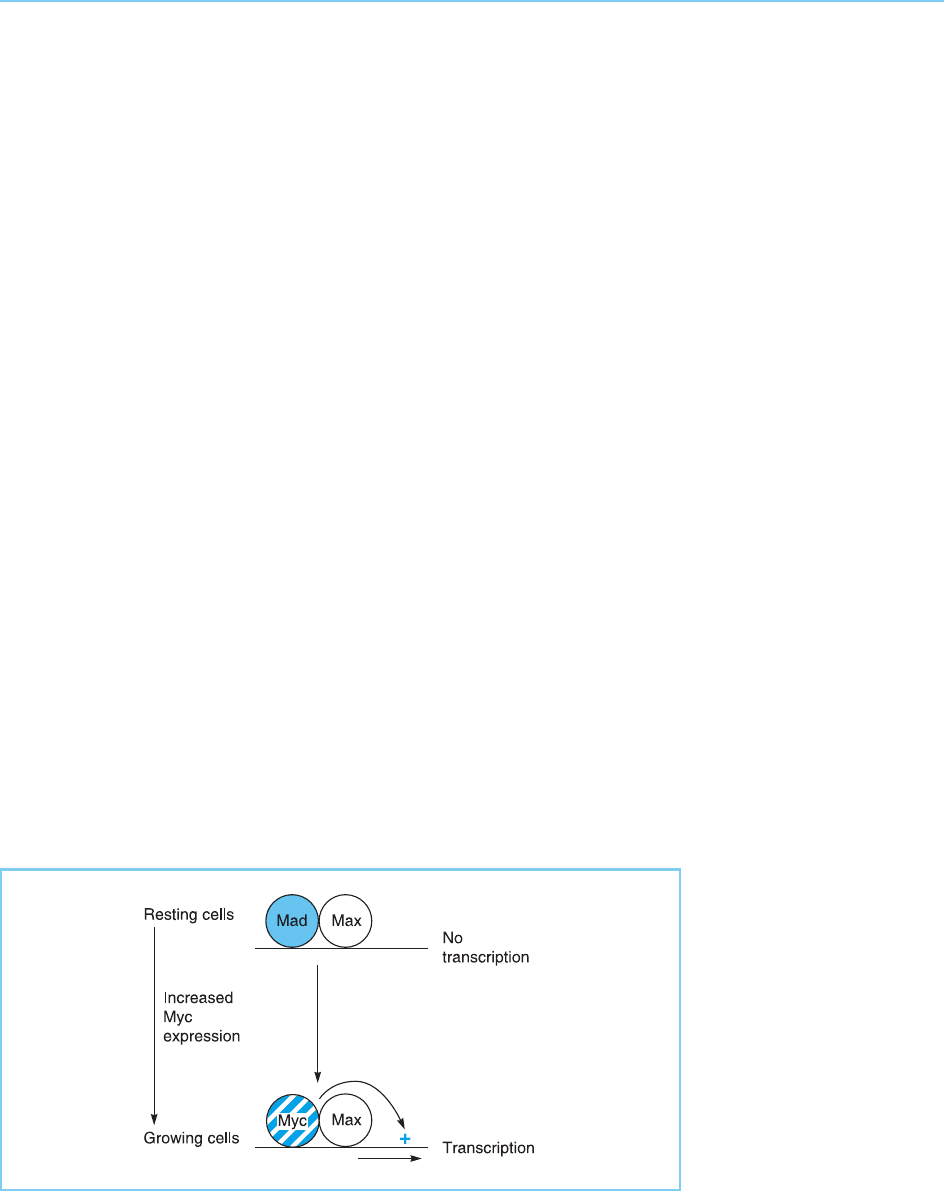
Mad and not Myc will be expressed and the expression of Myc dependent
genes will be repressed by Mad/Max homodimers. In contrast expression will
be activated by Myc/Max hete rodimers as the cells receive signals to prolifer-
ate resulting in increased Myc expression and decreased Mad expression (Fig.
9.22). Clearly the over-expression of the Myc gene, which is observed in many
cancer cells, would result in a similar production of activating Myc/Max het-
erodimers leading to gene activation. Hence, as in the case of the Fos/Jun
system, transformation by the Myc oncogene appears to depend primarily on
its over-expression resulting in the activation of genes required for cellular
growth.
Interestingly, it has been shown that Myc can also interact with another
transcription factor, Miz-1 (Myc interacting zinc finger protein-1), which is a
zinc finger protein (see Chapter 4, section 4.3 for discussion of this type of
protein). Un like the situation with Max, however, in the absence of Myc, Miz-1
acts as an activator of genes promoting growth arrest. In the presence of Myc,
however, this activity of Miz-1 is inhibited resulting in the repression of these
genes (Peukert et al., 1997). Hence the rise in Myc levels in transformed cells
stimulates the activity of growth promoting genes via Myc/Max-mediated
gene activation and represses growth inhibitory genes via a repres sion of
Miz-1 activity.
As well as regulating growth by altering the tr anscription of specific pro-
tein-coding genes by RNA polymerase II, another means by which Myc can
alter growth has recently been demonstrated. Thus, it has been shown that
Myc interacts with the TFIIIB transcription factor which is essential for tran-
scription by RNA polymerase III (see Chapter 3, section 3.4) and stimulates
the transcription of the genes encoding tRNA and 5S ribosomal RNA
(Gomez-Roman et al., 2003). Since these RNAs are essential for protein synth-
TRANSCRIPTION FACTORS AND HUMAN DISEASE 311
Figure 9.22
In resting cells, Myc-
dependent genes will be
repressed by a Mad/Max
heterodimer. As cells
begin to grow, the
expression of Myc
increases resulting in the
formation of Myc/Max
heterodimers which
activate transcription.
