Latchman. Eukariotic Transciption Factors
Подождите немного. Документ загружается.

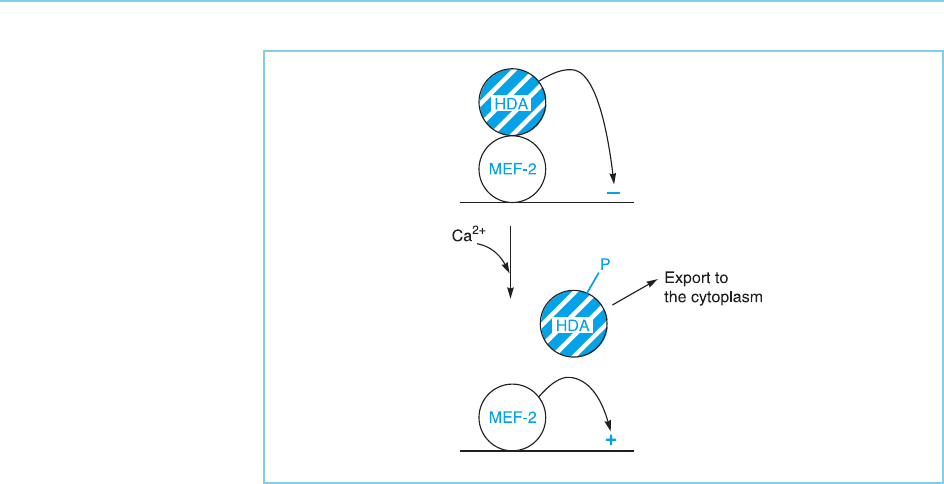
turn, regulate chromatin structure. Thus, in the absence of calcium stimula-
tion, the MEF2 transcription factor is bound to the promoters of muscle-
specific genes. However, gene activation does not occur since histone
deacetylase enzyme s are bound to MEF2 and, as discussed in Chapter 1 (sec-
tion 1.2.3), a lack of acetylate d histones produces a tightly packed chromatin
structure incompatible with transcription. However, in response to calcium,
kinase enzymes are activated and phosphorylate the histone deacetylases. This
phosphorylation results in the histone deacetylase enzymes being exported
from the nucleus, allowing MEF-2 to fulfill its function and activate muscle-
specific gene expression (Fig. 8.25) (for reviews see Stewart and Crabtree,
2000; McKinsey et al., 2002).
This ability of calcium to activate a kinase which then phosphorylates a
target protein is evidently in contrast to the direct binding of calcium to
the DREAM transcription factor which was discussed in section 8.2.1
REGULATION OF TRANSCRIPTION FACTOR ACTIVITY 271
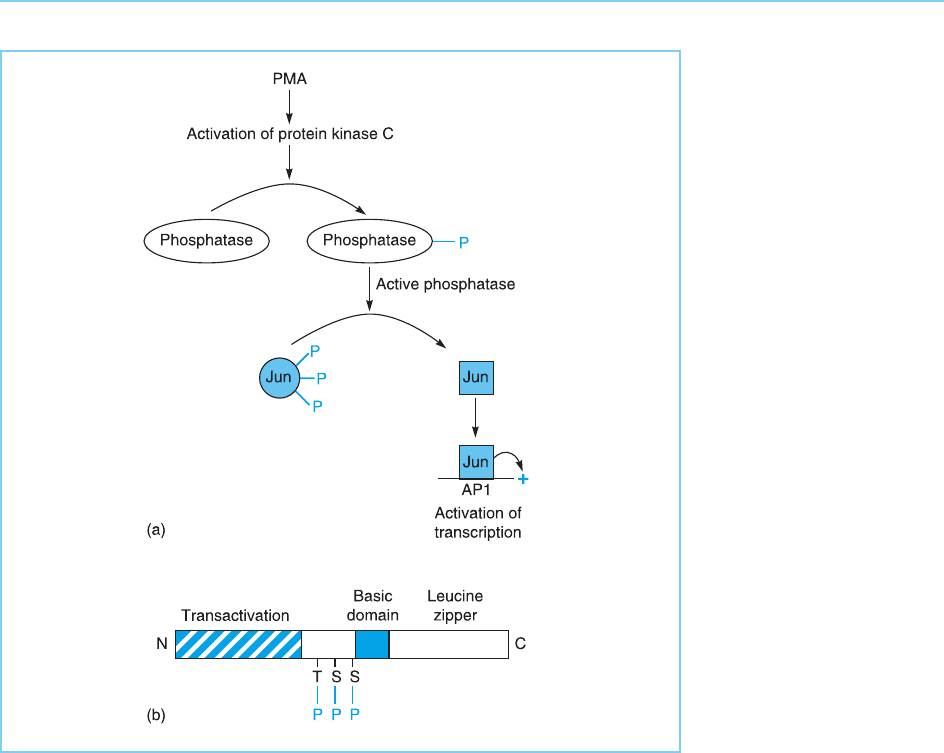
Figure 8.24
(a) Activation of Jun
binding to DNA by
dephosphorylation. The
dephosphorylation of Jun
protein following PMA
treatment increases its
ability to bind to AP-1
sites and activate PMA-
responsive genes. This is
likely to be mediated via
the PMA-dependent
activation of protein
kinase C which, in turn,
phosphorylates a
phosphatase enzyme
allowing it to
dephosphorylate Jun. ( b)
Position in the Jun
protein of the two serine
(S) and one threonine (T)
residues which are
dephosphorylated in
response to PMA. Note
the close proximity to the
basic domain (shaded)
which mediates DNA
binding. The positions of
the transactivation
domain and leucine
zipper are also indicated.
(compare Fig. 8.4 and Fig. 8.25) and illustrate s the fact that a specific stimulus
can use multiple mechanisms to activate transcription.
Hence, protein modification by phosphorylation can have a wide variety of
effects on transcription factors, either stimulating or inhibiting their activity
and acting via a direct effect on the ability of the factor to enter the nucleu s,
bind to DNA, associate with another protein or activate transcription or by
an indirect effect affecting the activity of an inhibitory protein or a histone-
modifying enzyme. The directness and rapidity of this means of transcription
factor activation evidently renders it of part icular importance in the response
to cellular signalling pathways.
8.4.3 ACETYLATION
In view of the directness and rapidity of using post-translational modification
as a means of modulating the activity of transcription factors, it is not surpris-
ing that other transcription factor modificat ions apart from phosphorylation,
are used in this way.
In particular, acetylation of transcription factors, particularly on lysine
residues has now been defined as an important means of regulating their
activity. Thus, although acetylation was initially defined as a modification
able to modulate histone activity (see Chapter 1, section 1.2.3), it has now
been shown also to occur for transcription factors themselves (for review see
Freiman and Tjian, 2003).
272 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 8.25
The ability of MEF-2
transcription factor to
activate gene transcription
can be blocked by
histone deacetylase
enzymes (HDA) which
deacetylate histones and
thereby block
transcription. Following
calcium treatment, the
histone deacetylases are
phosphorylated and
exported from the
nucleus, allowing MEF-2
to activate gene
transcription.
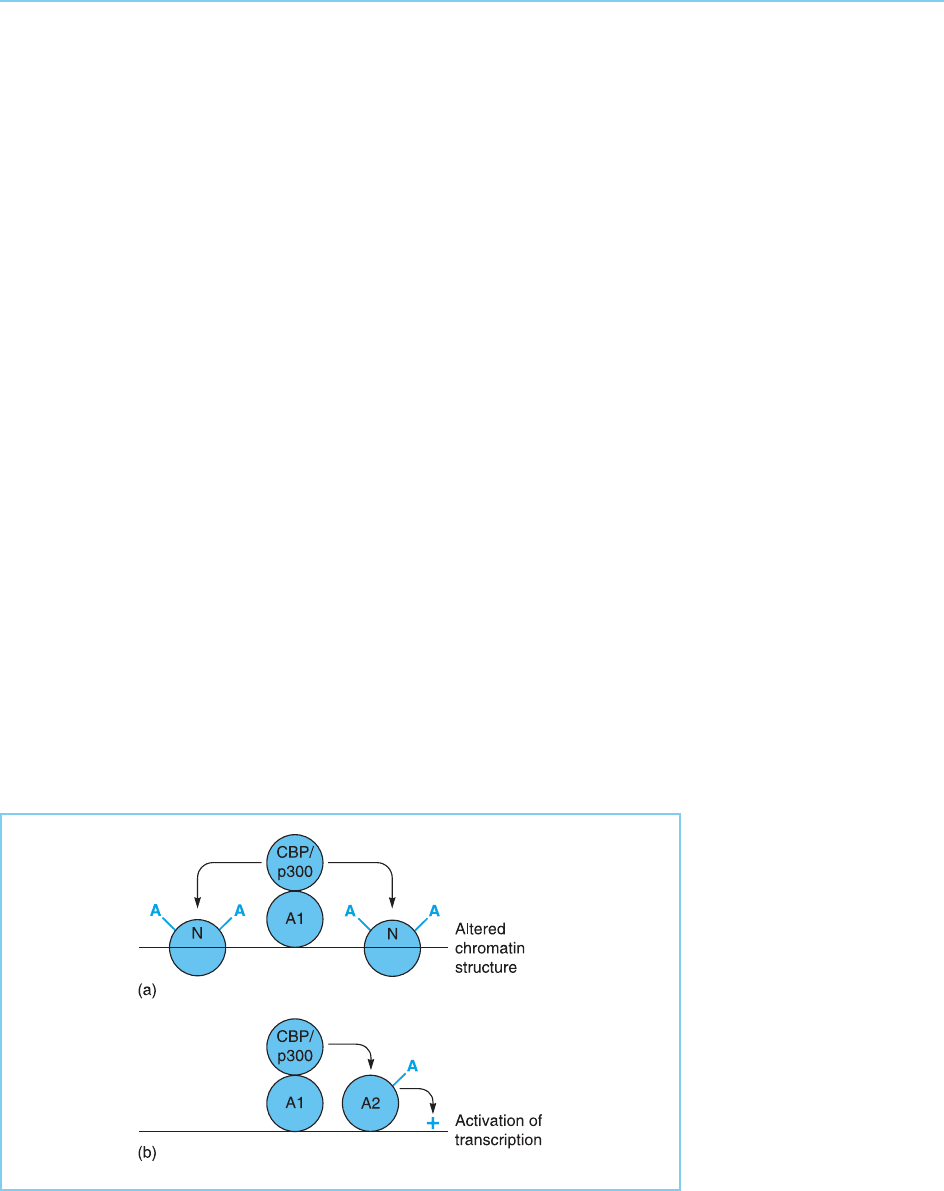
Thus, the addition of acetyl residues to the C-terminal domain of the p53
protein (see Chapter 9, section 9.4.2) increases the activity of p53 (Gu and
Roeder, 1997; Luo et al., 2000), although the precise manner in which acetyla-
tion enhances the ability of p53 to stimulate transcription is currently unclear
(for review see Prives and Manley, 2001). This acetylation of p53 is carried out
by the p300 co-activator molecule which, as described in Chapter 5 (section
5.4.3), associates with p53 as well as with a wide variety of other transcription
factors. This finding indicates that as well as acetylating histones and thereby
modifying chromatin structure (see Chapter 1, section 1.2.3), p3 00 and the
related CBP co-activators may also use their acetyltransferase activity to acet-
ylate specific transcription factors and thereby modify their activity (Fig. 8.26).
Hence, acetylation can modulate the activity of p53 by targeting its C
terminus. However, the N terminus of p53 can be modified by phosphoryla-
tion and thi s reduces its ability to bind to the MDM2 inhibitory protein (see
Chapter 9, section 9.4.2), thereby enhancing the stability of p53. Therefore,
the activity of p53 can be modified by phosphorylation and by acetylation,
indicating that different post-translational modifications can target the same
transcription factor molecule.
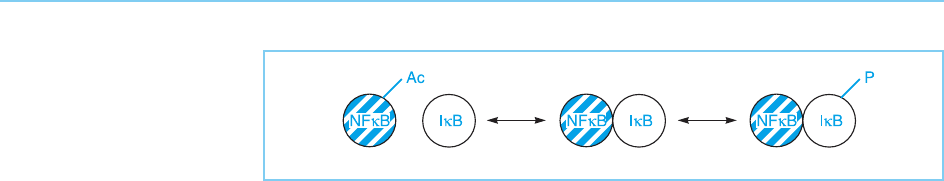
Acetylation also occurs in the NFB/IB system which also involves regu-
lated phosphorylation as discussed above (section 8.4.2). Thus, it has been
shown that NFB is acetylated and that this inhibits its interaction with IB
(Chen et al., 2001). Hence, interaction of NFB with IB requires both
deacetylated NFB and dephosphorylated IB (Fig. 8.27).
As well as targeting the same transcription factor (as in the case of p53) or
two interacting transcription fact ors (as in the case of NFB/IB), there is
REGULATION OF TRANSCRIPTION FACTOR ACTIVITY 273
Figure 8.26
Possible mechanisms of
action of CBP/p300.
Following recruitment to
DNA by an activating
molecule (A1) the acetyl
transferase activity of
CBP/p300 may either (a)
acetylate histones
producing a more open
chromatin structure or (b)
acetylate another
activating transcription
factor (A2) allowing it to
stimulate transcription.
evidence that the phosphorylation and acetylation systems can interact with
one another. Thus, for example, the ATF-2 transc ription factor has been
shown to have histone acetyltransferase activity and this activity is stimulated
by ATF-2 phosphorylation (Kawasaki et al., 2000).
8.4.4 METHYLATION
As with acetylation, methylation has been shown to play an important role in
the modification of histones (see Chapter 1, section 1.2.3) and as described in
Chapter 6 (section 6.4.1), the polycomb repressor complex contains an activ-
ity capable of methylating histones. However, as with acetylation, methylation
has also been shown to occur for transcription factors. Thus, for example, the
STAT-1 transcription factor is modified by the addition of methyl groups to
specific arginine residues and this stimulates its DNA binding ability (Mowen
et al., 2001). As described in section 8.4.2, the activity of STAT-1 is also
modified by phosphorylation, indicating that, as with acetylation, methylation
and phosphorylation can target the same molecule.
As well as affecting transcription factor s which bind to DNA, methylation
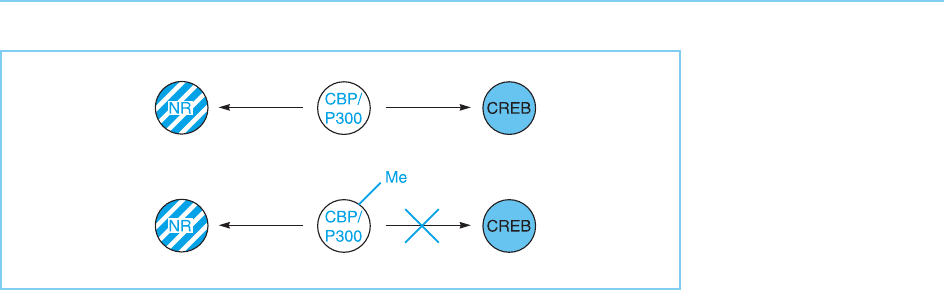
can also affect co-activators such as CBP and the related p300 factor, discussed
in Chapter 5 (section 5.4.3). Thus, both these factors are modified by methyla-
tion on specific arginine residues (for review see Gamble and Freedman,
2002). Most interestingly, such methylation affects the ability of CBP/p300
to bind to the variou s transcription factors with which they interact. Thus,
methylation abolishes the ability of CBP/p300 to bind to the CREB factor but
has no effect on its ability to bind to nuclear receptors, such as the steroid
receptors. Hence, the competition betw een different transcription factors for
binding to CBP/p300 (see Chapter 6, section 6.5) can be altered by modifica-
tion of the co-activator, resulting in a different balance between the different
factors under different conditions (Fig. 8.28).
As in the case of STAT-1, CBP is modified by phosphor ylation, as well as by
acetylation. Thus, phosphorylation of CBP on serine 436 enhances its ability
to interact with the AP-1 (see Chapter 9, section 9.3.1) and Pit-1 (see Chapter
4, section 4.2.6) transcription factors (for review see Gamble and Freedman,
2002).
274 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 8.27
Either acetylation of
NFB or phosphorylation
of IB can inhibit the
NFB/IB interaction.
The pos t-translational modification of co-activators can therefore modulate
their interaction with different activating molecules, allowing them preferen-
tially to activate different pathways under different conditions. This effect
evidently parallels the phosphorylation of transcription factors such as
CREB which affects their ability to interact with CBP/p300 and thus produce
transcriptional activation (see Chapter 5, section 5.4.3 and section 8.4.2 of this
chapter).
8.4.5 UBIQUIT INATION
Although phosphorylation, acetylation and methylation all involve the addi-
tion of relatively small chemical groups to the transcription factor molecule, it
is possible for a much larger entity to be added. Thus, many proteins in the
cell, including transcription factors, become modified by the addition of ubi-
quitin, which is itself a 76 amino acid protein. This small protein is linked to
the transcription factor by a covalent bond between the C terminal of ubiqui-
tin and an internal lysine residue of the transcription factor (for review see
Freiman and Tjian, 2003).
In many cases, this ubiquitination serves to target the molecule for degra-
dation, since it is recognized by the proteolytic machinery of the cell as mark-
ing the protein for destruction. Indeed, in the NFB/IB case discussed
above (section 8.4.2), phosphorylation of IB leads in turn to its ubiquitina-
tion and hence targets it for destruction, releasing NFB to activate gene
expression (Fig. 8.29) (for review see Maniatis, 1999; Karin and Ben-Neriah,
2000).
An interesting example of such ubiquitin-mediated control of gene expres-
sion is provided by the hypoxia inducible factor, HIF-1 (for review see Bruick
and McKnight , 2001; Kaelin, 2002). This factor consi sts of two subunits, HIF-
1 and HIF-1 and is activated when cells are exposed to low oxygen. It then
activates the expression of genes that are required in this situation. This
REGULATION OF TRANSCRIPTION FACTOR ACTIVITY 275
Figure 8.28
The ability of CBP/p300
to bind to different
transcription factors is
affected by the
methylation of CBP/
p300 which blocks
binding to the CREB
factor while not affecting
binding to the nuclear
receptors (NR).
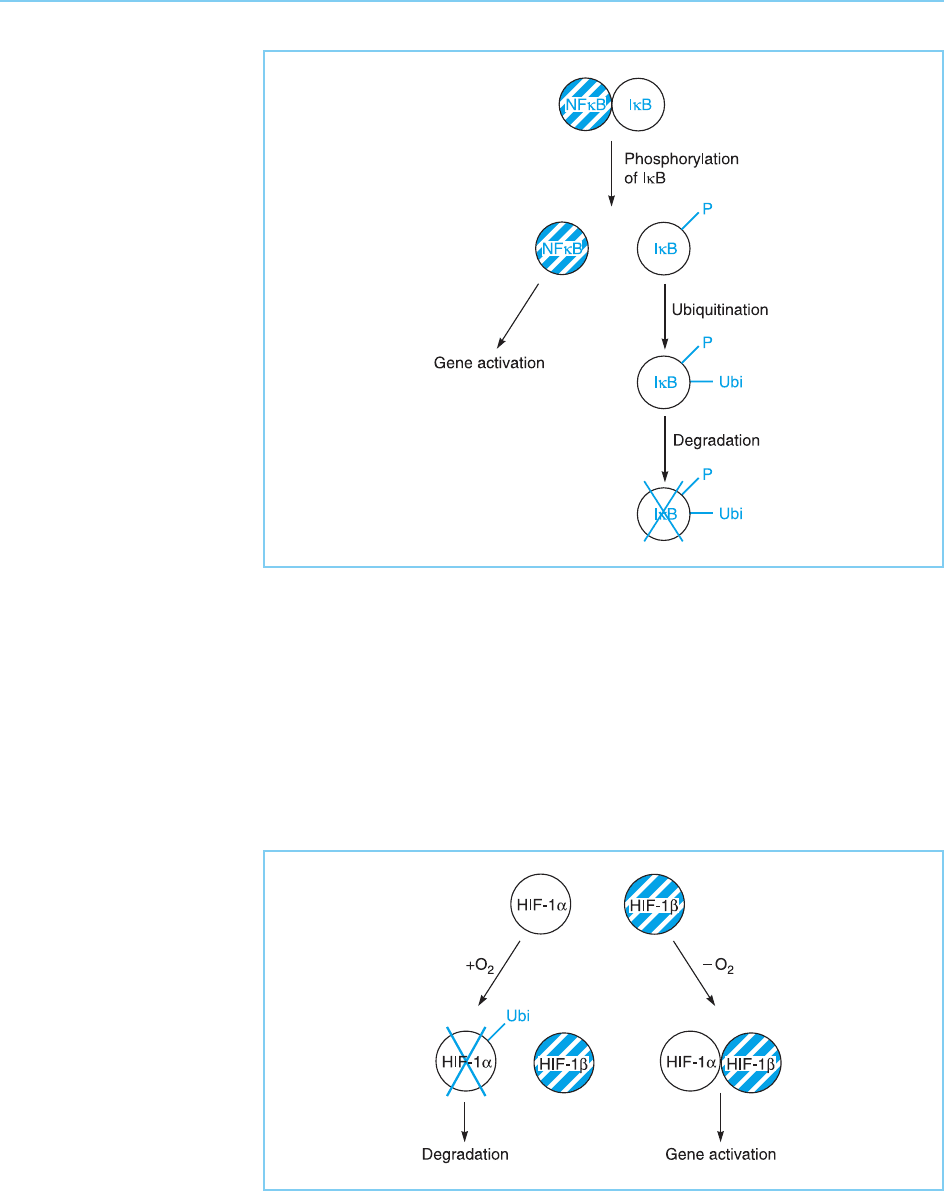
activation of HIF-1 is controlled at the level of protein degradation. In the
presence of oxygen, the HIF-1 subunit is rapidly ubiquitinated and degraded.
When oxygen levels fall, HIF-1 is no longer ubiquitinated and can therefore
associate with HIF-1 and activate gene transcription (Fig. 8.30).
This obviously leads to the question of how the ubiquitination of HIF-1 is
regulated by oxygen. It has been shown that, in the presence of oxygen, HIF-
1 is modified by the addition of a hydroxyl (OH) group to a proline amino
276 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 8.29
Phosphorylation of IBis
followed by its
ubiquitination which
targets it for destruction.
Figure 8.30
In the presence of
oxygen, the HIF-1
factor is modified by
addition of ubiquitin (Ubi)
and then degraded. In the
absence of oxygen this
addition of ubiquitin does
not occur and the HIF-1
is stabilized, allowing it to
dimerize with HIF-1 and
activate transcription.
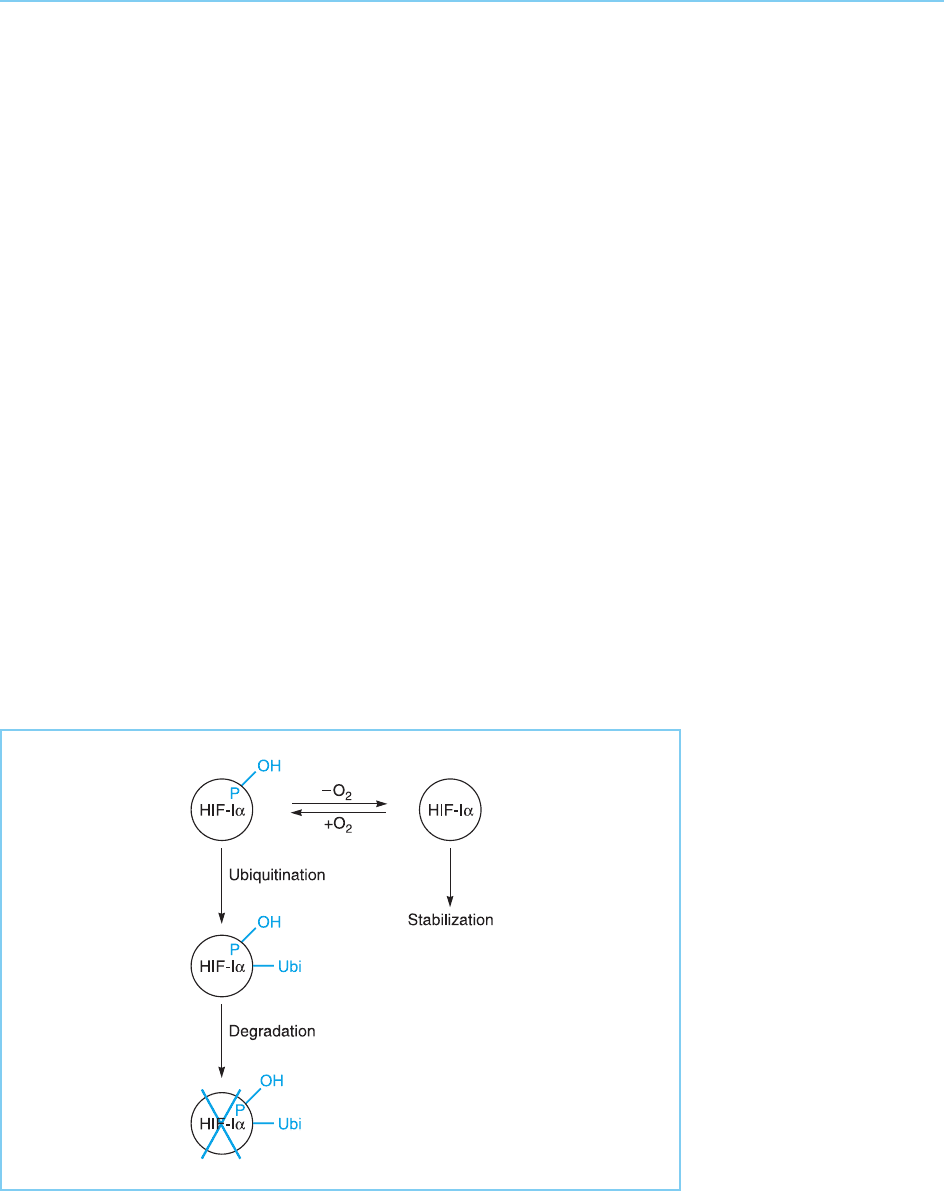
acid by a proline hydroxylase enzyme. This novel transcription factor modifi-
cation allows the HIF-1 to be recognized by the von Hippel-Lindau anti-
oncogene product (VHL) (see Chapter 9, section 9.4.4) which is part of a
multi-protein complex necessary for the addition of ubiquitin. Following a
fall in oxygen levels, the proline hydroxylation of HIF-1 does not occur
since the activity of the proline hydroxylase enzyme is directly regulated by
oxygen. Hence, the VHL produ ct cannot bind and HIF-1 is stabilized (for
review see Semenza, 2001; Zhu and Bunn, 2001) (Fig. 8.31).
In this case, therefore, a novel transcription factor modification is recog-
nized by the VHL protein and leads to further modification by ubiquitination.
This is evidently analogous to the phosphorylation of IB discussed above,
which is necessary for its subsequent ubiquitination. The structural basis for
the role of hydroxyproline in regulating the interaction of HIF-1 and VHL
has recently been defined. Thus, the hydroxyproline res idue on HIF-1 inserts
into a pocket in VHL allowing only hydroxyproline-modified HIF-1 to bind
to VHL (Hon et al., 2002; Min et al., 2002) (Fig. 8.32).
Interestingly, the pocket in VHL which binds the hydro xyproline has been
shown to be a hot spot for mutations which inactivate VHL and result in
cancer. Hence, the anti-oncogenic function of VHL appears to involve its
ability to bind to proteins such as HIF-1 via hydroxyproline residues.
Indeed, patients with cancer caused by mutation of VHL show expression
of HIF-1-activated genes even in the presence of oxygen (see Chapter 9,
section 9.4.4 for further discussion).
REGULATION OF TRANSCRIPTION FACTOR ACTIVITY 277
Figure 8.31
Oxygen induces the
modification of HIF-1
by addition of a hydroxyl
group (OH) on a proline
(P) amino acid which
results in its
ubiquitination and
degradation.
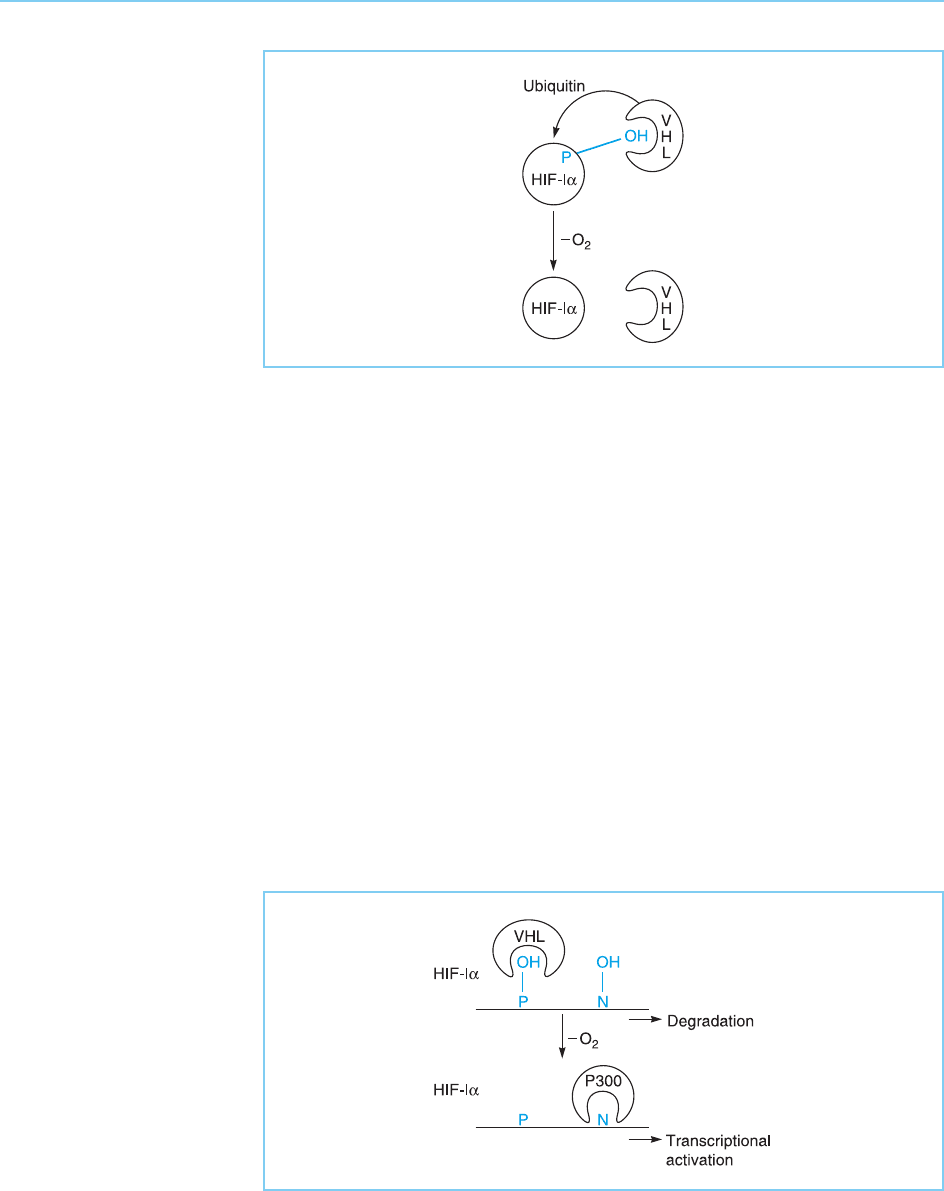
In the case of HIF-1 therefore, a novel modification involving the hydroxy-
lation of proline residues stimulates ubiquitination and consequent degrada-
tion. In addition however, HIF-1 is also modified by a further novel
modification involving addition of a hydroxyl group to an asparagine amino
acid. Like hydroxylation of proline, this modification is also inhibited by
reduced oxygen levels. However, rather than controlling protein stability,
the loss of the hydroxyl group on asparagine facilitates the binding of the
p300 transcriptional co-activator (for review see Bruick and McKnight, 2002;
Kaelin, 2002). This binding of p300 enhances the ability of HIF-1 to activate
transcription (see Chapter 5, section 5.4.3 for discussion of CBP/p300).
Hence, reduced oxygen levels stabilize the HIF-1 protein by inhibiting
hydroxylation of proline and enhance the ability of the stabilized protein to
activate transcription by inhibiting hydroxylation of asparagine (Fig. 8.33).
As discussed in Chapter 6 (section 6.4.2), HIF-1 is not the only target for
ubiquitination by the VHL complex. Thus, the phosphorylated form of the
278 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 8.32
Proline hydroxylation
allows recognition of HIF-
1 by the von Hippel
Lindau (VHL) protein
which catalyses the
addition of ubiquitin.
Figure 8.33
In the HIF-1 factor
removal of oxygen not
only blocks the addition
of hydroxyl residues to
proline (P), preventing
VHL binding, but also
blocks addition of
hydroxyl residues to
asparagine (N) promoting
the binding of the p300
co-activator molecule.
This allows the stabilized
protein to stimulate
transcription.
large subunit of RNA polymerase II is also ubiquitinated by the VHL complex
resulting in its degradation. This specifically blocks the elongation step of
transcription, since this phosphorylated form of RNA polymer ase II is speci-
fically required for tr anscriptional elongation (see Chapter 3, sectio n 3.1). As
with HIF-1, the ubiquitination of the large subunit of RNA polymerase II also
requires prior prolin e hydroxyla tion of the polymerase subu nit (Kuznetsova et
al., 2003) suggesting that this may be a general mechanism for targeting of
proteins by VHL, accounting for its importance in its anti-oncogenic function
(see above).
The use of ubiquitination to target proteins such as NFB, HIF-1 or the
large subunit of RNA polymerase II for degradation is not unique to tran-
scription factors but is widely used in the turnover of a vari ety of different
proteins. However, recently a further role of ubiquitin has emerged which is
specific to transcription factors. Thus, it has been shown that modification by
ubiquitination may be necessary for activation domains to stimulate transcrip-
tion (see Chapter 5, section 5.2 for a discussion of activation domains). In
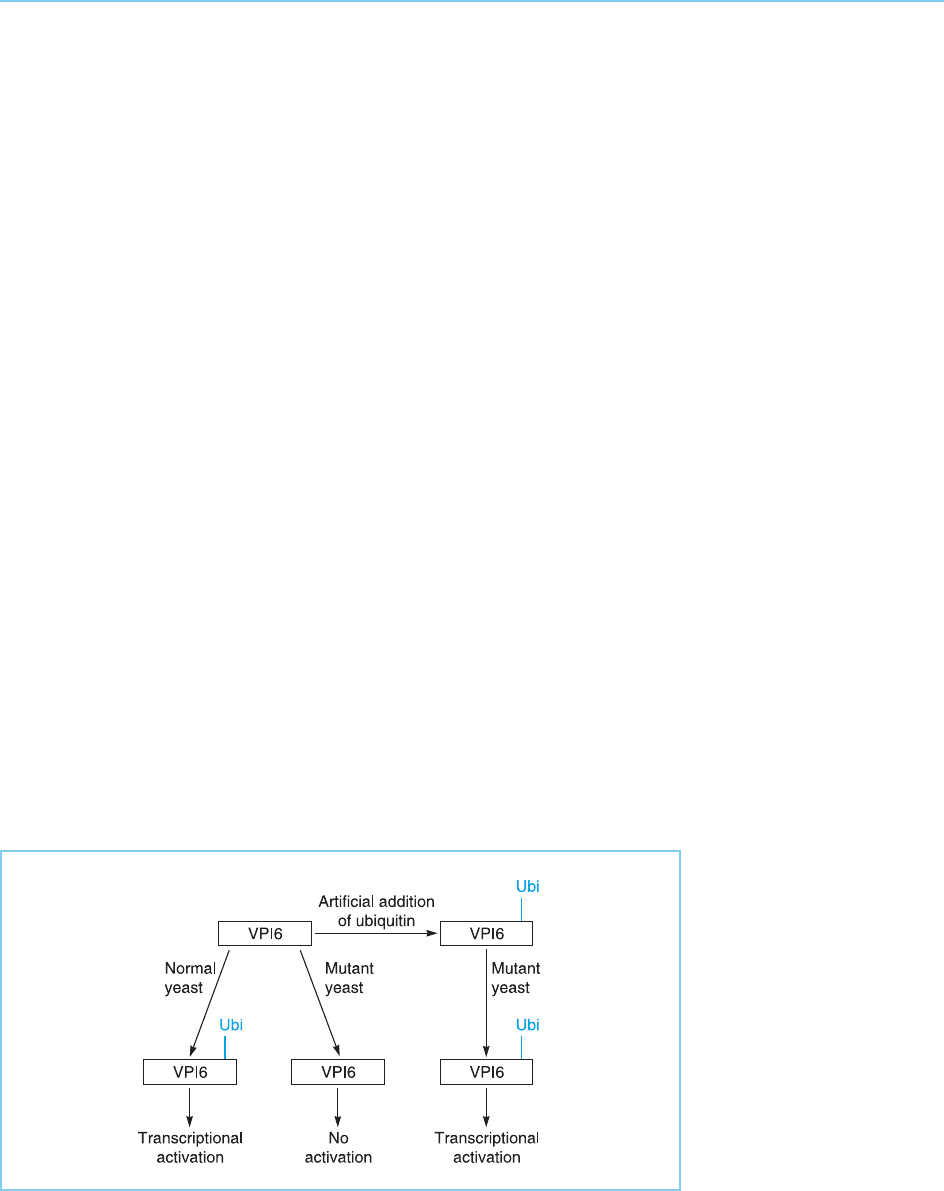
experiments in yeast, the VP16 acidic activation domain could not activate
transcription in a yeast strain which could not add ubiquitin to the VP16
protein. However, if a modified VP16 was prepared in which ubiquitin had
already been added to the activation domain, then transcription was activated
(Salghetti et al., 2001; Fig. 8.34).
This indicates that modification of the VP16 activation domain by ubiqui-
tination is necessary for it to activate transcription. This effect is not unique to
VP16, with the heat shock factor discussed in section 8.3.1 having been show n
to be modified by addition of the ubiquitin-related protein SUMO-1.
Moreover, this modification stimulates its ability to activate transcription
REGULATION OF TRANSCRIPTION FACTOR ACTIVITY 279
Figure 8.34
The transcriptional
activator VP16 can
activate transcription in
normal yeast which can
modify it by the addition
of ubiquitin (Ubi) but not
in mutant yeast which
cannot carry out this
modification. However, if
the VP16 is modified by
artificial addition of
ubiquitin, then it can
activate transcription even
in the mutant yeast.
(Hong et al., 2001). Hence, modification by addition of ubiquitin or SUMO-1
appears to be widespread among transcription factors (for review see Freiman
and Tjian, 2003).
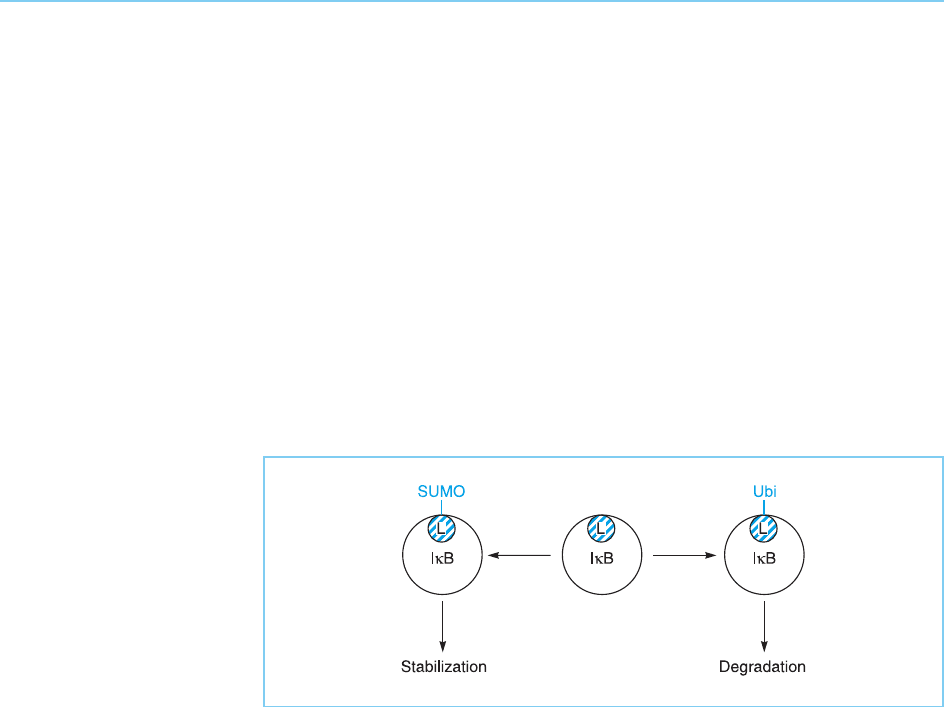
Interestingly, modification of a specific lysine residue in IB by addition of
SUMO-1 has been shown to prevent the addition of ubiquitin and thereby
protect IB from degradation (Desterro et al., 1998) (Fig. 8.35). Hence, dif-
ferent modifications of the same residue may produce opposite effects on
transcription factor activity, providing a further mechanism for regulating
such activity. As lysine residues are the target for acetylation (section 8.4.3)
as well as for addition of ubiquitin or SUMO-1, several different modification
enzymes may compete to modify a specific lysine amino acid in a transcription
factor with different consequences for its functional activity (for review see
Freiman and Tjian, 2003).
Although the regulation of transcription factor activation domains by ubi-
quitin may appear unrelated to the role of ubiquitin in protein degradation,
this may not be the case. Thus, it has been proposed that modification of an
activator by ubiquitination allows it to activate transcription but also targets it
for subsequent destruction, after activation has occurred. This would limit the
potentially dangerous process of uncontrolled transcriptional activation by
ensuring that the activator was rapidly degraded after it had achieved its
function of transcriptional activation (for reviews see Tansey, 2001;
Conaway et al., 2002) (Fig. 8.36).
The modification of transcription factors by ubiquitin therefore offers
a means of regulating both their degradation and their activity. When taken
together with modification by phosphorylation, acetylation and methy-
lation discussed above, it is clear that the control of transcription factor
activity by post-translational modification is of critical importance,
particularly in allowing gene expression to be modulated by specific signalling
pathways.
280 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 8.35
Modification of a specific
lysine residue (L) in IB
by addition of ubiquitin
promotes degradation of
the protein. In contrast,
addition of the ubiquitin-
like protein SUMO-1 to
the same lysine residue
blocks addition of
ubiquitin and hence
stabilizes IB.
