Latchman. Eukariotic Transciption Factors
Подождите немного. Документ загружается.


esis by the ribosome and hence for cellular growth, this provides a further
means by which transcriptional regulation by Myc can regulate cellular
growth.
9.3.4 OTHER ONCOGENIC TRANS CRIPTION FACTORS
In view of the likely need for multiple different transcription factors to reg-
ulate genes involved in cellular growth processes, it is not surprising that
several other genes encoding transcription factors have also been identified
as oncogenes as well as playing a key role in gene expression in specific cell
types. Thus, for example, the myb oncogene and the maf oncogene, both of
which were originally isolated fr om avian retroviru ses, play key roles in gene
regulation in monocytes and erythroid cells respectively (for reviews see Graf,
1992; Blank and Andrews, 1997; Motohashi et al., 1997). Simi larly, the rel
oncogene of the avian retrovirus Rev-T is a member of the NFB family of
transcription factors discussed in Chapter 8 (for review see Baeurale and
Baltimore, 1996; Foo and Nolan, 1999) while the Bcl-3 oncogene is a member
of the IB family which interacts with the NFB proteins (Bours et al., 1993).
Interestingly, the Bcl-3 factor illustrates another facet of the mechanisms by
which transcript ion factor genes become oncogenic. This factor was not iden-
tified as a retroviral oncogene but on the basis that it was located at the break
point of chromosomal rearrangements which resulted in its translocation to a
position adjacent to the immunoglobulin gene in some B cell chronic leukae-
mias. A number of other transcription factors have also been shown to be
capable of causing cancer when translocated in this way. This can occur
because their expression is increased due to their being translocated to a
highly expressed locus such as the immunoglobulin gene loci in B cells or
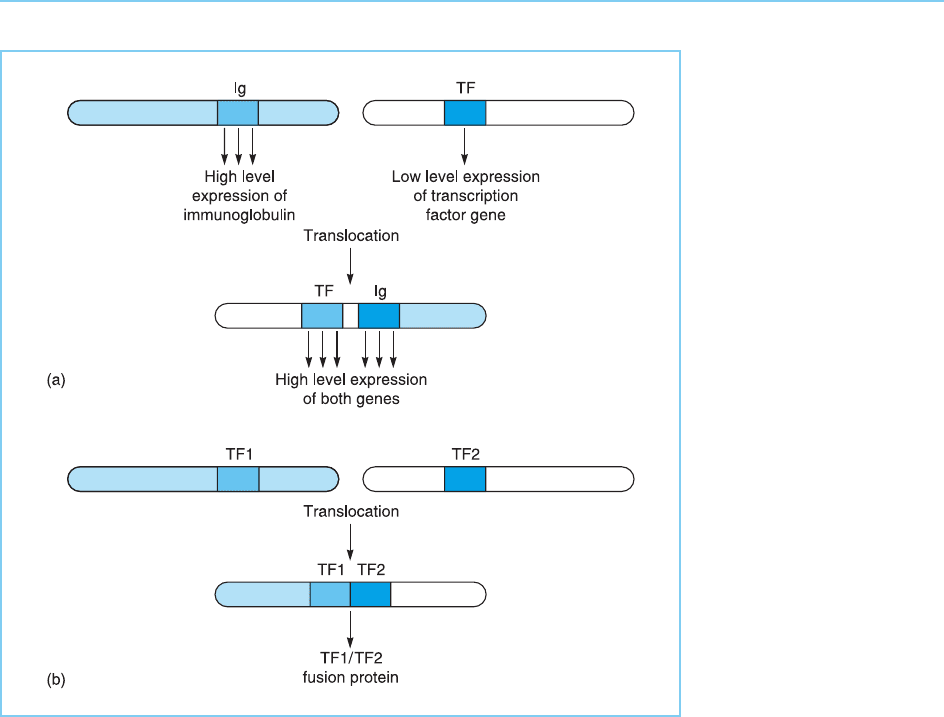
the T-cell receptor gene loci in T cells (Fig. 9.23a). Alternatively it can occur
because the translocation results in the production of a novel form of the
transcription factor due to its truncation or its linkage to another gene
(encoding either another transcription factor or another class of protein)
following the translocat ion (Fig. 9.23b).
Factors translocated in these ways include both factors which were origin-
ally identified in oncogenic retroviruses and others which had not previously
been shown to have oncogenic potential (for reviews see Rabbits, 1994;
Latchman, 1996; Look, 1997). Thus for example, expression of the c-myc
oncogene (section 9.3.3) is dramatically increased by its translocation into
the immunoglobulin heavy chain locus which occurs in the human B-cell
malignancy known as Burkitt’s lymphoma (for review see Spencer and
Groudine, 1991) while the gene encoding the Ets transcription factor, dis-
cussed above (section 9.3.1), is fused to the gene for the platelet derived
312 EUKARYOTIC TRANSCRIPTION FACTORS
growth factor receptor to create a novel oncogen ic fusion protein in patients
with chronic myelomonocytic leukaemia (for review see Sawyers and Denny,
1994).
Similarly, expression of the homeobox gene Hox11 (see Chapter 4 section
4.2.5) is activated in cases of acute childhood T-cell leukaemia while the CBP
co-activator (see Chapter 5, section 5.4.3) is fused to the MLL gene in acute
myeloid leukaemia (Sobulo et al., 1997). Interestingly, the PBX factors, which
are the mammalian homolog ues of the Drosophila extradenticle factor dis-
cussed in Chapter 4 (section 4.2.4), were originally identified on the basis
of the fact that the gene encoding PBX1 was found fused to the E2A gene
(which encodes the E12 and E47 proteins discussed in Chapter 4, section
4.5.2) in a human leukaemia (for review see Mann and Chan, 1996).
These findings provide further evidence that transcription factor genes are
not only rendered oncogenic by transfer into a retrovirus but are also
involved in the causation of human cancers playing a key role, for example,
TRANSCRIPTION FACTORS AND HUMAN DISEASE 313
Figure 9.23
Chromosomal
translocations can result
in cancer when (a) the
gene encoding a
transcription factor is
translocated next to a
highly transcribed locus
such as the
immunoglobulin gene (Ig)
and is therefore
expressed at a high level
or (b) the translocation
results in the fusion of
the genes encoding two
different transcription
factors resulting in a
fusion protein with
oncogenic properties.
in the oncogenic effects of the chromosome translocations which are charac-
teristic of specific cancers.
9.4 ANTI-ONCOGENES AND CANCER
9.4.1 NATURE OF ANTI-ONCOGENES
As noted in section 9.2, a number of genes exist whose normal function is to
encode proteins that function in an opposite manner to those of oncogenes,
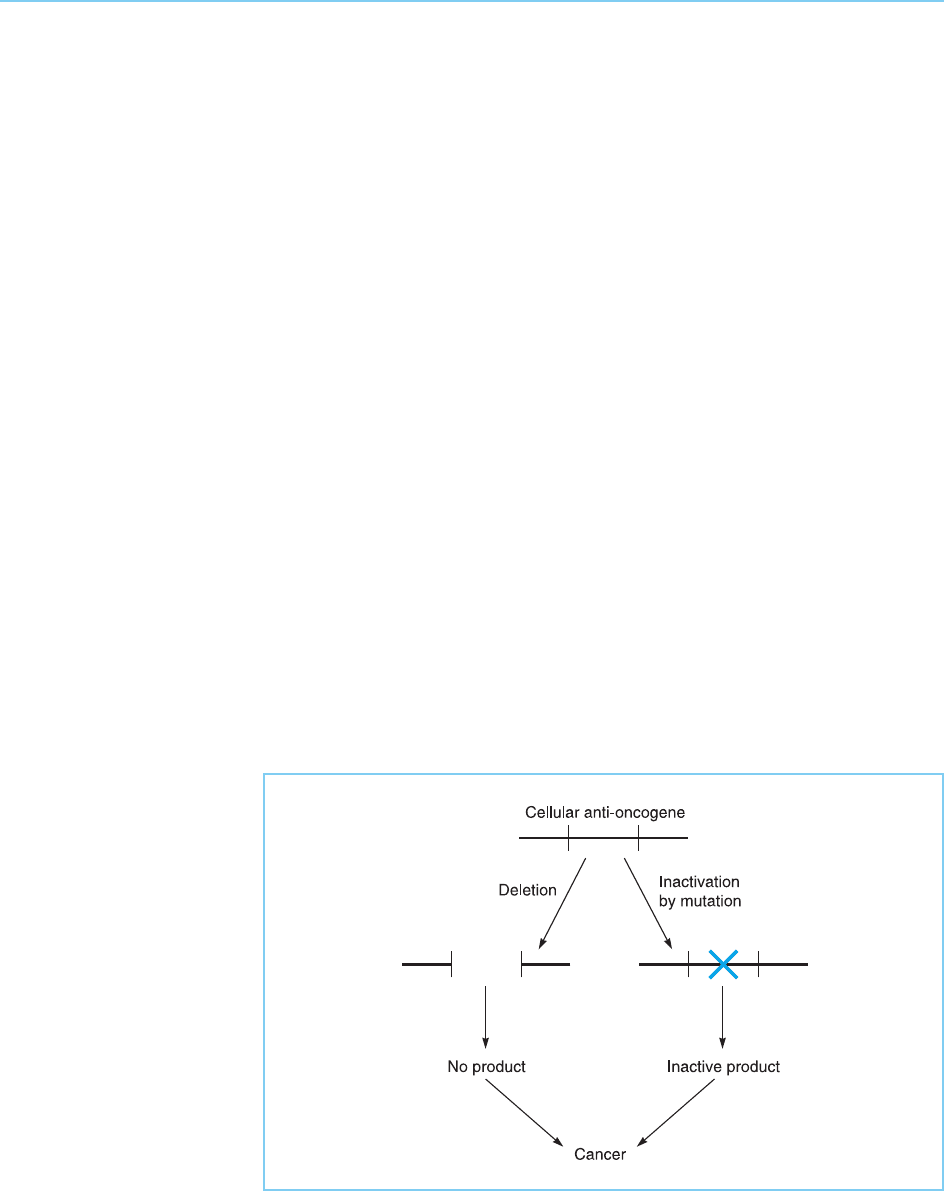
acting to restrain cellular growth. The deletion or mutational inactivation of
these anti-oncogenes (also known as tumo ur suppressor genes) therefore
results in cancer (for reviews see Knudson, 1993; Fearon, 1997; Hunter,
1997) (Fig. 9.24). This effe ct evidently parallels the production of cancer by
the over-expression or mutational activation of cellular proto-oncogenes
(compare Figs. 9.6 and 9.24).
A number of anti-oncogenes of this type have been defined and several
encode transcription factors. The two best characterized of these act by dif-
ferent mechanisms. Thus, p53 acts by binding to the DNA of its target genes
and regulating their expression, whereas the retinoblastoma gene product
(Rb-1) acts pr imarily via protein–protein interactions with other DNA binding
transcription factors. The p53 and Rb-1 proteins are therefore discussed in
sections 9.4.2 and 9.4.3 as examples of these two mechanisms of action. Other
anti-oncogenes encoding transcription factors are discussed in section 9.4.4.
314 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.24
Cancer can result from
the deletion of specific
anti-oncogenes or their
inactivation by mutation.

9.4.2 p53
The gene encoding the 53 kilo-dalton protein kn own as p53 is mutated in a
very wide variety of human tumours, especially carcinomas (for review see Ko
and Prives, 1996; Levine, 1997; Vogelstein et al., 2000; Haupt et al., 2002;
Sharpless and DePinho, 2002). In normal cells expression of this protein is
induced by agents which cause DNA damage and its over-expression results in
growth arrest of cells containing such damage or their death by the process of
programmed cell death (apoptosis). Hence p53 has been called the ‘guardian
of the genome’ (Lane, 1992), which allows cells to proliferate only if they have
intact undamaged DNA. This would prevent the development of tumours
containing cells with mutations in their DNA and the inactivat ion of the
p53 gene by mutation would therefore result in an enhanced rate of tumour
formation. In agreement with this idea, mice in which the p53 gene has been
inactivated do not show any gross abnormalities in normal dev elopment but
do exhibit a very high rate of tumour formation, leading to early death (for
review see Berns, 1994).
The molecular analysis of the p53 gene product showed that it contains a
DNA binding domain and a region capable of activating transcription. The
majority of the mutations in p53 which occur in human tumours are located in
the DNA binding domain (Friend, 1994; Anderson and Tegtme yer, 1995).
These mutations result in a failure of the mutant p53 protein to bind to
DNA, indicating that this ability is crucial for the ability of the normal p53
protein to regulate cellular growth and suppress cancer.
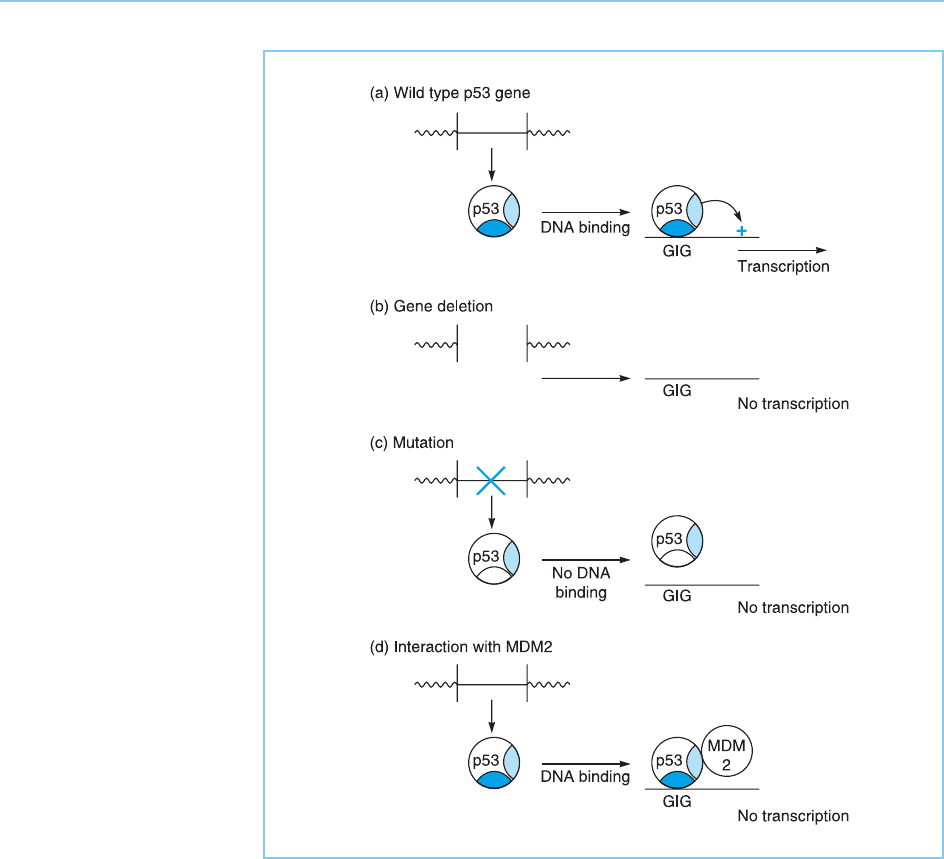
The p53 protein therefore functions, at least in part, by activating the
expression of genes whose protein products act to inhibit cellular growth
(Fig. 9.25a). The absence of functional p53 either due to gene deletion (Fig.
9.25b) or to its inactivation by mutation (Fig. 9.25c) results in a failure to
express these genes leading to uncontrolled growth.
In addition, functional p53 can also be prevented from activating gene
transcription by interaction with the MDM2 oncoprotein (Fig. 9.25d). Thus
MDM2 masks the activation domain of p53 preventing it activating transcrip-
tion (Fig. 9.26a). Moreover, MDM2 when bound to p53 also actively inhibits
transcription by interacting with the basal transcriptional complex to reduce
its activity (Thut et al., 1997) (Fig. 9.26b).
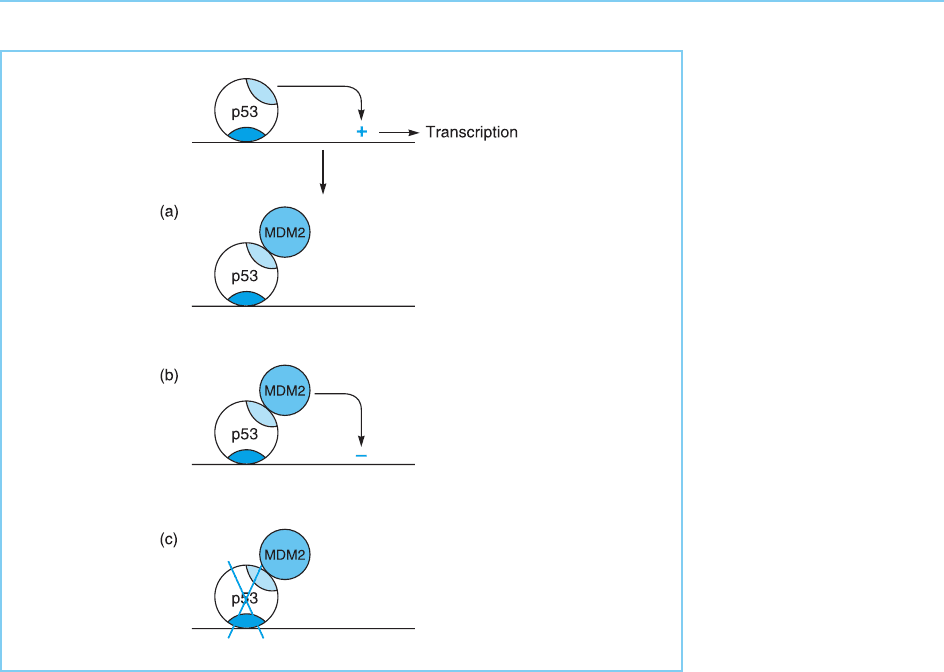
The major inhibitory effect of the interaction of MDM2 with p53, however,
is that it results in the rapid degradation of p53. Thus, MDM2 causes the
addition of ubiquitin residues to p53, thereby promoting its degradation
(Haupt et al., 1997; for review see Lane and Hall, 1997) (Fig. 9.26c). Hence,
several of the different inhibitory mechanisms, discussed in Chapter 6, are
involved in the inhibitory effect of MDM2 on p53 (Fig. 9.26) (f or review see
TRANSCRIPTION FACTORS AND HUMAN DISEASE 315
Oren, 1999). Interestingly, the addition of ubiquitin to p53, targeting it for
degradation is paralleled by the addition of the ubiquitin-related protein
SUMO-1 to MDM2. This modification of MDM2 paradoxically enhances its
ability to add ubiquitin to p53 and thereby induce p53 degradatio n
(Buschmann et al., 2000) (see Chapter 8, section 8.4.5 for discussion of the
regulation of transcription factors by the addition of ubi quitin or SUMO-1).
The inhibitory effect of MDM2 on p53 brought about by these multiple
mechanisms is of particular importance in many human soft tissue sarcomas
where the p53 gene is intact and encodes wild type p53 but the protein is
functionally inactivated due to the high levels of MDM2 resulting from ampli-
316 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.25
The ability of wild type
p53 to activate genes
encoding growth inhibiting
proteins (GIG) (panel a)
can be abolished by
deletion of the p53 gene
(panel b), mutations in
the DNA binding domain
(solid) which prevent it
binding to DNA (panel c)
or by the interaction of
functional p53 with the
MDM2 protein which
prevents it from activating
transcription (panel d).
fication of the mdm2 gene encoding it. Indeed, the major function of MDM2,
even in normal cells, may be to inhibit the action of p53 by interacting with it.
Thus mice in which the gene encoding MDM2 is inactivat ed are non-viable but
can be rendered viable by the addi tional inactivation of the p53 gene (de Oca
Luna et al., 1995).
Interestingly, both partners in the p53/MDM2 interaction are subject to
modification by phosphorylation and these modifications affect their interac-
tion with one another (for reviews see Prives, 1998; Mayo and Donner, 2002).
Thus, following exposure to DNA damage/stress, p53 is phosphorylated. This
enhances its retention in the nucleus and inhibits its interact ion with MDM2,
so allowing it to activate transcription of its target genes. Conversely, stimuli
that inhibit apoptosis, lead to phosphorylation of MDM2. This promotes its
movement from the cytoplasm to the nucleus and hence allows it to inhibit
p53 and its pro-apoptotic effect (Fig. 9.27) (for review see Gottifredi and
Prives, 2001).
As well as affecting binding to MDM2, phosphorylation also enhances the
binding of p53 to the Pin1 protein (Zheng et al., 2002). Pin1 is a member of
TRANSCRIPTION FACTORS AND HUMAN DISEASE 317
Figure 9.26
Multiple mechanisms by
which MDM2 inhibits p53
involving (a) masking of
its activation domain (pale
blue), (b) direct inhibition
of transcription by MDM2
itself and (c) targeting
p53 for degradation by
proteolytic enzymes.
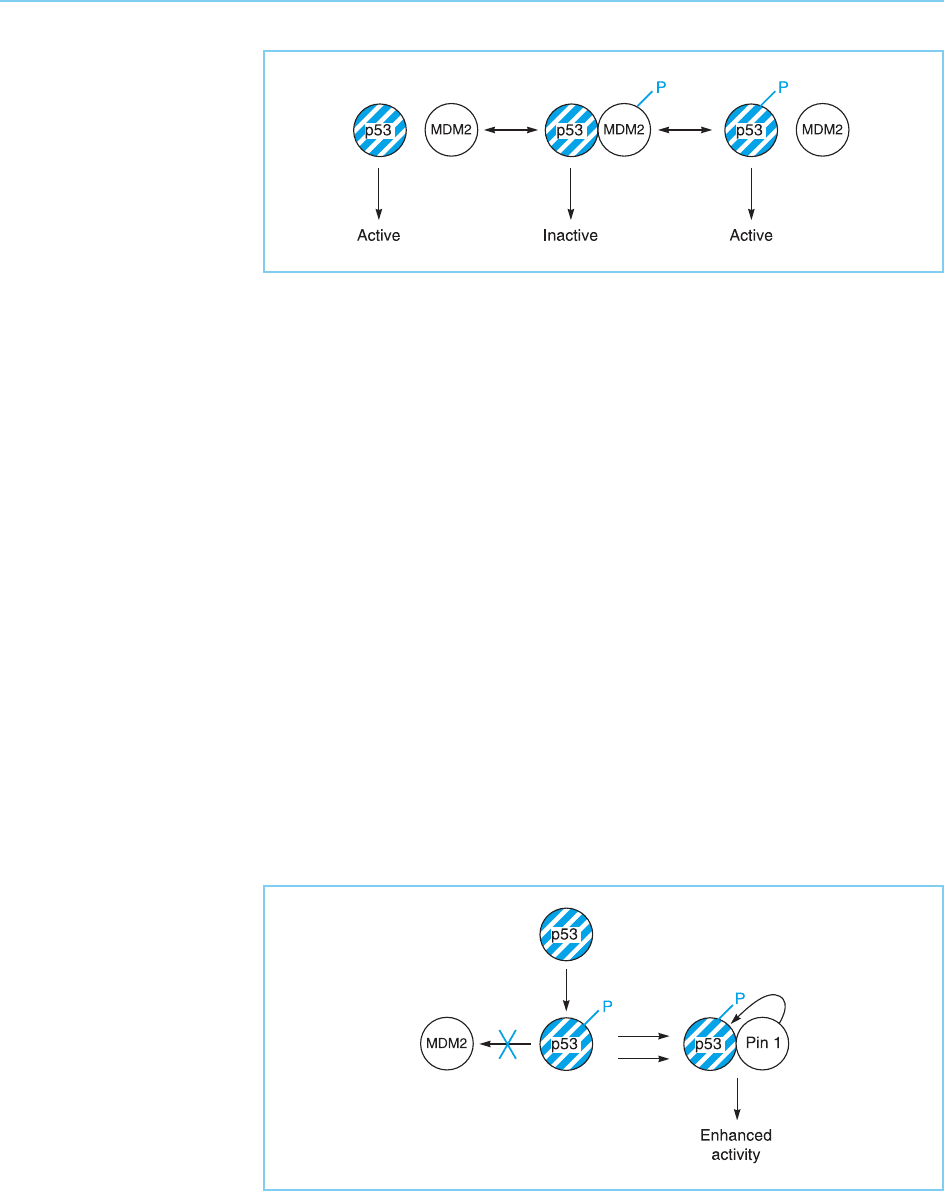
the class of proteins known as peptidyl prolyl isomerases which have the
ability to change the structure of the peptide bond between proline residues
and adjacent amino acids in a process known as cis-trans isomerization. In the
case of p53, interaction with Pin1 and the consequent isomerization of
peptide bonds within the p53 protein, stimulates the DNA binding and trans-
activation ability of p53. This therefore provides a second mechanism for
phosphorylation to stimulate the activity of p53 (Fig. 9.28) (for further
details of the effect of phosphorylation on transcription factors see Chapter
8, section 8.4.2).
It should be noted that this effect of a prolyl isomerase enzyme on a
transcription factor is not unique to p53. Thus, DNA binding activity of the
c-myb proto-oncogene protein (see section 9.3.4 ) has been shown to be nega-
tively regulated by its interaction with the peptidyl prolyl isomerase, Cyp40
(for review see Hunter, 1998).
Hence, signals such as DNA damage/stress can activate p53 by inducing its
phosphorylation. As noted in Chapter 8 (section 8.4.3) p53 is also subject to
acetylation, which stimulates its activity (for review see Prives and Manley,
2001). Recently, it has been demonstrated that histone deacetylase enzymes,
318 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.27
The interaction of p53
and MDM2, which
inactivates p53, is
promoted by
phosphorylation of MDM2
but inhibited by
phosphorylation of p53.
Figure 9.28
Phosphorylation of p53
blocks its interaction with
MDM2 so stabilizing the
protein and also
enhances its interaction
with the peptidyl prolyl
isomerase Pin1, which
stimulates the activity of
p53.
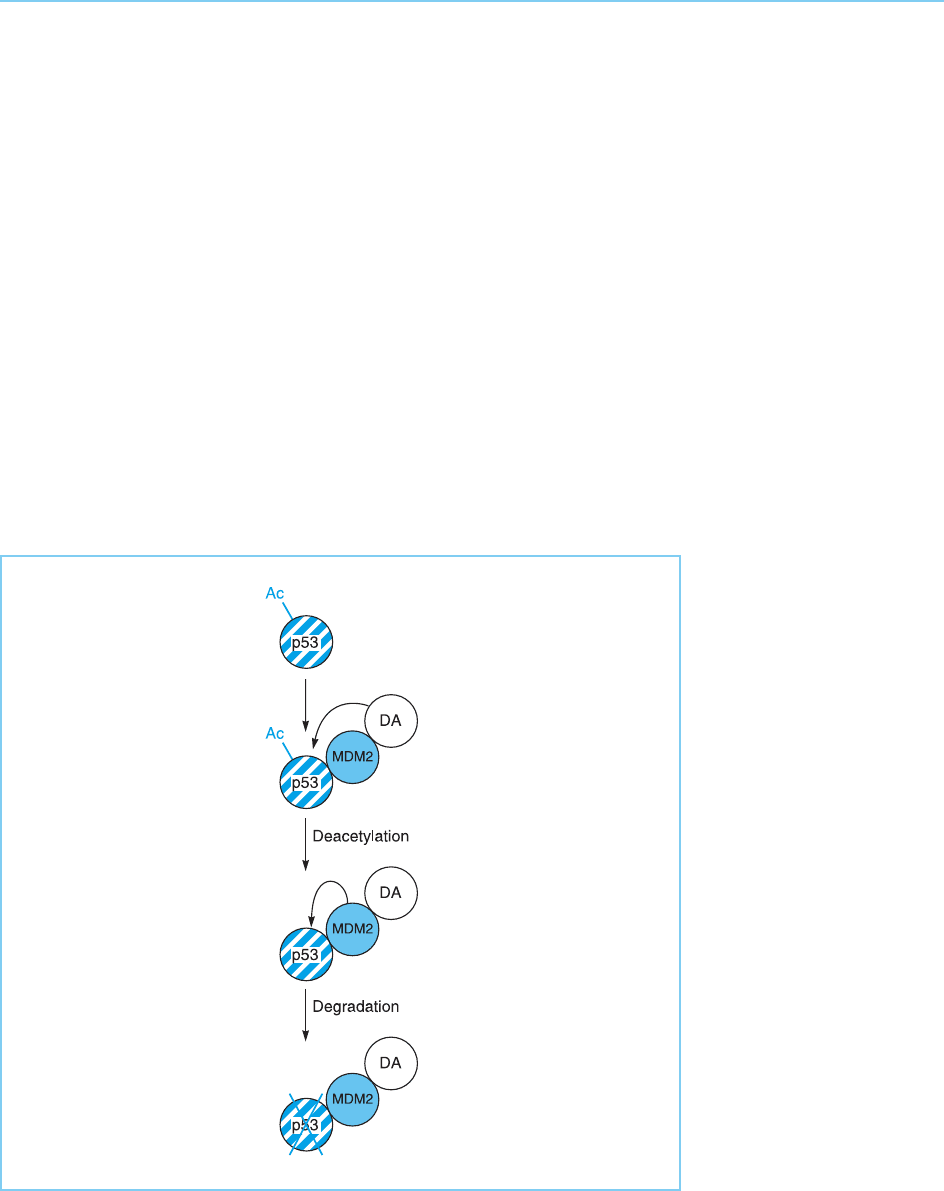
such as Sir2, can specifically deacetylate p53, thereby reducing its ability to
activate transcription (Luo et al., 2001; Vaziri et al., 2001). Indeed, it appears
that MDM2 exists in a complex with a histone deacetylase enzyme and that
deacetylation of p53 actually enhances its degradation by MDM2 (Ito et al.,
2002; Li et al., 2002). Hence, this inhibitory complex can deacetylate p53,
reducing its activity and targeting it for degradation by MDM2 (Fig. 9.29).
The activity of p53 is thus regulated, in part, by the balance between its
acetylation by molecules such as the p300 co-activator (as discussed in Chapter
8, section 8.4.3) and its deacetylation by molecules such as Sir2. In turn, this
represents part of the multiple modifications used to alter the activity of the
p53/MDM2 system which include many of the modifications that can affect
transcription factors, such as phosphorylation, acetylation and modifica tion
by addition of ubiquitin or SUMO-1 (see Chapter 8, section 8.4 for a discus-
sion of the modulation of transcription factor activity by post-translational
modifications).
TRANSCRIPTION FACTORS AND HUMAN DISEASE 319
Figure 9.29
Binding to p53 of a
complex of MDM2 and a
histone deacetylase
enzyme (DA) results in
deacetylation of p53
which promotes its
subsequent degradation
by MDM2.
The interaction of p53 with the MDM2 oncogenic protein is paralleled by
its interaction with the transforming protein s of several DNA viruses. Indeed
p53 was originally discovered as a protein that interacted with the large T
oncoprotein of the DNA tumour virus SV40. The functional inactivation of
p53 produced by this interaction appears to play a critical role in the ability of
these DNA viruses to transform cells paralleling the similar action of MDM2.
These interactions suggest that functional antagonism between oncogene and
anti-oncogene products is likely to be critical for the control of cellular growth
with changes in this balance which activate oncogenes or inactivate anti-
oncogenes resulting in cancer.
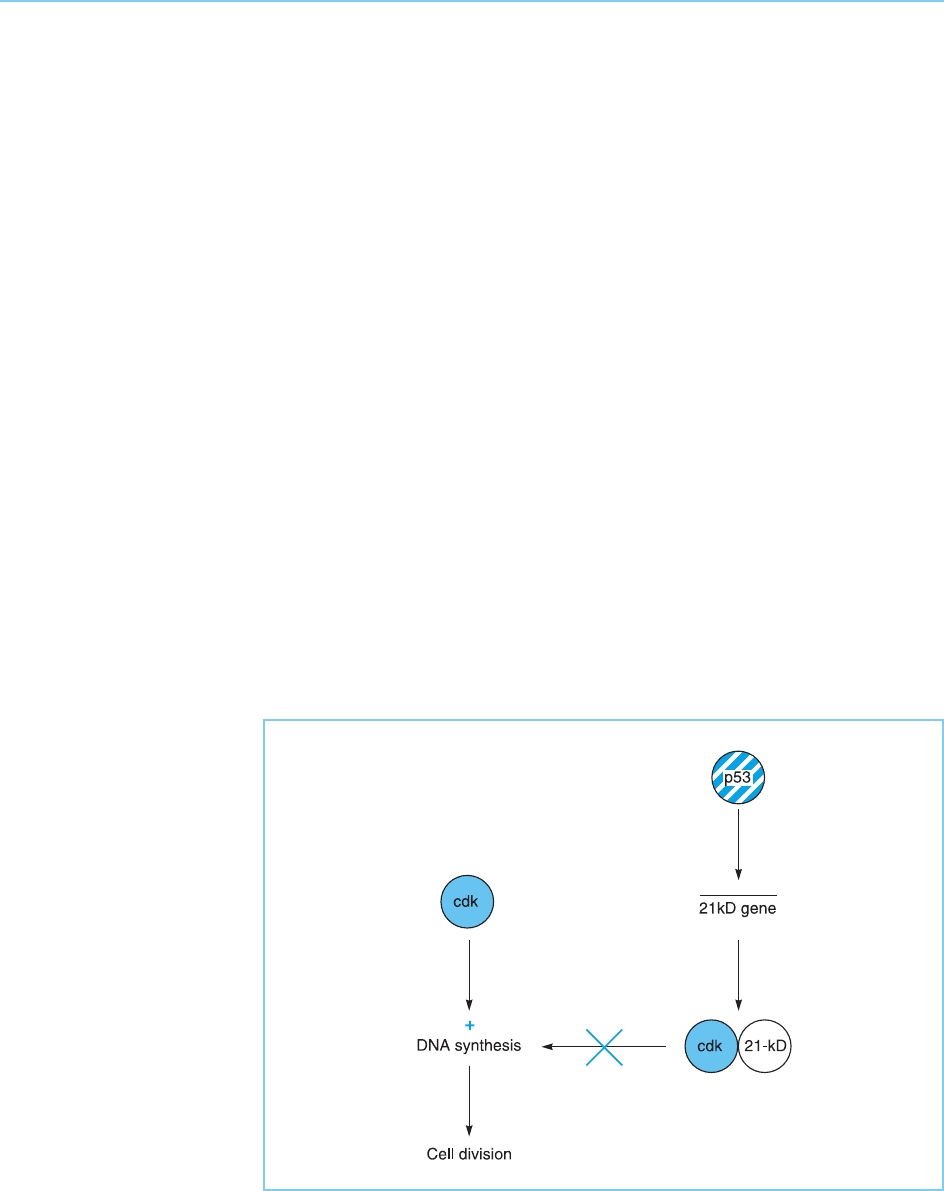
These considerations evidently focus attention on the genes that are acti-
vated by p53. One such gene is that encoding a 21 kilo-dalton protein (p21)
which acts as an inhibitor of cyclin-dependent kinases (for review of p53-
dependent genes see Ko and Prives, 1996; Vogelstein et al., 2000). As the
cyclin-dependent kinases are enzymes that stimulate cells to enter cell divi-
sion, the finding that p53 stimulates the expression of an inhibitor of these
enzymes is entirely consistent with its role in restraining growth, since the
inhibition of the cyclin-dependent kinases will prevent cells replicating their
DNA and undergoing cell division (Fig. 9.30).
Interestingly, p53 also stimulates expression of the mdm2 gene whose pro-
tein product interferes with the activity of p53 as described above. This effect
is likely to be part of a negative feedback loop in which p53, having fulfilled its
320 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 9.30
p53 activates the gene
for the 21kD inhibitor of
cyclic dependent kinases
(cdk). This inhibitor then
prevents the cyclin
dependent kinases from
stimulating DNA
synthesis and consequent
cell division.
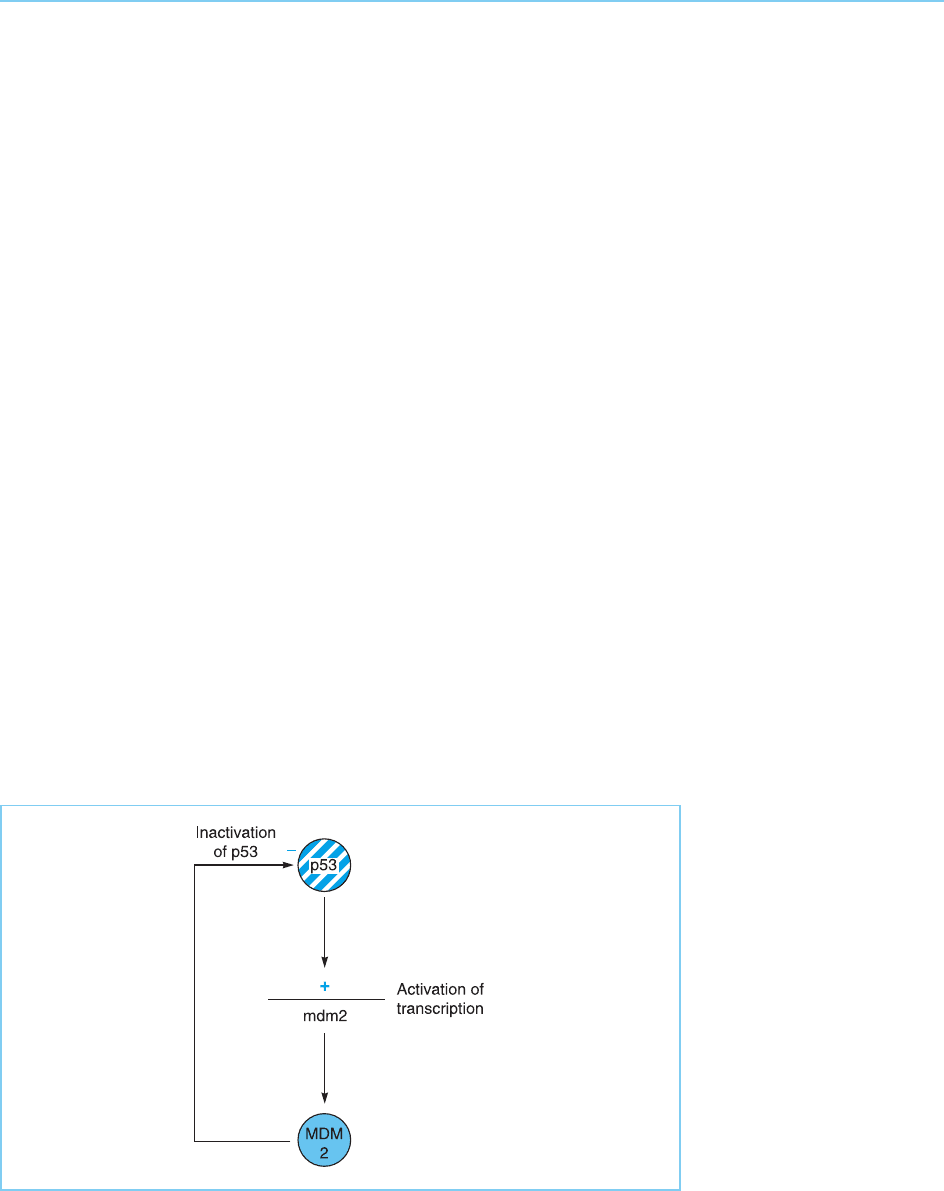
function, activates mdm2 expression resulting in p53 inactivation (Fig. 9.31)
(for review see Oren, 1999). This would allow, for example, cells which had
repaired the damage to their DNA to inactivat e p53 and res ume cell division.
Similarly, p53 also stimulates the expression of the bax gene whose protein
product stimulates programmed cell death or apoptos is allowing p53 to pro-
mote the death of cells whose damaged DNA is irreparable. Further studies
have also identified several genes involved in the generation of toxic reactive
oxygen species whose expression is induced by p53 during this process, indi-
cating that p53 may also promote apoptosis by inducing the production of
these species (Wyllie, 1997).
As with AP1 (section 9.3.1) transcriptional activation by p53 requires the
CBP co-act ivator or the closely related p300 protein (Avantaggiati et al., 1997).
Hence, as with AP1 and the steroid receptors (see Chapter 6, section 6.5), AP1
and p53 can compete for CBP/p300 resulting in antagonism between the
oncogenic activity of AP1 and the anti-oncogenic activity of p53.
Hence the p53 gene product plays a key role in regulating cellular growth
by binding to DNA and activating the expression of specific genes (for review
see Almog and Rotter, 1997). Its inactivation by mutation or by interaction
with oncogene products is likely to play a critical role in most human cancers.
Interestingly, two novel p53-related proteins which encod e transcription fac-
tors known as p73 and p63, have been described (for reviews see Lohrum and
Vousden, 2000; Morrison and Kinoshi ta, 2000; Yang et al., 2002). It is cur-
rently unclear whether either p63 or p73 play a role as anti-oncogenes whose
inactivation results in human cancers. However, inactivation of p63 or p73 in
knock out mice results in gross developmen tal abnormalities whereas this is
TRANSCRIPTION FACTORS AND HUMAN DISEASE 321
Figure 9.31
p53 activates the gene
for the MDM2 protein
which acts in a negative
feedback loop to
inactivate p53 by the
mechanisms illustrated in
Figure 9.26.
