Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

754
Chapter
I3
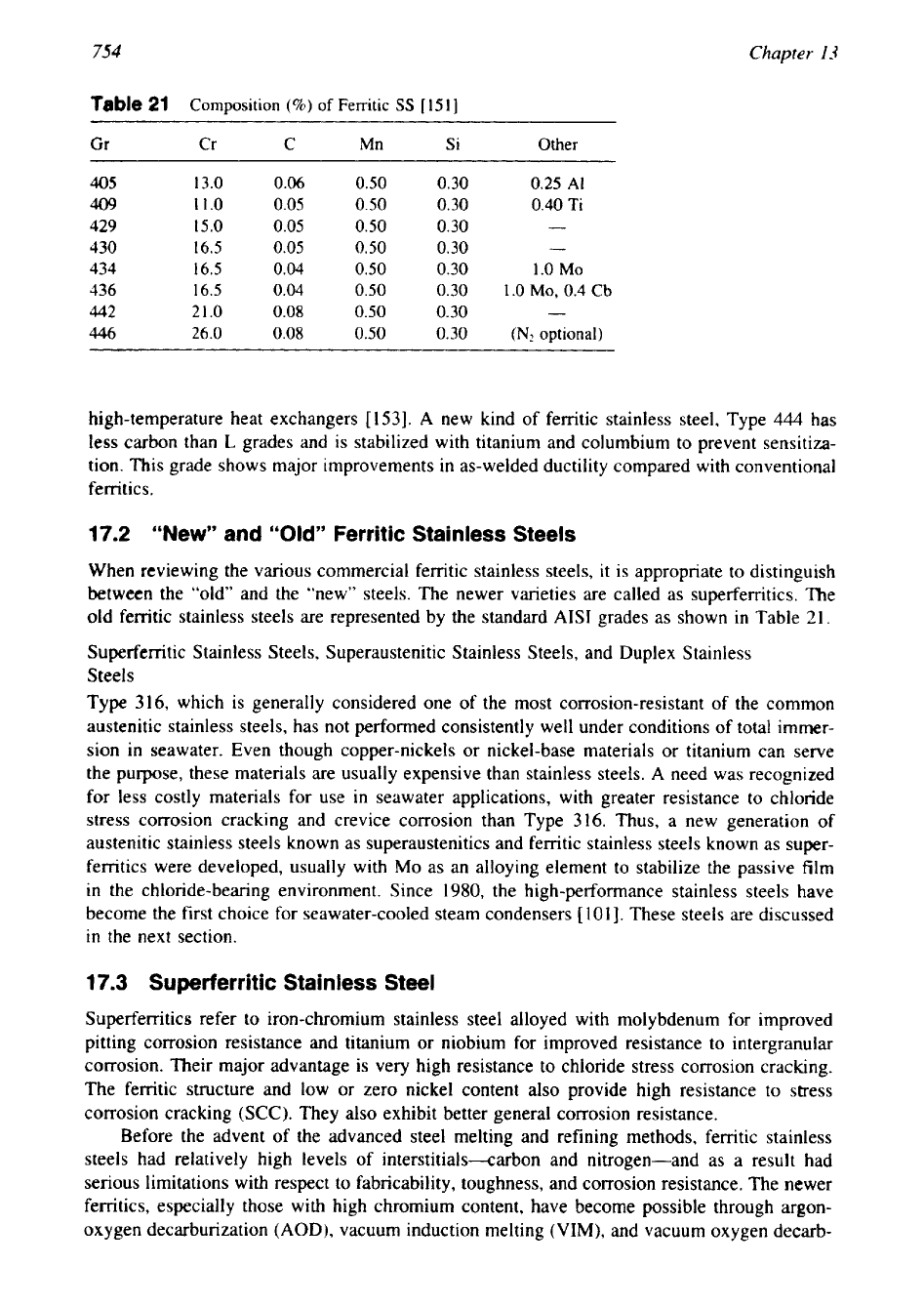
Table
21
Composition
(%I
of
Ferritic
SS
[I511
Gr
Cr
C
Mn
Si
Other
405
13.0
0.06
0.50
0.30
0.25
A1
409
11.0
0.05
0.50
0.30
0.40
Ti
429
15.0
0.05
0.50
0.30
-
430
16.5
0.05
0.50
0.30
-
434
16.5
0.04
0.50
0.30
1.0
MO
436
16.5
0.04
0.50
0.30
1.0
MO,
0.4
Cb
442
21.0
0.08
0.50
0.30
-
446
26.0
0.08
0.50
0.30
(N,
optional)
high-temperature heat exchangers [153]. A new kind of ferritic stainless steel, Type
444
has
less
carbon than
L
grades and is stabilized with titanium and columbium to prevent sensitiza-
tion. This grade shows major improvements
in
as-welded ductility compared
with
conventional
ferrit ics,
17.2
“New” and “Old” Ferritic Stainless Steels
When reviewing the various commercial ferritic stainless steels, it is appropriate to distinguish
between the “old” and the “new” steels. The newer varieties are called as superferritics. The
old ferritic stainless steels are represented by the standard AISI grades as shown
in
Table
21.
Superferritic Stainless Steels, Superaustenitic Stainless Steels, and Duplex Stainless
Steels
Type
316,
which is generally considered one of the most corrosion-resistant of the common
austenitic stainless steels, has not performed consistently well under conditions
of
total immer-
sion in seawater. Even though copper-nickels or nickel-base materials or titanium can serve
the purpose, these materials are usually expensive than stainless steels.
A
need was recognized
for less costly materials for use
in
seawater applications, with greater resistance to chloride
stress corrosion cracking and crevice corrosion than Type 316. Thus, a new generation of
austenitic stainless steels known as superaustenitics and ferritic stainless steels known as super-
ferritics were developed, usually with
MO
as an alloying element to stabilize the passive film
in
the chloride-bearing environment. Since
1980,
the high-performance stainless steels have
become the first choice for seawater-cooled steam condensers
[
1011.
These steels are discussed
in
the next section.
17.3
Superferritic Stainless Steel
Superferritics refer to iron-c~omium stainless steel alloyed with molybdenum for improved
pitting corrosion resistance and ti~ium or niobium for improved resistance to intergranular
corrosion. Their major advantage is very high resistance to chloride stress corrosion cracking.
The ferritic structure and low or zero nickel content
also
provide high resistance to stress
corrosion cracking
(SCC),
They also exhibit better general corrosion resistance.
Before the advent of the advanced steel melting and refining methods, femtic stainless
steels had relatively high levels of interstitials-carbon and nitrogen-and as a result had
serious limitations with respect to fabricability, toughness, and corrosion resistance.
The
newer
ferritics, especially those with high c~omium content, have become possible through argon-
oxygen decarburization (AOD), vacuum induction melting
(VIM),
and vacuum oxygen decarb-
755
Material Selection and Fabrication
urization (VOD). All are capable of producing low interstitials content [154]. During the refin-
ing stage, care is taken to reduce interstitials to very low levels andor to “tie up” carbon and
nitrogen by stabilizing them with elements such as titanium or niobium.
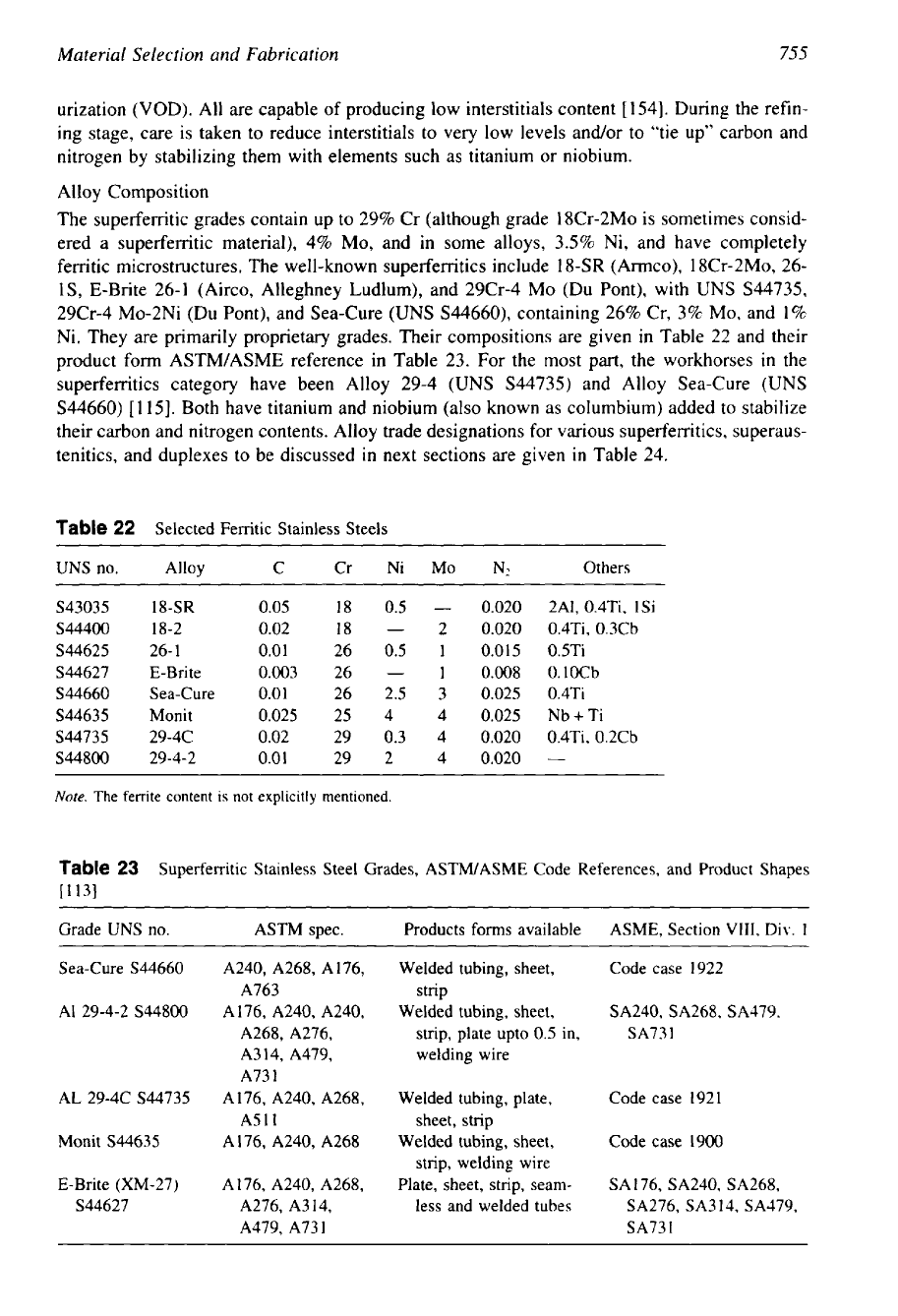
Alloy Composition
The superferritic grades contain up to 29% Cr (although grade 18Cr-2Mo is sometimes consid-
ered a superferritic material), 4% MO, and in some alloys, 3.5% Ni, and have completely
ferritic microstructures. The well-known superferritics include
18-SR
(Armco), 18Cr-2M0, 26-
lS,
E-Brite 26-1 (Airco, Alleghney Ludlum), and 29Cr-4 MO (Du Pont), with
UNS
S44735,
29Cr-4 MO-2Ni (Du Pont), and Sea-Cure (UNS S44660), containing 26% Cr, 3% MO, and
1%
Ni. They are primarily proprietary grades. Their compositions are given in Table
22
and their
product form ASTM/ASME reference in Table 23. For the most part, the workhorses
in
the
superferritics category have been Alloy 29-4 (UNS S44735) and Alloy Sea-Cure (UNS
S44660) [115]. Both have titanium and niobium (also known as columbium) added to stabilize
their carbon and nitrogen contents. Alloy trade designations for various superferritics, superaus-
tenitics, and duplexes to be discussed in next sections are given
in
Table 24.
Table
22
Selected Ferritic Stainless Steels
UNS no. Alloy C Cr Ni MO
N2
Others
S43035
18-SR
0.05
18
0.5
-
0.020
2A1,
0.4Ti, 1Si
S44400 18-2 0.02
18
-
2
0.020 0.4Ti, 0.3Cb
S44625
26-
1
0.01
26
0.5
1
0.015 0.5Ti
S44627
E-Brite 0.003
26
-
1
0.008 0.1OCb
S44660
Sea-Cure 0.01
26 2.5
3
0.025 0.4Ti
S44635
Monit 0.025
25 4 4
0.025 Nb+Ti
s44735
29-4C 0.02
29
0.3 4
0.020 0.4Ti, 0.2Cb
S44800
29-4-2
0.01
29 2 4
0.020
-
Note.
The ferrite content
is
not explicitly mentioned.
Table
23
Superferritic Stainless Steel Grades, ASWASME Code References, and Product Shapes
11
131
Grade UNS no. ASTM spec. Products
forms
available ASME, Section
VIII,
Div.
1
Sea-Cure S44660 A240, A268, A176,
Welded tubing, sheet,
Code case 1922
A763
strip
AI 29-4-2 S44800 A176, A240, A240,
Welded tubing, sheet,
SA240, SA268, SA479,
A268, A276,
strip, plate upto
0.5
in,
SA73
1
A314, A479,
welding wire
A73
1
AL 29-4C S44735
A176, A240, A268,
Welded tubing, plate,
Code case 192
1
A511
sheet, strip
Monit S44635 A176, A240, A268
Welded tubing, sheet,
Code case 1900
strip, welding wire
E-Brite (XM-27) A176, A240, A268,
Plate, sheet, strip, seam-
SA 176, SA240, SA268,
S44627 A276, A314,
less and welded tubes
SA276, SA3 14, SA379,
A479, A731
SA73
1

756
Chapter
13
Table
24
Alloy Trade Designations
~
Alloy UNS
no.
Registered trade name Producers
S44635
Moni
t
Uddeholm Steel Corp.
S44627
E-Brite
Allegheny Ludlum Steel Corp.
s44735
AL 29-4C
Allegheny Ludlum Steel Corp.
S44660
Sea-Cure
Trent Tube Div.
of
Colt
Ind.,
Crucible
Steel
Co.
S44800
AL 29-4-2C
Allegheny Ludlum Steel Corp.
S3
1254
254
SMO
Avesta Jarnverks AD
NO8904 AL 904L
Allegheny Ludlum Steel Corp.
NO8366
AL-6X
Allegheny Ludlum Steel Corp.
NO8367 AL-6XN
Allegheny Ludlum Steel Corp.
NO8925
1925hM0, 25-6M0
VDM Technologies, Inco-Alloys Internationals
NO8026 20M0-6
Carpenter Technology
Note.
This
table gives only partial information on
various
alloy manufactures.
Applications
Superferritics have found extensive applications as tubing for power plant condensers, and heat
exchangers handling chloride solutions, seawater, and brine. Types 18Cr-2M0, E-Brite
26-
1,
Sea-Cure, and AL 29-4 are used in the petrochemical and chemical industries and
in
seawater
applications. Because of its high chromium content, E-Brite
26-
1
offers substantial resistance
to oxidation and sulfidation at elevated temperatures. The E-Brite alloy is being used in air
preheaters and heat recuperators.
Physical Properties
The ferritic stainless steels offer two physical properties that are of particular importance
[
1541:
1.
Their thermal conductivity is about
50%
higher than that of the austenitics, or about half
that of carbon steel.
2.
Their thermal expansion coefficient is low, roughly equal to that of carbon steel and about
30%
less than that of type
304
stainless steel.
Strength.
The strength of ferritics is compared with austenitic stainless steels as follows
[I%]:
1.
The room-temperature yield strength in the annealed condition is 2040% higher than that
of the austenitic steels.
2.
The rate of work hardening of the ferritic steels is lower.
3.
At high temperature, the ferritic steels have lower strength than the austenitics, but their
oxidation resistance is very good.
Toughness and Embrittling Phenomena.
Toughness of the ferritic stainless steels
is
influ-
enced by three embrittling mechanisms
[
15
11:
(1)
“‘885°F (475°C) embrittlement,”
(2)
sigma
phase precipitation, and
(3)
high-temperature embrittlement. Each of these conditions lowers
the ductility and toughness of the ferritic stainless steels at room temperature and hence im-
poses restriction on the fabricator and user in various operations such as hot forming, brazing,
welding, and heat treating
in
order to minimize high-temperature exposure
[
1551.
“885’F
(475°C) Embrittlement.”
All ferritic stainless steels, with the possible exception
of Type
409,
are susceptible to this embrittlement phenomenon when exposed in the tempera-
ture range of 720-950°F (400-540°C). Peak hardening and embrittlement occur at approxi-
mately 885°F as a result of the formation and precipitation of alpha prime, a coherent chro-

757
Material Selection and Fabrication
mium-rich particle in the iron-rich matrix [151]. Consequently, an increase in hardness and
tensile strength is observed, concurrent with substantial decrease in ductility and impact resis-
tance [156]. The phenomenon is observed at chromium levels
in
excess of about 12%. In order
to overcome this form of embrittlement during processing, fast cooling is recommended
through the 700-950°F temperature range. Consequently, neither the new nor the old ferritics
should be exposed to this temperature range
[
15 1,1541.
Sigma Phase Precipitation.
Sigma phase may
form
in the higher chromium ferritic steels
(15 to 20%) when they are exposed for prolonged periods at
1100
to 1500°F. The precipitation
results in room-temperature embrittlement as well as loss of corrosion resistance
[
15
I]. Forma-
tion of sigma phase may be minimized or prevented by a proper selection of composition, or
sigma phase may be transformed into austenite and ferrite by suitable heating, followed by
water quenching or rapidly cooling by other means.
High-Temperature Embrittlement.
When high-chromium stainless steels of intermediate
and high interstitial content are heated normally above about 1000°C and cooled to room
temperature, precipitation of carbide and nitride can result in embrittlement as well as suscepti-
bility to intergranular corrosion, especially in alloys containing high interstitial elements
[
15 11.
Ductile-Brittle Transition.
The superferritic stainless steels with body-centered cubic (bcc)
crystal structure exhibit a ductile-to-brittle transition temperature (DBTT) that for many grades
is close to room temperature. The interstitial elements carbon and nitrogen have a potential
effect on the DBTT; a low interstitial elements content is essential for a low DBTT and hence
good toughness at room temperature. For this reason, all the new ferritic stainless steels have
very low interstitial elements content, obtained by special melting techniques
[
15 11. The new
grades are acceptably tough as both sheet and tubing, and therefore these alloys are typically
used in thin sections, such as tubing, light-wall pipe, sheet, and strip. However, VIM-produced
superferritics have extremely low carbon and nitrogen levels and thus improved toughness.
These materials are usually used in all product forms up to plate thickness of about
0.50
in
(12.7 mm) [loll.
Corrosion Resistance
Because the chromium content of the ferritic stainless steels ranges from
I1
to
29%,
the general
corrosion resistance can
vary
from moderate to excellent [151]. An important feature of the
ferritic stainless steels is their resistance to chloride stress corrosion cracking. Because the
austenitic steels are highly susceptible to SCC, the ferritic steels provide a more economical
alternative than the nickel-base alloys for applications requiring resistance to SCC. Superfer-
ritic stainless steels offer outstanding resistance to chlorides, alkalies, nitric acid, uredammo-
nium carbomate, amines, and organic acids
[
1521. Additions of molybdenum increase the resis-
tance to pitting corrosion by producing a more stable passive film. The pitting resistance
equivalent (PRE) number
is
calculated using the formula
PRE
=
%Cr
+
3.3(%Mo)
Intergranular Corrosion in Ferritic Stainless Steels.
A major obstacle to the use of ferritic
stainless steels has been their susceptibility to intergranular corrosion
[
1571. Ferritic stainless
steels are sensitized when exposed to a temperature
of
about 1800°F (982°C) due to welding
or improper heat treatment, and this makes them susceptible to intergranular corrosion. The
mechanism responsible for intergranular corrosion is generally accepted as being the same as
that in austenitic stainless steels [158]. This problem is overcome by alloying with titanium
andor columbium to form the carbides of these elements. Lowering the carbon content below

7.58
Chapter
13
0.03%,
as in austenitic stainless steels, is not effective. Even with
0.01%
maximum carbon
content, it is necessary to add carbide-stabilizing elements
[
15
11.
Fabricability
The ductility and toughness of the ferritic stainless steels are low at room temperature. The
sequence of fabrication operations, and often the design, should consider the relatively low
toughness and ductility of the material at room temperature. Cold-forming operations must
also be commensurate with possibly reduced ductility and toughness
[
1551.
Welding
The two most important factors in making good welds in the superferritics are
(1)
the mainte-
nance of alloy purity and
(2)
that ferritic weld metal structure cannot be refined by heat treat-
ment
[103].
Superferritic stainless steels tend to retain the problem of conventional ferritic
steels in relation
to
grain growth
in
HAZ, grain coarsening in the weld metal, and
loss
of
toughness after welding and reduced corrosion resistance due to sensitization and sigma phase
precipitation. The problems faced while welding ferritic stainless steels are summarized by
Walker
[
1591:
Grain growth.
Coarse-grained weld metal.
Reduced impact strength.
Reduced corrosion resistance due to sensitization and precipitation
of
sigma phase.
Being highly magnetic, they are subjected to arc blow during welding. The following measures
will help to reduce some of these problems
[
1591:
TIG welding with good shielding and backing.
Extreme cleanliness of weld preparation.
Autogenous welding or matching filler.
Low heat input to minimize grain growth.
Since the high quality of these steels is based on low carbon and nitrogen contents,
it
is
essential that the pick up of these elements during the welding process should be avoided due
to the risk of chromium carbide and nitride formation, resulting in loss of corrosion resistance
[103,140].
Avoid the pickup of oxygen and hydrogen
also.
Careful shielding, front and back,
with dry argon or helium is necessary
[
1541.
Cleanliness and extraordinary inert gas shielding
are essential for successful welding, particularly when matching filler metal is used.
Low heat input is necessary to limit grain growth. The grain growth problems can also be
minimized by using austenitic filler materials, and still there is some grain growth
in
the
HAZ;
this can be reduced by keeping preheat temperature below 200°C
[
1371.
The ferritics are less prone to welding defects than the austenitics, but their notch sensitiv-
ity makes any defects that are present much more detrimental. Therefore, special care must be
taken to avoid undercutting or poor penetration, and thorough inspection of welds is essential
[154].
Sugace
Preparation and
Joint
Design.
Surface preparation and cleaning before and after
welding are important for assured weld quality and corrosion resistance. Edge preparation of
weld joints includes removal of all contaminated metal by machining or grinding the thermally
cut surfaces. Weld joints and filler metal surfaces must be free of surface contaminants.
A
nonchlorinated, residue-free solvent, such as methyl ethyl ketone (MEK), is useful for
this

Material Selection and Fabrication
759
purpose. Cleaning should immediately precede welding. Optimum corrosion resistance is ob-
tained by mechanically or chemically removing the heat tint after welding.
Good Practices
for
Welding Ferritic Stainless Steels.
Beigay et al. [140] discuss good prac-
tices for ferritic stainless steel welding. Some of the good practices include the following:
1.
Minimize contamination by running stringer beads. Stringer beads are preferred over a
“weaving” technique.
2.
Keep ends of filler rods protected in shielding
gas.
Filler metal must not be pulled
in
and
out of the shielding gas. Once pulled away from the shielding gas, the contaminated tip
must be removed before the wire is used again.
3.
Welding techniques that minimize contamination must be employed.
4.
Allow shielding gas to flow after extinguishing the arc, protecting the weld bead and filler
tip until both cool sufficiently to prevent oxidation.
5.
To protect weld puddles, shield with pure argon or helium, or by vacuum. Avoid shielding
gases containing carbon dioxide, nitrogen, or oxygen. Prefer gas metal arc welding and
gas tungsten arc welding, because they shield the welding area well.
6.
Brush tack welds with a stainless steel wire brush, then weld to complete the root pass.
Finish the weld using the same shielding used for tack welds and root pass.
7.
Between passes, remove oxides with brushes having bristles
of
stainless steel, not ordinary
carbon steel. Carbon steel bristles, or grinding wheels containing carbon, will carbonize
and crack stainless steel welds
[150].
The gas tungsten arc welding (GTAW) process is required to achieve the degree of shield-
ing necessary.
Filler Metals.
Although matching filler metals have been used successfully
in
a few cases,
the most satisfactory results are obtained with low-carbon, austenitic stainless steel or nickel-
base fillers.
PWHT.
This structure cannot be refined by heat treatment; however, the use of low heat
inputs during welding will restrict the amount of martensite formation, and following by an
annealing heat treatment will be useful.
ia
DUPLEX
STAINLESS STEELS
As the name implies, duplex stainless steels have a mixed microstructure of ferrite and austen-
ite in the proportion of 50:
50.
For these steels, the chromium content may
vary
from 22 to
27% and molybdenum 2 to 4%, depending upon corrosion resistance required. Typical newer
varieties of duplex stainless steels include Alloy 2205, known as Code Plus Two,
44LN,
and
Ferralium 255 (S32550), which also contain
2%
copper, 7 MO-PLUS
(UNS
S32950),
Atlas
948,
and 253
ME.
Of
these varieties, Ferralium 225 is regarded as the only seawater duplex
stainless steel
[loll.
7 MO-PLUS is being used very successfully
in
both tube-side and shell-
side cooler condensers for nitric acid production
[115].
Because of their higher strength and
better resistance to stress corrosion cracking than conventional austenitic stainless steels, du-
plexes are now gaining acceptance.
18.1
Composition
of
Duplex Stainless Steels
Duplex stainless steels usually contain high chromium (22-27%) and
MO
(24%).
The compo-
sitions are carefully controlled to maintain the proper balance of austenites and ferrites. The
high chromium content makes duplex alloys exhibit strong resistance to oxidation. The nickel
content varies from 4 to 7%. Because of lower nickel content, duplexes costs less. The addition
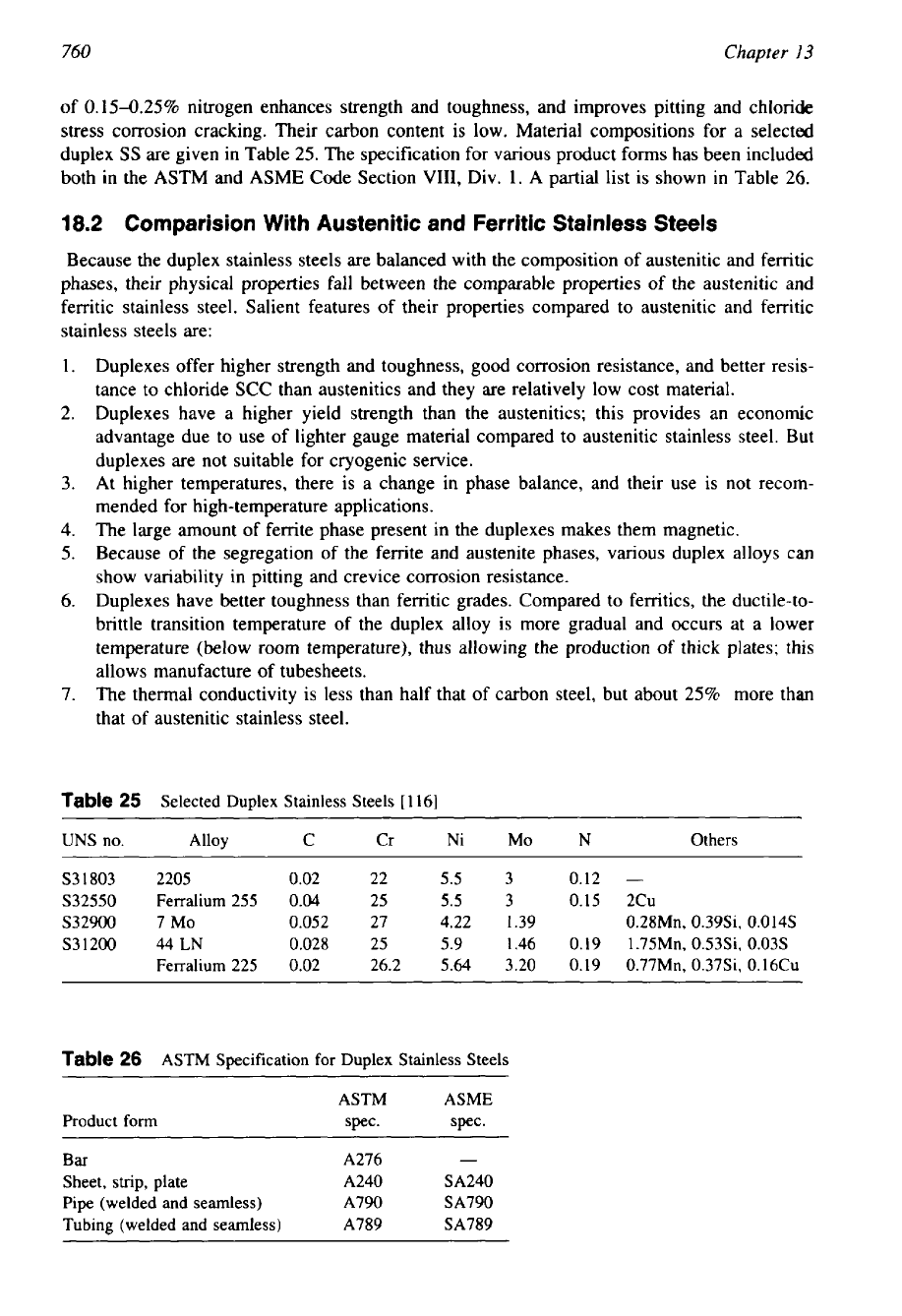
760
Chapter
I3
of 0.15425% nitrogen enhances strength and toughness, and improves pitting and chloride
stress corrosion cracking. Their carbon content is low. Material compositions for a selected
duplex
SS
are given in Table 25. The specification for various product forms has been included
both in the ASTM and ASME Code Section
VIII,
Div. 1.
A
partial list is shown in Table
26.
18.2
Cornparision With Austenitic and Ferritic Stainless Steels
Because the duplex stainless steels are balanced with the composition of austenitic and ferritic
phases, their physical properties fall between the comparable properties of the austenitic and
ferritic stainless steel. Salient features of their properties compared to austenitic and ferritic
stainless steels are:
1.
Duplexes offer higher strength and toughness, good corrosion resistance, and better resis-
tance to chloride
SCC
than austenitics and they are relatively low cost material.
2.
Duplexes have a higher yield strength than the austenitics; this provides an economic
advantage due to use of lighter gauge material compared to austenitic stainless steel. But
duplexes are not suitable for cryogenic service.
3.
At higher temperatures, there is a change in phase balance, and their use is not recom-
mended for high-temperature applications.
4.
The large amount of ferrite phase present in the duplexes makes them magnetic.
5.
Because of the segregation of the ferrite and austenite phases, various duplex alloys can
show variability in pitting and crevice corrosion resistance.
6.
Duplexes have better toughness than ferritic grades. Compared to ferritics, the ductile-to-
brittle transition temperature of the duplex alloy is more gradual and occurs at a lower
temperature (below room temperature), thus allowing the production of thick plates; this
allows manufacture of tubesheets.
7.
The thermal conductivity
is
less than half that of carbon steel, but about
25%
more than
that of austenitic stainless steel.
Table
25
Selected Duplex Stainless Steels
[
1161
UNS
no.
Alloy C Cr Ni MO N Others
~~~~~~~~ ~ ~ ~ ~
S31803 2205 0.02 22
5.5
3 0.12
-
S32550 Ferralium 255
0.04
25
5.5
3 0.15 2Cu
S32900 7 MO
0.052
27 4.22 1.39 0.28Mn, 0.39Si,
0.0
14s
S31200 44LN 0.028 25 5.9 1.46
0.19
1.75Mn, 0.53Si, 0.03s
Ferralium 225 0.02 26.2
5.64
3.20 0.19 0.77Mn, 0.37Si, 0.16Cu
Table
26
ASTM Specification for Duplex Stainless Steels
ASTM ASME
Product form spec. spec.
Bar
A276
-
Sheet,
strip,
plate
A240 SA240
Pipe (welded and seamless) A790 SA790
Tubing (welded and seamless) A789 SA789

Material Selection and Fabrication
761
8.
The coefficient of thermal expansion is about 40% less than that of austenitic stainless
steel, but equal to that of carbon steel.
18.3 Corrosion Resistance
of
Duplex Stainless Steels
Although their nickel contents
(4
to
7.5%)
are less than those of austenitic stainless steels,
their SCC resistance is much better than would be expected from nickel content alone. Duplex
stainless steels have two important advantages over Types 304 and 3 16 stainless steels
[
1
151:
(1) good resistance to chloride stress corrosion cracking, and
(2)
higher mechanical properties
with good ductility. Because the duplexes have a higher yield strength than the austenitics,
SCC resistance may usually be maximized with a
50
:
50
microstructure. Because nickel is a
factor for providing corrosion resistance in reducing environments, the duplexes show less
resistance in these environments than the austenitics.
The high chromium and molybdenum contents of the duplex stainless are particularly
important in providing resistance in oxidizing environments and are also responsible for the
better pitting and crevice corrosion resistance, particularly in chloride environments. The pit-
ting resistance equivalent
(PRE)
number is calculated using the formula
[
1 161:
PRE
=
%Cr
+
3.3(%Mo)
+
16(%N)
Duplexes are produced with low carbon contents, usually less than 0.03%, and hence, inter-
granular corrosion due to sensitization of weld metal as a result
of
carbide precipitation gener-
ally is not a problem.
Laboratory corrosion tests
include Critical pitting temperature by ASTM G-48A, ASTM
A262-C (Huey Test) for IGC, and ASTM G30 and G36 for SCC.
18.4 Process Applications
The duplex alloys have excellent resistance to oxidizing and reducing acids, sulfuric acid below
25%
concentration, organic and acid mixtures, seawater and sour oil and gas wells, and alkali
solutions
[
1601. Duplexes are applied successfully in applications containing corrosive chlo-
rides, like oil and gas plant components, pulp and paper plants, chemical processing as heat
exchangers, tanks, pressure vessels, columns, flue gas scrubbers, boiler tubes, digester preheat-
ers, evaporators, etc.
18.5 Welding Methods
Welding methods used for austenitic
SS
can be used except for the use of pure argon shielding
during welding to protect cerium addition. The welding processes most applicable are GTAW,
GMAW, PAW, SMAW, and SAW [161].
Weldability
The goal in welding duplex
SS
is to obtain weld metal and HAZ having the same corrosion
resistance, toughness, and absence
of
intermetallics. The trick in welding the duplex
SS
is
avoiding the formation of excess ferrite. The advantage of corrosion resistance and mechanical
properties can be achieved in welded assemblies when the welding practice ensures formation
of a
50
:
50
austenitic-ferrite phase both in the weld metal and in the HAZ. The following
problems are normally faced while welding duplex stainless steels
[
1591:
High ferrite phase and hence reduced toughness.
Liquation cracking.
Grain growth in HAZ.

762
Chapter
I3
Reduced corrosion resistance due to sigma phase, and carbonitrides precipitates.
Precipitation of chromium nitrides, which reduce corrosion resistance.
To overcome these problems, the following measures will help
[
1591:
Overalloyed filler metal with higher Ni content and with a guaranteed
PRE
value of not less
than
40
[
1621.
Controlled heat input.
Controlled interpass temperature (limited to 200°C maximum).
Balancing the Austenite and Ferrite Phases.
Balanced phases can only be ensured if the
cooling rate
is
slow enough to allow austenite to reform as the weld cools. If the rate
is
too
slow, embrittling phases may form; on the other hand, if it
is
too fast intermetallics could form.
Autogenous welding will increase the ferrite phase in the weldment and adjacent areas of the
base metal. Subsequent annealing will tend to restore the balance of phases in the base metal.
Heat Input.
High weld heat inputs, preheat, and interpass temperatures promote coarse-
grained weld deposits and HAZ and the tendency to precipitation is enhanced [161]. Welding
heat input should be controlled
to
limit the ferrite level, whereas
too
low a heat input produces
too much ferrite, and it has been suggested that heat inputs above
1.5
kl/mm must be employed
[163].
Liquation Cracking.
Duplex
SS
is susceptible to HAZ liquation cracking due to the for-
mation of low-melting-point films of
S
and
P
along the grain boundaries, and the effect is
aggravated in the presence of restraint and residual stresses.
Precipitations
of
Secondary Phases.
Duplexes are susceptible to the precipitation of
sigma phase due to the higher concentration
of
ferrite-forming elements. Base metals with low
carbon, balanced ferrite-austenite phases, and adequate nitrogen speed the reformation
of
aus-
tenite during cooling, and avoid secondary sigma phase formations.
Precipitation
of
Chromium Nitrides.
Due to the increased amounts of nitrogen that are
now used
to
strengthen the austenite, chromium nitrides (Cr,N) are usually formed in the
weldments intragranularly, along with sigma phase. This causes the formation
of
secondary
austenite in the surrounding material due to reduction in concentration
of
ferrite-forming ele-
ments. This secondary austenite causes the localized loss of corrosion resistance due to its low
chromium content
[
1031.
Welding Consumables.
When consumables that match the parent plate material composi-
tions are used, the final weld metal microstructure may be low in austenite and high in ferrite,
causing a
loss
in toughness unless a slow cooling rate is ensured. Satisfactory phase balance
is obtained by use of cbiisumables that are overmatched in nickel by
2
to
3%.
Welding Practices.
Welding practices should ensure cleanliness, provide inert gas shield-
ing, and avoid carbon contamination. Since ferrite is susceptible for hydrogen embrittlement,
low-hydrogen welding practices should be followed. It is not necessary to heat-treat these
duplexes after welding.
Welding Practices to Retain Corrosion Resistance.
Welding practices to retain corrosion
resistance include the following
[
1611:
1. Special attention is need to control weld spatter, slag residues, and oxide formation, since
these have adverse effects on resistance to pitting and crevice corrosion.
2.
As far as possible, postweld cleaning is to be done; when cleaning is not possible, restrict
oxygen to values of
10
ppm and
25
ppm maximum inert backing gases. To promote arc
stability and penetration, small additions of
CO2
and
0:
may be made to argon shields
when employing GMAW technique.

Material Selection and Fabrication
763
18.6 Nondestructive Testing (NDT) of Duplex
SS
Radiographic and dye penetrant inspection procedures are readily applicable. NDT by conven-
tional ultrasonic techniques with angle beam shear waves can be difficult, owing to the anisot-
ropy of the parent materials and weld deposits, especially the tendency to epitaxial columnar
structures in the latter
[
16 11. Improved techniques using angle beam compression wave probes
and creep wave probes at diminished frequencies are reported.
19 SUPERAUSTENITIC STAINLESS STEELS WITH
MO
+
N
Superaustenitic stainless steels are alloys with a face-centered cubic (fcc) structure, highly
alloyed, usually containing substantial amounts of chromium, molybdenum, and nitrogen,
along with sufficient nickel to stabilize a fully austenitic microstructure. They are classified by
their molybdenum content, which is in the range of 4.5-7%. The addition of 0.3 to
0.5%
N
provides yield strength typically twice that of conventional stainless steels
[
1 161. Relatively
high nickel content (18-31%) and high chromium content and MO content give the alloys
excellent resistance to SCC. Copper is intentionally added to some of the alloys and improves
resistance to reducing media such as hot phosphoric acid, acetic acid, and dilute sulfuric acid.
In this section, superaustenitics are covered under two headings: 4.5% MO superaustenitic
steels, and 6% MO superaustenitic steels.
19.1
4.5%
MO
Superaustenitic Steels
The 4.5% MO austenitic alloys like AL 904L, Ciramet, 254SLX, and JS700 have demonstrated
sufficient resistance to corrosion in seawater to perform reasonably well as tubing. They are
readily weldable and workable, available in a wide range of product forms such as tubing,
sheet, plate, and forgings. Nitrogen content in the range of 0.4 to
0.5%
gives good resistance
to pitting and crevice corrosion [115]. The high nickel (25%) and molybdenum (4.5%) contents
provide good resistance to chloride SCC. They also exhibit resistance to intergranular corro-
sion. Chlorination to control microbiological fouling is necessary to minimize under-deposit
corrosion; flange faces and gasketed surfaces are subject to crevice corrosion in brackish water
and seawater
[
1641.
19.2 6%
MO
Superaustenitic Stainless Steel
The 6% MO superaustenitics are now well established in the chemical process industries. They
contain about 20% chromium, nickel contents range from 18 to 25%, and they are enhanced
with more than 0.10% nitrogen. Nitrogen addition serves to improve strength, stabilize the
austenitic structure, and improve pitting corrosion resistance. 6% MO superaustenitics exhibit
excellent toughness and ductility characteristic of the
300
series austenitics. Several
6%
MO
superaustenitic stainless steels, their product forms, and ASTM/ASME Code references are
listed in Table 27 and their compositions in Table 28 along with Types 304 and 3 16. Although
there are differences in their chemistry to pennit three different
UNS
numbers (UNS S31254,
N08367, and N08926) for these 6% MO superaustenitics, for most applications they have simi-
lar corrosion resistance and are used more or less interchangeably
[
1
151. Superaustenitics have
an iron content less than
50%
and therefore do not fall under the jurisdiction of ASTM Com-
mittee A1 on Steel, Stainless Steel, and Related Alloys, except for 254
SMO.
Corrosion Resistance
In general, the high-alloy superaustenitic steels show superior resistance to uniform corrosion,
pitting and crevice corrosion, and stress corrosion cracking
[
1651. The 6% MO superaustenitics
