Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.


724
Chapter
13
where vTr40 is the transition temperature for embrittlement weld metal, and AvTr40 is
the
change in transition temperature for weld metal that has been step-cooled to cause ternper
embrittlement.
Control of Temper Embrittlement of Weld Metal
Control of temper embrittlement in weld metal of 2.25Cr- 1Mo is considerably more difficult
than with plate and forgings. Higher silicon and manganese contents are necessary to sound
weld metal. Basic fluxes generally provide the minimum susceptibility to temper embrittlement
consistent with high-temperature strength requirements [88,96]. The other factors that may
affect the low temperature toughness and temper embrittlement are discussed next
[88,96]:
1
It is well known that reduction of grain size is effective for improving the low-temperature
toughness of the weld metal. Addition of nitrogen results in reduction in grain size, which
in
turn improves low-temperature toughness.
2.
It is known that reduction
of
oxygen content in weld metal leads to improved toughness.
C, Si, Mn, and Ti are generally used as the deoxidants. Be cautious because extremely low
oxygen content can cause a reduction in toughness.
3.
Control of chemical compositions and impurities
in
the weld metal. The influencing ele-
ments are phosphorus, arsenic, antimony, and tin. In the case of manganese, the recom-
mended range for the weld metal is
0.7-1.0%
Mn.
4.
The best temperature range for the PWHT is
1250-1300°F
(677-704°C). The
CVN
values
can be improved by prolonging the time of PWHT.
15.9
Postweld Heat Treatment (Stress Relief)
ASME boiler and pressure vessel code requires postweld heat treatment, or stress relieving,
primarily to soften the HAZ, minimizing the presence of hard zones and stabilizing its micro-
structure. Otherwise, hydrogen attack or creep embrittlement could occur in service. Various
stress-relieving requirements are specified by end users and fabricators of
A387
grade steels.
Some specifications have increased the stress-relieving temperatures and hold times for these
steels because
of
the interest in stabilizing the microstructure of the heat-affected zones after
fabrication and accounting for possible weld repairs of the vessels while in service. However,
as stress-relieving temperatures and times are increased, the capability of the chemical compo-
sition of each grade of steel to achieve the tensile requirements of the specification using a
normalized and tempering heat treatment is limited [95]. It is found that there is deleterious
influence of extensive stress relief treatments
on
CVN toughness levels.
Larson-Miller Tempering Parameter
The Larson-Miller parameter is usually employed to obtain some idea of the change in a given
property
of
a material during a heat-treatment process performed at different temperatures and
with different holding times [8,96]. It is widely used because of its usefulness in summarizing
the heat-treating characteristics of low-alloy steels and in estimating their long-time strengths
at given temperatures
[
181.
Cr-Mo steel has a hardened structure in the as-welded condition. This is given postweld
heat treatment for changing it to a softened structure (tempered bainite) before use. By soften-
ing the structure, the toughness recovers gradually, but the heat-treatment parameter commonly
known as the Larson-Miller parameter
P
=
T(20
+
log
t),
where
T
is the absolute temperature
in Kelvin
(OK)
and
t
is heat-treatment time in hours, becomes large. Excessive increase in this
parameter causes reduction in the toughness. Therefore, it is necessary to carefully examine
the heat- treatment conditions.

Material Selection and Fabrication
725
15.10 Reheat Cracking in Cr-Mo and Cr-Mo-V Steels
Phosphorus and sulfur were found to enhance the reheat cracking (RC) susceptibility of Cr-
MO steels. For a particular alloy, there exists a critical phosphorus content below which embrit-
tlement will not take place. Measures such as (1) reduction of P and
S,
(2) addition of a small
quantity of titanium (0.07%), which decreases the
RC
susceptibility due to phosphorus effects,
and (3) the addition of calcium or rare earth metals, in accordance with sulfur, improve resis-
tance to reheat cracking.
15.11 Modified 9Cr-1
MO
Steel
Small additions of niobium (0.06-0.10%) and vanadium
(0.18-0.25%)
to the standard 9Cr-
1Mo
elevated-temperature steel result in a steel that is stronger and more ductile. This new
steel exhibits improved long-term creep properties, lower thermal expansion, higher thermal
conductivity, and better resistance to stress corrosion cracking
[97].
Proposed applications for
the modified 9Cr- 1 mo steel include boilers, reaction vessels, breeder reactor systems, pressure
vessels for coal liquefaction and gasification
,
oil refining hydrotreating equipment, and geo-
thermal energy systems.
15.1
2
Advanced 3Cr-MO-Ni Steels
Advanced 3Cr-MO-Ni steels have been developed for use in thick-section pressure vessels,
specifically for coal liquefaction and gasification, by minor alloy modifications to commercial
2.25Cr-1Mo (ASTM A387, Grade 22, Class
2
steel)
[98].
Specifically, the new alloy shows
improved hardenability (i.e., fully bainitic microstructures following normalizing of 400-mm
plates), enhanced strength, superior hydrogen attack resistance, and better Charpy V-notch
impact toughness, with comparable ductility, creep rupture resistance, and temper embrittle-
ment resistance.
16
STAINLESS STEELS
Stainless steels
(SS)
are those alloy steels that have a normal chromium content
of
not less
than 12%, with or without other alloy additions. Stainless steels are more resistant to rusting
and staining than plain carbon steel and low-alloy steels. They have superior corrosion resis-
tance because of relatively high contents of chromium. These metals are available in both
wrought and cast forms.
16.1 Classification and Designation of Stainless Steels
Stainless steel
(SS)
may be classified in five families, according to metallurgical structure:
1.
Martensitic
SS.
2. Austenitic
SS.
3. Ferritic
SS.
4.
Duplex
SS.
5.
Precipitation hardening (PH)
SS.
The first four groups are characterized by the predominant metallurgical phase present
when the stainless steel is placed in service. In iron and steel, the body-centered cubic lattice
is called ferrite and the face-centered cubic lattice is austenite,
so
the steels are described as
ferritic or austenitic depending on their major structural constituent.
As
the name implies,
duplex stainless steel consists of both austenite and ferrite phases in
50:
50
proportion. The

726
Chapter
I3
fifth group contains those stainless steels that can be strengthened by an aging heat treatment.
This steel is generally not used
in
heat exchanger applications. Therefore
it
is not covered here.
Designations
Wrought stainless steels are assigned designations by the American Iron and Steel Institute
(AISI) according to composition: the Cr-Ni-Mn austenitic stainless steels as
2xx
series, and
the Cr-Ni austenitic stainless steels as
3xx
series and 4xx series. The precipitation hardening
grades are assigned designations based on their Cr and Ni contents.
ASTM Specification for Stainless Steels
Most of the AISI types-austenitic, duplex, ferritic, and martensitic stainless steels, plus some
special stainless like superferritic and superaustenitic steels-are included in A240.
Guidance for Stainless Steel Selection
Guidelines for selection of the types of available stainless steels are discussed by Brown
[99]
and DeBold
[
1001.
According to Brown, the following guidelines will serve to select
a
proper
grade for the service under consideration:
Select the level of corrosion resistance required for the application.
Select the level of strength required.
For special fabrication problems, select one of the basic alloy modifications that provides the
best fabrication characteristics.
Do
a cost-benefit analysis to include the initial raw material price, cost of installation, and the
effective life expectancy of the finished product.
Determine the availability
of
the raw material at the most economical and practical choice.
DeBold
[
1001
discusses the approach to select stainless steels to meet corrosion resistance and
strength.
16.2
Martensitic Stainless Steel
The martensitic stainless steels are on the lower scale of corrosion resistance, because they
contain only 11-18% Cr, with carbon content usually less than 0.4%. The lower limit on
chromium is governed by corrosion resistance, and the upper limit by the requirement for the
alloy to convert fully to austenite on heat treatment [loll. The key feature of this family is
that it can be hardened by heat treatment. Its utility as heat exchanger material in aqueous
environments is limited. But these steels do exhibit a useful combination of strength, ductility,
toughness, and corrosion resistance in mild environments. Resistance to corrosion is obtained
only when the material is fully hardened and tempered
[
1021. AISI 410 is the most widely
used of the martensitic grades. It is occasionally used
in
heat exchangers.
16.3
Austenitic Stainless Steel Properties and Metallurgy
Austenitic stainless steels make up approximately
80-90%
of the stainless steels
in
use today
[
1031. The common austenitic stainless steels type 300 series are low carbon, iron, and chro-
mium alloys sufficiently alloyed with nickel and sometimes manganese or nitrogen, or combi-
nations of these elements, to have an austenitic structure, most or all of it when the steel is
cooled rapidly to room temperature. The chromium content
is
between 15 and 32%, nickel
between
8
and
37%,
and carbon is
restricted to a maximum of
0.03%.
Chromium provides
oxidation resistance and resistance to corrosion in certain media. An important source book on
stainless steels is Llewellyn
[
1041.

Material Selection and Fabrication
72
7
Types of Austenitic Stainless Steel
The conventional types of 3xx stainless steel include types such as 304, 304L, 309, 310, 316,
316L, 321, 347, and 348. The basic alloy Type 304 stainless steel contains
18%
chromium and
8%
nickel. It has moderate strength, excellent toughness, and moderate corrosion resistance.
Additional resistance to chloride pitting was achieved by the addition of
MO,
creating Types
316 and 317. Types 316
(18%
Cr,
12%
Ni, 2.5% MO) and 317 (18% Cr,
15%
Ni, 3.5% MO)
have greater resistance to corrosion in chloride environments than Type 304.
Alloy Development
The 18Cr-8Ni austenitic stainless steels have been successfully used in fresh water and mildly
corrosive industrial conditions for more than
50
years. The corrosion resistance, weldability
,
and strength of the austenitic family of alloys have been constantly improved for more demand-
ing industrial applications by changing the basic chemical composition
[
1051.
Such features
include:
1.
Molybdenum is added to enhance corrosion resistance in chloride environments, such as,
AISI Types 316 and 317. These steels possess a greatly increased resistance to chemical
attack as compared to that of the basic Cr-Ni Type 304.
2.
Low-carbon steels (Types 304L, 316L, and 317L) are resistant to carbide precipitation in
the 800-1 600°F range and can thus undergo normal welding without reduction
in
corrosion
resistance. These steels are generally recommended for use below 800°F.
3.
Nitrogen is added to compensate for the reduced strength of the low-carbon-grade steels (L
grades); it increases strength at all temperatures-cryogenic through elevated-improves
localized corrosion resistance in acid chloride solutions, and improves pitting resistance
and phase stability. Nitrogen addition also improves passivation characteristics, and en-
hances the effects of other alloy elements additions, particularly, Cr and
MO,
that add
corrosion resistance
[106].
Nitrogen addition may be of the order of
0.1
to 0.25%. Maxi-
mum nitrogen content in austenitic stainless steels is typically restricted to 0.25% by
weight to avoid problems such as ingot porosity, hot workability, and nitride precipita-
tion that are associated with excess nitrogen content. Nitrogen addition is denoted by the
suffix N.
4.
Chromium is increased to enhance pitting and crevice corrosion resistance.
5.
Nickel is added to stabilize the austenitic microstructure and to improve resistance to stress
corrosion cracking as well as general corrosion in reducing environments. The effect of
nickel on SCC of stainless steels is explained by the Copson curve.
6.
Stabilized grades: The addition
of
titanium and niobium forms stable carbides, which pre-
vents chromium depletion by the formation of complex chromium carbides, thereby avoid-
ing sensitization of weldments or heat-treated parts; examples are Type 321 (Ti stabilized)
and Type 347 (Nb stabilized).
7.
LR stands for low residuals, and in this case the restrictions are on carbon for corrosion
resistance. Reducing the carbon also allows the
Nb
to be reduced and
so
minimizes the
risk
of
Nb-rich interdendritic films. There
is
also restriction on Si,
S,
and
P
for liquation
cracking resistance. Manganese is usually raised to improve resistance to solidification
cracking.
Stainless Steel for Heat Exchanger Applications
Austenitic stainless steels are used primarily because of their low cost, corrosion resistance,
and good mechanical properties over a broad temperature range from cryogenics to high tem-
perature. They have been applied successfully in a large variety of environments including
acids, fresh water, and seawater. On the other hand, the martensitic and ferritic stainless steels

728
Chapter
I3
have acquired a more restricted field of application due to low toughness at room temperature.
Factors that favor austanitic stainless steels selection as heat exchanger material include the
following
[
107,1081:
High resistance to uniform corrosion, such as erosion corrosion.
Resistance to high-pH solutions.
Resistance to oxidation and sulfidation.
Suitability for intended fabrication techniques.
Can be easily maintained and cleaned to remove fouling deposits by most of the chemical and
mechanical means without damage.
Compatible with other materials commonly used for fabrication
of
heat exchangers.
Stability of properties in service.
Generally compatible with the process fluids.
Toughness at cryogenic temperature and strength at elevated temperatures.
Resistance to galling and seizing.
Moderate thermal conductivity.
Dimensional stability.
Newer Stainless Steels for Heat-Exchanger Service.
New steel making technologies like
argon-oxygen deoxidation (AOD) and vacuum induction melting
(VIM)
in the last two decades
have introduced a variety of new grades of ferritic, austenitic, and duplex stainless steel with
low impurity elements, and a wide range of alloying elements tailored to the requirements of
specific applications.
Against these
good
properties, the following are the demerits of stainless steels
[
1091:
1.
Sensitive to crevice corrosion under deposits.
2.
Sensitive to pitting corrosion and stress corrosion cracking in the presence of chloride ions
if temperature exceeds
50°C.
3.
Sensitization leads to intergranular corrosion.
4.
Sensitive to fouling.
Properties of Austenitic Stainless Steels
Stainless steels are known for excellent fabricability, weldability, good mechanical properties
(strength, toughness, and ductility) over a broad temperature range, and corrosion resistance in
many environments. Other relevant properties are lower melting points, higher electrical resis-
tance, lower thermal conductivity, and higher coefficients of thermal expansion than carbon
steels.
Mechanical Properties at Cryogenic Temperature and Elevated Temperature.
Although, aus-
tenitic stainless steels are used primarily because of their high corrosion resistance, they also
possess excellent mechanical properties over a wide range of temperature from cryogenic
to
elevated temperature.
Cryogenic Applications.
Unlike ferritic materials, austenitic stainless steels do not ex-
hibit ductile-to-brittle transition. They maintain a high level
of
toughness at cryogenic tempera-
tures. Austenitic stainless steels types such as
304, 304L, 316, 316L,
and
347
are used in
cryogenic applications for liquid gas storage and transportation vessels.
Elevated Temperature Strength.
Austenitic stainless steels exhibit good creep rupture
strength at temperatures up to
600°C.
If still higher creep strength and elevated temperature
strength are required, addition of
V,
Nb,
and Ti is necessary. Addition of these elements can
lead to an increase in strength, but also a reduction in the low-temperature toughness. Stainless
steels also exhibit high-temperature oxidation resistance due to the oxide layer formed on the
surface.
729
Material Selection and Fabrication
Alloying Elements and Microstructure
The microstructures most important in weldable stainless steels are ferrite and austenite. Al-
though chromium and nickel are the principal alloying elements in austenitic stainless steels,
other elements are added to meet specific requirements and therefore consideration must be
given to their effects on microstructure. Molybdenum, columbium, and titanium promote the
formation of delta ferrite in the austenitic matrix and also form carbides similar to that of
chromium. These elements are known as ferrite-forming elements. On the other hand, copper,
manganese, cobalt, carbon, and nitrogen have a similar effect to nickel and promote the forma-
tion of austenite. These elements are known as austenite-forming elements.
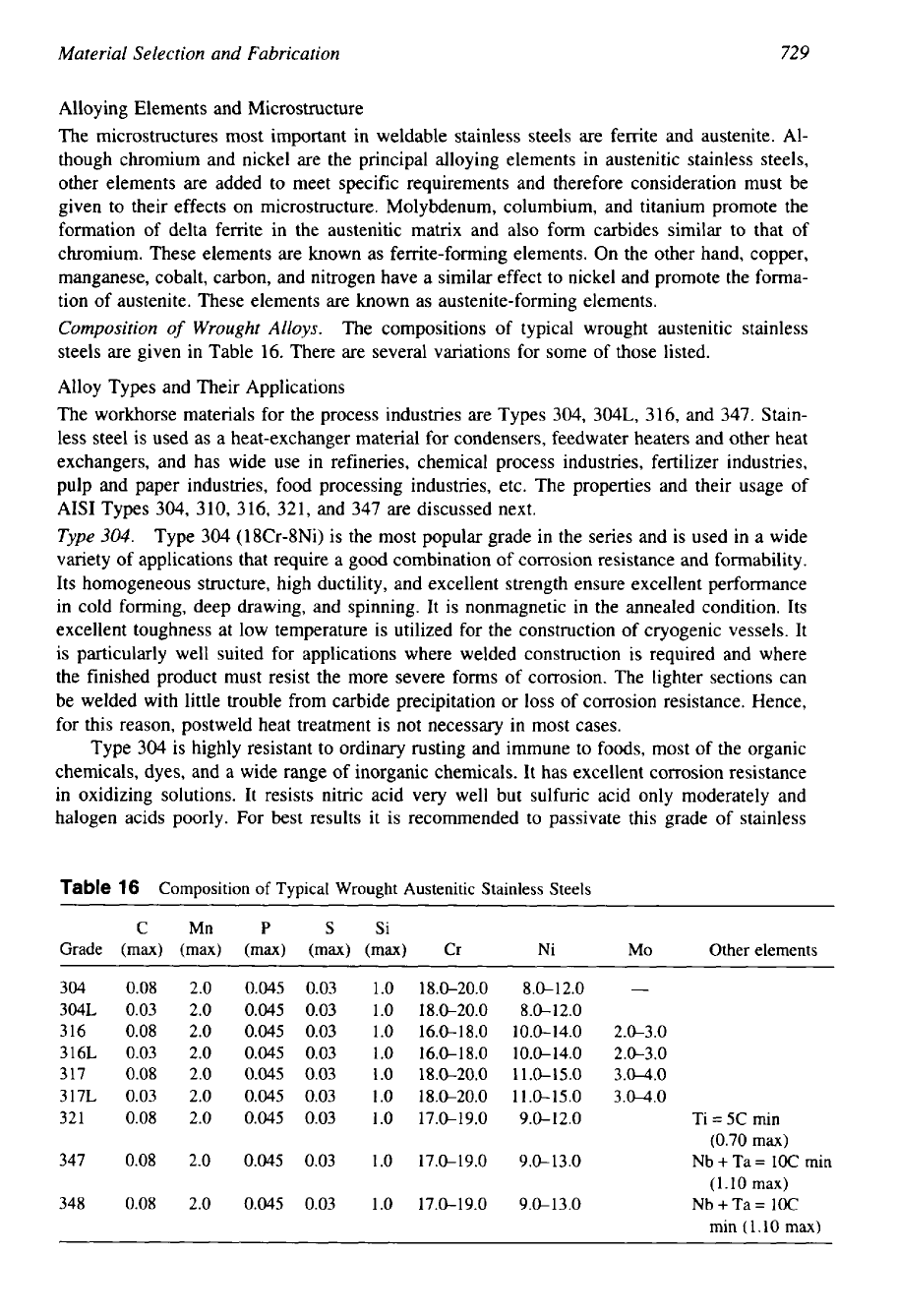
Composition
of
Wrought Alloys.
The compositions of typical wrought austenitic stainless
steels are given in Table 16. There are several variations for some of those listed.
Alloy Types and Their Applications
The workhorse materials for the process industries are Types 304, 304L, 316, and 347. Stain-
less steel is used as a heat-exchanger material for condensers, feedwater heaters and other heat
exchangers, and has wide use in refineries, chemical process industries, fertilizer industries,
pulp and paper industries, food processing industries, etc. The properties and their usage of
AISI Types
304,
3 10, 3 16, 321, and 347 are discussed next.
Type
304.
Type 304 (18Cr-8Ni) is the most popular grade in the series and is used in a wide
variety of applications that require
a
good combination of corrosion resistance and formability
.
Its homogeneous structure, high ductility, and excellent strength ensure excellent performance
in cold forming, deep drawing, and spinning. It is nonmagnetic in the annealed condition. Its
excellent toughness at low temperature is utilized for the construction of cryogenic vessels. It
is particularly well suited for applications where welded construction is required and where
the finished product must resist the more severe forms of corrosion. The lighter sections can
be welded with little trouble from carbide precipitation or loss of corrosion resistance. Hence,
for this reason, postweld heat treatment is not necessary in most cases.
Type 304 is highly resistant to ordinary rusting and immune to foods, most of the organic
chemicals, dyes, and
a
wide range of inorganic chemicals. It has excellent corrosion resistance
in oxidizing solutions. It resists nitric acid very well but sulfuric acid only moderately and
halogen acids poorly. For best results it is recommended to passivate this grade of stainless
Table
16
Composition
of
Typical Wrought Austenitic Stainless Steels
C
Mn P
S
Si
Grade
(max) (max)
(max) (rnax)
(rnax)
Cr
Ni
MO
Other elements
304
0.08 2.0
0.045
0.03 1.0
18.0-20.0
8.0-12.0
304L
0.03 2.0
0.045 0.03 1.0
18.0-20.0 8.0-12.0
3 16
0.08 2.0
0.045 0.03 1.0
16.0-1 8.0
10.0- 14.0
2.0-3.0
316L
0.03
2.0
0.045
0.03 1.0
16.0-1 8.0
10.0-14.0
2.0-3.0
317
0.08 2.0
0.045
0.03 1.0
18.0-20.0
11.0-15.0
3.04.0
317L
0.03 2.0
0.045
0.03 1.0
18.0-20.0
11.0-15.0
3.04.0
321
0.08 2.0
0.045
0.03 1.0
17.0-19.0
9.0-12.0
Ti
=
5C min
(0.70
max)
347
0.08 2.0
0.045 0.03
1.0
17.0-19.0
9.0-1 3.0
Nb
+
Ta
=
1OC
min
348
0.08 2.0
0.045 0.03 1.0
17.0-19.0
9.0-13.0
(1.10
max)
Nb
+
Ta
=
1OC
min
(1.10
max)

730
Chapter
13
steel to retain its corrosion resistance. It resists scaling up to 1600°F. For intermittent heating
and cooling applications, temperatures should not exceed 1500°F. The maximum temperature
for continuous service is 1650°F
[
1
101.
Type
310.
Type 3 10 (25Cr-20Ni) represents the most highly alloyed composition in the popu-
lar range of austenitic stainless steels and exhibits the greatest resistance to corrosion and
oxidation.
Type
316.
The addition of molybdenum gives this grade the highest resistance to pitting
corrosion of any of the chromium-nickel grades, which makes this grade particularly suitable
for applications involving severe chloride corrosion. Therefore, alloys 3 16 and 3 16L are “work-
horse” materials in both the chemical industries and pulp and paper industries
[
11
11.
Type
316
has excellent resistance to sulfates, chlorides, phosphates, and other salts. Nevertheless, the
resistance of Types 316 and 316L to pitting and crevice corrosion is often not high enough,
particularly in stagnant or slow-moving seawater (~1.5
m/s).
for this reason,
in
the last two
decades, several more highly alloyed grades have been developed, They are known as superfer-
ritic, duplex, superaustenitic stainless steels.
Type 3 16 is used for applications requiring high strength and creep resistance at elevated
temperatures. Its molybdenum content provides higher creep strength than Type 304. Type
3
I6
has excellent resistance to oxidation and has a low rate of scaling in ordinary atmosphere
at
temperatures up to 1650°F in continuous service and 1500°F when used intermittently
(1
10).
It
can
be
welded without difficulty and usually does not require subsequent heat treatment.
This steel is used for applications requiring high strength and creep resistance at the elevated
temperatures. Its molybdenum content provides several times the resistance to creep that
is
available with Type 304. This grade is used for chemical and pulp handling equipment, and
photograpic and food processing equipment.
Because of their freedom from chromium carbide precipitation and intergranular
attack
Type 321 (18%Cr, 10.5%Ni, Ti), Type 347 (18%Cr, 11% Ni, Nb), and Type
348
(
8%Cr
11% Ni, Nb) are often referred to as stabilized stainless steels.
16.4
Mechanism
of
Corrosion Resistance
Stainless steels owe their corrosion resistance to a relatively thin passive surface film that
provides a physical barrier between the steel and the service environment. This layer, only
20-
30 angstroms thick, of hydrated chromium oxide (Cr203), is extremely adherent and resistant
to chemical attack [15]. The passive films are formed on the surface interacting with the
environment, essentially containing
0:
or oxidizing conditions.
If
the passive film is damaged
by abrasion or scratching, a healing process or repassivation occurs almost immediately in the
presence of oxygen. On the other hand, stainless steel will show rapid corrosion attack in
reducing conditions or under crevices or local deposits, which produce an
0,
depleted zone
[161.
Another reason for loss in corrosion resistance of stainless steels is the formation of oxide
films on the surface due to bad cleaning after heat treatment. The oxide film is different from
passive film. Cleaning of heat-treated components should be done in an inert solution to avoid
the formation of oxide films, which render the
SS
susceptible to various forms of corrosion
[107].
Sigrna
Phase.
Sigma phase, an intermetallic compound, in stainless steels may significantly
reduce their ductility and toughness, and renders stainless steel susceptible to stress corrosion
cracking and other forms of corrosion.
The
sigma phase is one cause of low ductility creep failure of certain austenitic
welds[
1
121. When cooling a weld metal from
1800
to 1000”F, the rate of cooling must be

731
Material Selection and Fabrication
relatively rapid to avoid the formation of a sigma phase. Avoidance of prolonged time in this
temperature range will usually result in a weld metal containing very small amounts of sigma
phase, which is generally harmless. Like sensitization, precipitation of intermetallic phases can
be corrected by solution annealing. In newer varieties of austenitic stainless steels, namely,
superaustenitics, alloying with nitrogen retards sigma-phase formation and allows for the pro-
duction of thicker plates
[
1131.
Passive Versus Active Behavior.
In most natural environments, stainless steel will remain in
a passive state. When exposed to conditions that remove the passive film, stainless steels are
subject to the active state. Change to an active state usually occurs when chloride concentra-
tions are high, as in seawaters or in reducing solutions, or when there is oxygen starvation.
Oxygen starvation occurs when there is no free access to oxygen, such as
in
crevices and
beneath deposits.
Resistance to Chemicals.
Stainless steel alloys have excellent resistance to nitric acid, particu-
larly to all concentrations and temperatures. Type 304 is most widely used in nitric acid plants.
To
handle sulfuric acid without inhibitors, Type
3
16
stainless steel has limited application.
Stainless Steel
in
Seawater.
While Type 304
(
18Cr-8Ni) alloys have been satisfactorily used
in fresh water, the MO-containing Type 316 is preferred in saltwater. Variable results have
been obtained with Type
316
in seawater. In the case of seawater-cooled condenser tubes,
satisfactory use was obtained where tubes were cleaned regularly
in
service
to
prevent fouling.
However, the alloy content of Type 316 was found to be too lean to prevent pitting and crevice
corrosion
in
stagnant seawater. This is because of the breakdown of the passive film by the
chloride ions
in
the seawater under stagnanaow velocity conditions.
Resistance to Various
Forms
of
Corrosion.
In general, the corrosion and oxidation resistance
of stainless steels increases with chromium content, and the materials are used in a wide range
of aggressive media in the chemical and process industries. They are resistant to uniform
corrosion, erosion and erosion-corrosion, and high-pH solution
[
1091. However, under certain
conditions, stainless steel are attacked by localized corrosion forms.
Galvanic Corrosion.
Consideration should be given to when stainless steel is connected
to a more noble metal. However,
if
the stainless steel
is
passive in the environment, galvanic
corrosion may not take place. The most important aspect for the prevention of galvanic corro-
sion of stainless steel
is
to select the weldments and fasteners
of
adequate corrosion resistance
in the bulk of the material andor possessing larger exposed area.
Localized Forms of Corrosion.
Under certain conditions, stainless steels are susceptible
to highly localized forms of corrosion. For stainless steels, almost
60%
of equipment failures
in the chemical process industries are due to (1) pitting corrosion,
(2)
crevice corrosion, and (3)
stress corrosion cracking
(SCC)
[
1
141. Additionally, they also fail by intergranular corrosion.
Operating parameters and environmental factors such as pH, temperature, and chloride and
oxygen levels greatly affect alloy performance. Various forms of
SS
localized corrosion are
discussed next.
Pitting Corrosion. The corrosion resistance
of stainless steels relies on the stability and
the maintenance of continuity of passive films on the exposed surfaces. The stability of the
passive film to resist pitting initiation is controlled mainly by chromium and molybdenum
contents in stainless steels. Another element that improves resistance to pitting and crevice
corrosion is nitrogen
[
100,1151. Nitrogen addition is considerably cheaper than molybdenum
steels. The breakdown of the passive films takes place due to imperfections in the films,
mechanical damages, inhomogeneities in the metal surface such as inclusions or surface scale,
732
Chapter
I3
precipitates, second phases, and the presence of chloride ions in the environment
[
1
161.
High
chloride levels locally break down the passive film, as well as provide a good conductor that
promotes corrosion
[
1171.
The severity of attack is related to the chloride content and the
acidity, pH, and presence of oxygen and oxidizers
[
1
141.
Welding-related factorddefects such
as inclusions, secondary phases, compositional differences within the same phase, sensitization,
arc strikes, spatter, and local inhomogeneities in the base material act
as
potential sites where
pitting can initiate
[
118,1191.
In terms of microstructure, MnS inclusions are important sites
for pit initiation. Other features such as delta ferrite and sigma phase can also promote pitting
corrosion.
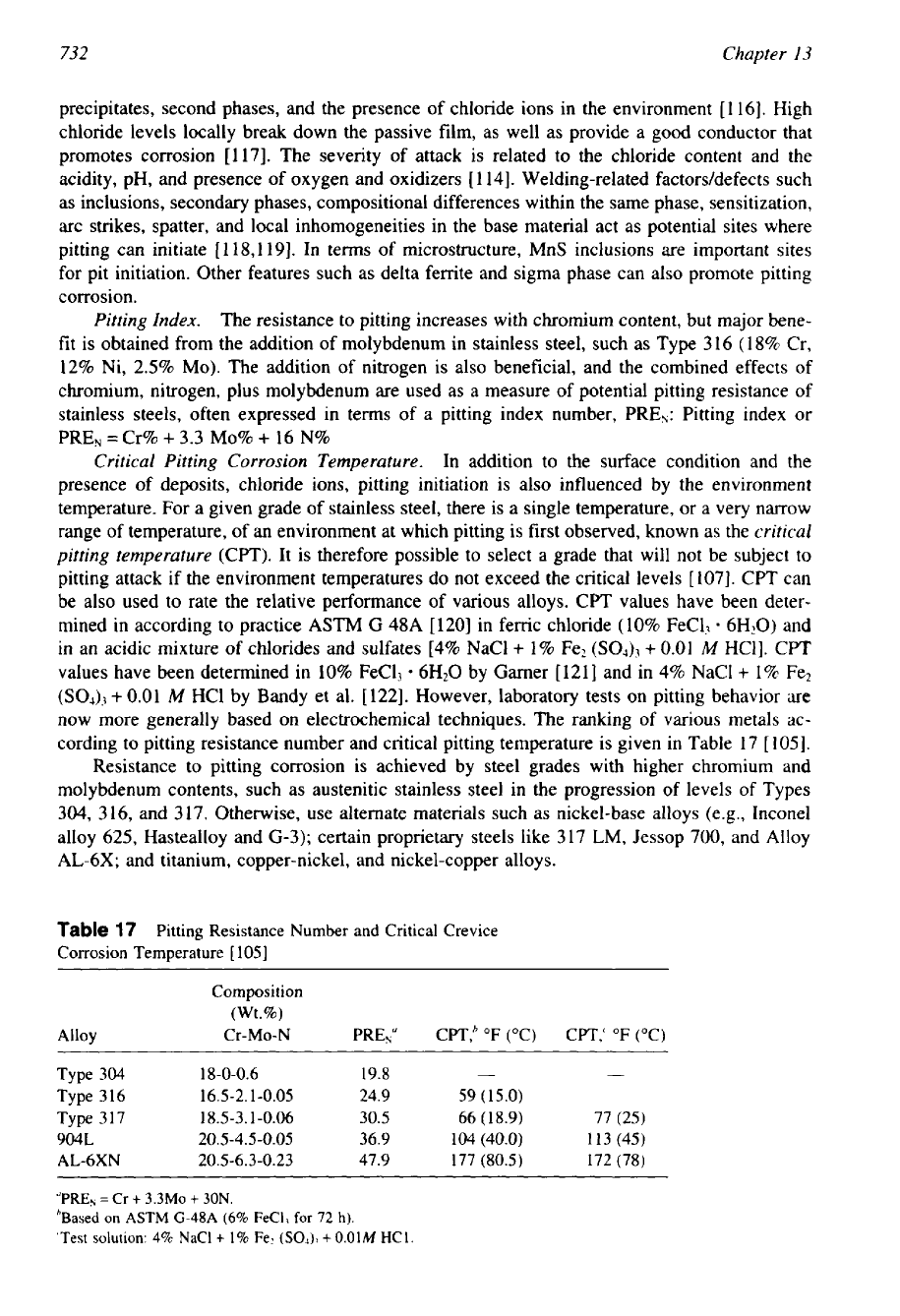
Pitting Index.
The resistance to pitting increases with chromium content, but major bene-
fit is obtained from the addition of molybdenum in stainless steel, such as Type
316 (18%
Cr,
12%
Ni,
2.5%
MO).
The addition of nitrogen is also beneficial, and the combined effects of
chromium, nitrogen, plus molybdenum are used as a measure of potential pitting resistance
of
stainless steels, often expressed in terms
of
a
pitting index number, PRE,: Pitting index
or
PRE,
=
Cr%
+
3.3
MO%
+
16
N%
Critical Pitting
Corrosion
Temperature.
In addition to the surface condition and the
presence of deposits, chloride ions, pitting initiation
is
also influenced by the environment
temperature. For a given grade of stainless steel, there is a single temperature, or a very narrow
range of temperature, of an environment at which pitting is first observed, known as the
critical
pitting temperature
(CPT). It is therefore possible to select a grade that will not be subject to
pitting attack if the environment temperatures do not exceed the critical levels
[
1071.
CPT can
be also used
to
rate the relative performance of various alloys. CPT values have been deter-
mined in according to practice
ASTM
G
48A
[
1201
in ferric chloride
(
10% FeC13
*
6H20)
and
in an acidic mixture of chlorides and sulfates
[4%
NaCl
+
1%
Fez
(SO4),
+
0.01
M
HCl]. CPT
values have been determined in 10% FeC1,
6H20
by Garner
[
1211
and in
4%
NaCl
+
1
%
Fez
(SO4),
+
0.01
M
HC1 by Bandy et al.
[
1221.
However, laboratory tests on pitting behavior
are
now more generally based on electrochemical techniques. The ranking of various metals ac-
cording to pitting resistance number and critical pitting temperature is given in Table
17
[
1051.
Resistance to pitting corrosion is achieved by steel grades with higher chromium and
molybdenum contents, such as austenitic stainless steel in the progression of levels of Types
304, 316,
and
317.
Otherwise, use alternate materials such as nickel-base alloys (e.g., Inconel
alloy
625,
Hastealloy and
G-3);
certain proprietary steels like
317
LM,
Jessop
700,
and Alloy
AL-6X;
and titanium, copper-nickel, and nickel-copper alloys.
Table
17
Pitting Resistance Number
and
Critical Crevice
Corrosion Temperature
[
1051
Composition
(Wt.%)
Alloy
Cr-MO-N
PRENu CPT,’
OF
(“C) CPT,‘
OF
(“C)
Type
304
18-0-0.6 19.8
- -
Type
316 16.5-2.1-0.05 24.9 59 (15.0)
Type
317 18.5-3.1-0.06 30.5 66 (18.9) 77 (25)
904L 20.5-4.5-0.05 36.9 104 (40.0) 113 (45)
AL-6XN 20.5
-6.3
-0.23 47.9 177 (80.5) 172 (78)
“PREN
=
Cr
+
3.3Mo
+
30N.
’Based
on
ASTM G-48A
(6%
FeC1, for
72
h).
‘Test
solution:
4%
NaCl
+
1%
Fez
(SO4),
+
0.01M
HC1
733
Material Selection and Fabrication
Crevice Corrosion. Crevices due to metal-to-metal joint, gasket, and fouling deposits,
which tend to restrict oxygen access, result in crevice corrosion. For austenitic stainless steels,
numerous factors affect both crevice corrosion initiation and propagation and include the fol-
lowing
[
1231:
Geometrical factors: crevice type (metal-to-metal, nonmetal-to-metal), crevice gap and
depth, exterior to interior surface area ratio.
Environmental factors: oxygen content, pH, chloride level, temperature, agitation, diffu-
sion and convection, crevice solution, biological influences.
Electrochemical reactions: metal dissolution, oxygen reduction, hydrogen evolution.
Metallurgical factors: alloy composition impurities, passive film characteristics.
Although it is unlikely that one can avoid design crevices, the problem can be minimized by
maintaining uniform flow velocity in the bulk
of
the heat exchanger and using higher chro-
mium and molybdenum grades, which are more resistant to crevice corrosion. Austenitic stain-
less steel grades with higher MO contents, like
316L, 904L,
and
254
SMO, ferritic grades like
18Cr-2M0, and duplex steel like
2205
exhibit resistance to crevice attack.
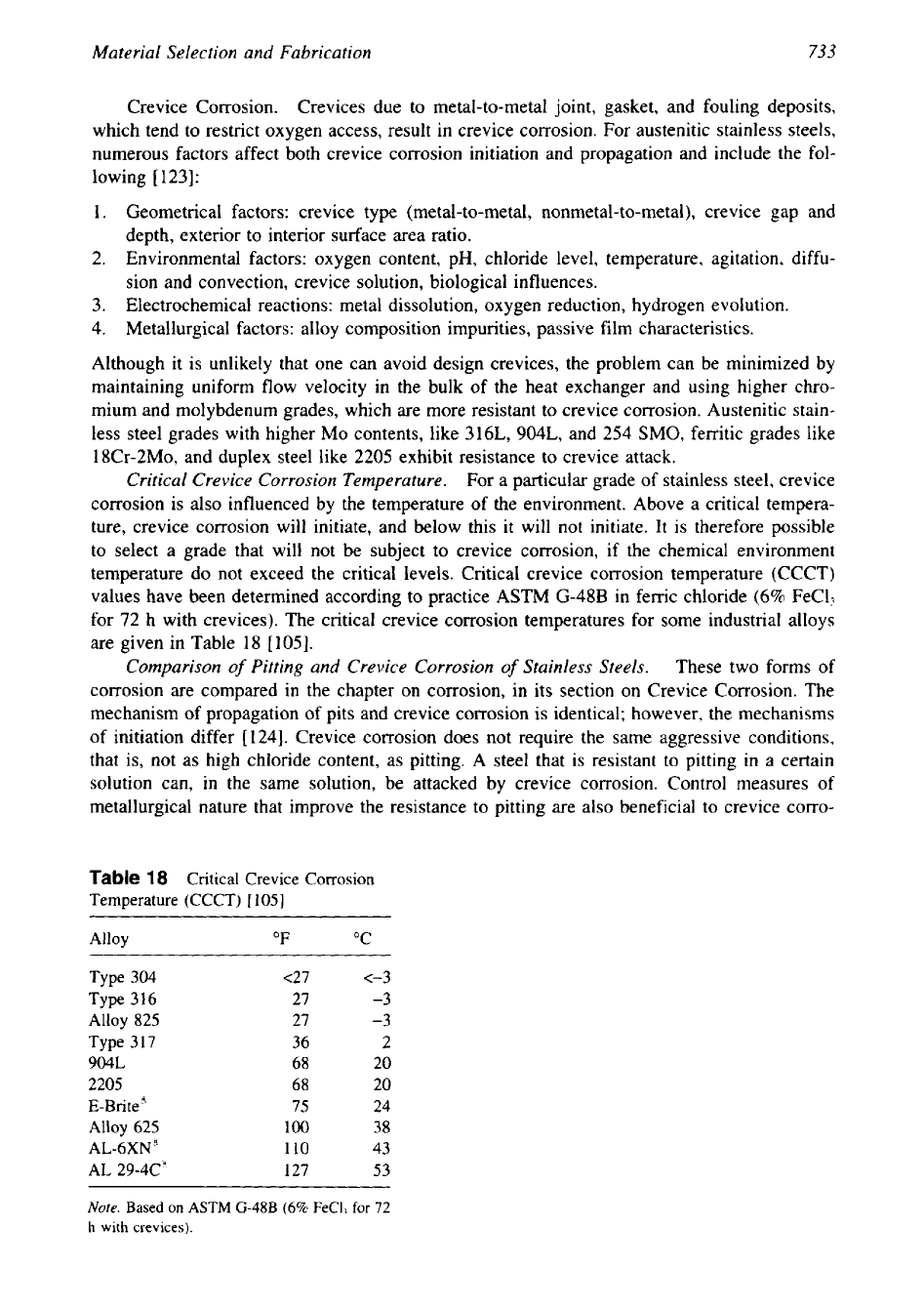
Critical Crevice Corrosion Temperature.
For a particular grade of stainless steel, crevice
corrosion is also influenced by the temperature of the environment. Above a critical tempera-
ture, crevice corrosion will initiate, and below this it will not initiate. It is therefore possible
to select a grade that will not be subject to crevice corrosion, if the chemical environment
temperature do not exceed the critical levels. Critical crevice corrosion temperature (CCCT)
values have been determined according to practice ASTM
G-48B
in ferric chloride
(6%
FeCl?
for
72
h with crevices). The critical crevice corrosion temperatures for some industrial alloys
are given in Table
18
[105].
Comparison
of
Pitting and Crevice Corrosion
of
Stainless Steels.
These two forms
of
corrosion are compared in the chapter on corrosion, in its section on Crevice Corrosion. The
mechanism of propagation of pits and crevice corrosion is identical; however, the mechanisms
of initiation differ
[
1241.
Crevice corrosion does not require the same aggressive conditions,
that
is,
not as high chloride content, as pitting.
A
steel that
is
resistant to pitting in
a
certain
solution can, in the same solution, be attacked by crevice corrosion. Control measures
of
metallurgical nature that improve the resistance
to
pitting are also beneficial to crevice corro-
Table
18
Critical Crevice Corrosion
Temperature (CCCT)
[
1051
Alloy
"F
"C
Type
304
c27
<-3
Type
316
27
-3
Alloy
825
27
-3
Type
317
36
2
904L
68
20
2205
68
20
E-Brite"
75
24
Alloy
625
100
38
AL-6XN'
110
43
AL
29-4C'
127
53
Note.
Based
on
ASTM
G-48B
(6%
FeCl]
for
72
h
with
crevices).
