Kuppan T. Heat Exchanger Design Handbook
Подождите немного. Документ загружается.

--
--
764
Chapter
13
Table
27
6% MO Austenitics, with ASTWASME References and Product Shapes
[
1671
ASME Section
Grade UNS no. ASTM specification Products forms available
VIII,
Div.
1
254 SMO S31254
A167, A182, A193, Plate, sheet, strip, bar, billet,
UHA 23
A194, A240, A249, wire, pipe, fittings, tube,
A267, A269, A312,
condenser tube
A358, A403, A409,
A473, A479
AL-6X NO8366
B675, B676, B688, B690,
Condenser tube
UNF-23.3
B69
1
AL-6XN NO8367
B366, B462, B472, B564, Plate, sheet, strip, bar, billet,
UNF-23.3
B675, B676, B688,
wire, pipe, fittings, tube,
B690, B691
pipe fittings, condenser
tube
1925hMo NO8925
B625, B649, B673, B674,
Plate, sheet, strip pipe, tub- UNF-23.3
B677
ing, bar, billet
25-6M0 NO8925
B625, B649, B673, B674,
Plate
UNF-23.3
B677
20M0-6 NO8026
B463, B464, B468, B474
Plate, sheet, bar, billet, strip, UNF-23.3
wire, tube, pipe
Table
28
Composition of Selected Superaustenitic Stainless Steels Along With Types
304
and 3 16
UNS No. Alloy C Cr Ni MO N Others
s30400
304 0.06 18
8
-
S3 1600
3 16 0.08 17
12 2.5
S3 1254
254SMO
0.02 20.0 18 6.2 0.2 0.75Cu
NO8367 AL-6XN
0.02 21 24.5 6.5 0.2 0.75Cu, max
1925hM0,
NO8926 25-6 MO
0.01 20 25
6.5 0.2 1.1cu
NO8366 AL-6X
0.018 21 24.5
6.5 1.39Mn, 0.4 1 Si
resist both localized corrosion and
SCC
in oxidizing chloride and
sulfidekhloride-containing
solutions, as well as a broad range
of
process chemicals. They are widely used in severe
seawater applications. The corrosion performance
of
6% MO
superaustenitics falls between
Types
316
and
3 17
stainless steels and the nickel-based alloys
625
and
C-276
[
1
151.
In order
to be resistant to some corrosive environments, some steels also contain additions
of
copper,
which improves its resistance to acids in general. The fully austenitic
6%
MO
steels are primar-
ily characterized by a high (Cr+Mo) content, with a pitting resistance equivalent given
as
[
1661:
PRE,
=
%Cr
+
3.3(%Mo)
+
30(%N)
exceeding
35
in all of the steels.
Applications
In process industries, the
6%
MO
superaustenitic stainless steels have replaced common austen-
itic stainless steels that have failed by pitting, crevice corrosion, and chloride stress corrosion

765
Material Selection and Fabrication
cracking
[
1671. They have been used extensively in the offshore and desalination industries,
in
seawater handling, in chlorine and chlorine dioxide stages and bleach plants in the pulp and
paper industries, and in flue gas desulfurization plants. Equipment fabricated of 6%
MO
austen-
itics includes pressure vessels, columns, seawater-cooled condensers, evaporators, heat ex-
changers, crystallizers, pumps, piping, and components.
Welding
In general, fully austenitic 6%
MO
superaustenitic stainless steels exhibit satisfactory weldabil-
ity. The main concern when using the superaustenitic stainless steels is adequate corrosion
resistance in welds. During welding, particular concern has to be paid to the following three
phenomena:
1. Hot cracking.
2.
Elemental microsegregation.
3.
Precipitation of intermetallic phases.
Since the carbon content of the 6%
MO
steels is low
(<0.03%),
the risk of chromium
carbide precipitation in the grain boundaries of HA2 and thus the susceptibility to intergranular
corrosion is negligible
[
1661.
Hot cracking.
Hot cracking occurs either directly during solidification, and is referred to as
liquation cracking, or upon reheating of successive weld runs, known as reheat cracking. Both
types of cracking are related to the solidification mode of weld deposit and the presence of
impurities such as sulfur and phosphorus [165]. Steels that exhibit a primary austenitic solidifi-
cation mode are more susceptible to hot cracking than those that exhibit ferritic or mixed
phases. Susceptibility to hot cracking is overcome by measures such as the use
of
fillers that
deposit weld metals with relatively low levels of trace elements like
P,
S,
and Sn and keep
stresses due to restraint during welding to a minimum [166]. Welding should be done with
low arc energy.
Molybdenum Microsegregation.
6%
MO
steels with high molybdenum levels show large mo-
lybdenum microsegregations in autogenous weld metals. The areas depleted in molybdenum
have a lower local pitting index and hence they are less resistant to chloride pitting corrosion
[
165,1661. Therefore, autogenous welds are not recommended for these steels, unless the com-
pleted equipment is to receive subsequent homogenization and solution annealing. To over-
come the reduction in corrosion resistance, fillers are overalloyed with molybdenum.
Precipitation
of
Intemetallic Phases.
When exposed to high temperature, the relatively high
Cr and
MO
contents of these steels may stimulate the precipitation of intermetallic phases such
as the chi or sigma phases. These intermetallics can reduce the steel’s corrosion resistance.
To
overcome this problem, limit the heat input and interpass temperatures. The heat input should
be limited to <15,000 J/cm and the interpass temperature should be restricted to 100°C, with
an absolute maximum
of
150°C [166].
Arc Welding Filler Metals.
If it is practicable to solution anneal and homogenize the weld
metal, use a matching filler metal. For applications in severe pitting or crevice corrosion envi-
ronments, use filler metals that are overalloyed with molybdenum
so
that the welds are more
resistant to corrosion than the base metal [162]. The use of overalloyed filler metals compen-
sates for the lean areas in the deposit caused by microsegregation. The filler metal most fre-
quently used is Alloy
625
with
9%
molybdenum, but some fabricators prefer alloy C-276 or
C-22 [115]. Welds made with these fillers can be used in the as-welded condition.
In
addition
to proper filler selection, certain measures are to be taken to obtain weld metal of required
qualities. They include the following
[
105,1661:

766
Chapter
13
1.
Keep the surface free from contaminations before and after welding. Vapor degreasing or
scrubbing with a solvent should be effective in removing all but paint. Alkaline cleaners
are required to remove paint.
2.
When using nickel-base filler metals, the proper weld preparation is also necessary. Exces-
sive dilution has to be prevented, especially in the root pass area. In order to ensure a
sufficiently high proportion of filler metal
in
the root pass, the root gap should be
2
to
3
mm.
3.
To inhibit the formation of oxidation during welding, gas shield the root side.
4.
The shielding gas for 6% MO superaustenitic stainless steels is
90-95%
hydrogen and
5-
10% pure nitrogen.
5.
Cleaning and pickling are necessary.
Welding
Processes.
The main welding processes employed are SMAW, and manual and auto-
matic GTAW with filler metals. GTAW without filler should only be considered in cases
where a subsequent solution anneal heat treatment is to be applied. GMAW, SAW, and auto-
matic plasma arc welding with filler metals are also used.
Pos&*eld Heat Treatment.
If the 6%
MO
superaustenitics need to be annealed or stress
re-
lieved, they must be given a
full
anneal and water quench. Solution annealing at temperatures
of 2
102
to 2282°F
(
1
150 to 1250°C) followed by quenching is also commonly used; this helps
in
better homogenization of the material properties of the weldments. Heat treatments that
permit the 6% MO austenitics to spend time in the 1300 to 1900°F (705 to 1040°C) temperature
range make the material susceptible to sigma phase precipitation with loss of corrosion resis-
tance and possibly toughness
[
1673. Depending on the alloy composition, stress relieving at a
temperature up to
1
112°F
(600°C)
for several hours may be used in special cases. In all these
cases, the fabricator should follow the material manufacturer’s recommendations.
19.3
Corrosion Resistance
of
Superaustenitic Stainless Welds
The factors that contribute to the loss of corrosion resistance of superaustenitic stainless steel
welds and the measures to overcome them are [105]:
1.
Microsegregation
of
molybdenum at localized regions, which most likely takes place when
the GTAW process involves autogenous welds, filler metal that has the same composition
as the base metal, and high heat input.
2.
Unmixed zones. High heat input welding can leave narrow bands of base metal adjacent
to the fusion line, which have been melted but not mixed with the overmatched filler
metal. This problem is overcome by controlling heat input and avoiding undercutting.
3.
Crevices, cracks, and microfissures. Crevice corrosion sites can also occur at the beginning
or end of weld passes, between weld passes, or under weld spatter.
4.
Sensitization and carbide precipitation. Low carbon content
(~0.02%)
provides an alloy
that is not susceptible to this problem. Molybdenum and nitrogen content increase the
level of carbon andor heat input that can be tolerated.
5.
Precipitation of intermetallic phases like sigma and chi phases.
6.
Surface contamination by embedded iron, which is removed by acid pickling or abrasive
blasting with clean sand or glass beads, and organic contamination, which is removed by
suitable solvent.
7.
Surface oxide scale or heat tint, which is to be removed by descaling.
8.
Shielding gases composition. Adding nitrogen at
3
to
5%
by volume
to
the torch
and
backing shielding gases enhance corrosion resistance.
9.
To impart optimum corrosion resistance to the weld metal, especially for the autogenous

Material Selection and Fabrication
767
welds, follow these measures: postweld anneal, postweld clean the surfaces, pickle for
optimum corrosion resistance, and passivate the surface.
20
ALUMINUM ALLOYS: METALLURGY
Aluminum is a reactive metal. It is known for its higher thermal conductivity, light weight,
corrosion resistance, and moderate cost. Its density is about one-third that of steel, which
provides a high strength to weight ratio. Untempered, commercially pure aluminum has a
tensile strength of 13000 lb/in' (89622 kPa) and cold working can double this strength. The
low mechanical properties can also be improved by alloying. Typical alloying elements include
magnesium, manganese, zinc, copper, and silicon. By taking advantage
of
cold working, heat
treatment, and aging, the strength of aluminum can be raised to 100,000 lb/in' (689,476 kPa)
[7]. With the addition of copper at 1.9 to 6% (2000 series), the heat-treated properties exceed
those of mild steel. Designing in aluminum is much like designing in other metals. Publications
from the Aluminum Association, Aluminum Welding Seminar Technical Papers, and others
[
1681 will be of great help.
20.1
Properties
of
Aluminum
Aluminum alloys have a high resistance to corrosion in most atmospheres, waters, and many
chemicals. They are nontoxic, permitting applications with foods, beverages, and pharmaceuti-
cal; and they are colorless, permitting applications with chemicals and other materials without
discoloration. Their corrosion products are not damaging to the ecology. Aluminum is fabri-
cated and joined readily by most of the metal joining processes. Aluminum is excellent for
cryogenic applications; most aluminum alloys have higher ultimate and yield strengths at -350
to
-450°F
(-2 12 to -268°C) than they have at room temperature. Other properties of aluminum
and its alloys for certain applications are high electrical conductivity, high reflectivity, and
nonmagnetic (ensures arc stability).
Aluminum for Heat Exchanger Applications
The unique combinations of properties provided by aluminum and its alloys make aluminum
one of the most versatile and economical materials for heat exchanger applications. The proper-
ties that favor aluminum for heat exchanger applications are:
Light weight and high specific strength. This offers automotive heat exchanger designers
the potential for compact low-cost heat exchangers over the more traditional copper and
brass materials
[
1691.
High thermal conductivity. The thermal conductivity of pure aluminum is about 60% that
of pure copper.
High resistance to atmospheric corrosion and environments like fresh water, saltwater,
and many chemicals and their solutions.
Depending on temper, it can be hot or cold formed; it has the ability to be formed into
various shapes, tubes, fin patterns, etc.
Ability to be joined by welding, brazing, and soldering.
Aluminum clad products protect pitting corrosion of the core alloys.
They retain toughness and strength, and exhibit high thermal conductivity at subzero
temperature. Aluminum maintains excellent strength and ductility to temperatures as low
as -321°F (-196°C). It also exhibits high thermal conductivity at cryogenic temperature.
For cryogenic services, aluminum alloy 3003 is generally used for the parting sheets,
corrugated fins, and edge bars that form the plate fin heat exchangers (PFHE) block.
Headers and nozzles are made from aluminum alloys 3003, 5154, 5083, 5086, or
5454.

768
Chapter
13
8.
It has hygienic and nontoxic qualities.
9.
It is nonmagnetic and nonsparking.
10.
There is availability in many product shapes and moderate cost.
Limitations
of
Aluminurn.
1.
Aluminum and its alloys rapidly lose strength at temperatures above 100°C; aluminum
does not resist fire as well as other materials. It is not generally used for
PFHE
process
temperatures above
1
50°C
particularly in high-pressure service
[
1701.
2.
Aluminum is susceptible to damage by rough handling, excessive vibration, and localized
unrelieved stresses.
Wrought Alloys Designations
A system of four-digit numerical designations is used to identify the various wrought aluminum
alloys, as shown in Table
29.
The first digit indicates the alloy group. The second digit indi-
cates a modification of the original alloy, or the impurity limit in the case of unalloyed alumi-
num. The third and fourth digits identify the alloy or indicate the aluminum purity.
Classification of Wrought Alloys
Wrought alloys are of two types: not heat-treatable, of the lxxx,
~XXX,
~XXX,
and Sxxx series,
and heat-treatable, of the
~XXX,
~XXX,
and 7xxx series. In the non-heat-treatable type, strength-
ening is produced by strain hardening. All non-heat-treatable alloys have a high resistance
to
general corrosion. Aluminum alloys of the 1 xxx series, representing unalloyed aluminum, have
a relatively low strength. Alloys
of
the 3xxx series (AI-Mn, Al-Mn-Mg) have the same desir-
able characteristics as those of the lxxx series and somewhat higher strength. Common
wrought alloys and their compositions are given in Table
30.
Ixxx
Series.
This grade
of
aluminum, with minimum
99.00%
purity, exhibits excellent corro-
sion resistance, high thermal and electrical conductivity, excellent formability, but low mechan-
ical properties. They are readily brazed and welded by all methods, and non-heat-treatable.
Moderate increase in strength may be obtained by strain hardening. They are used as fin stocks
in heat exchangers.
2xxx
Series.
These alloys are copper based. The copper content is in the range of
1.9
to 6%.
These alloys are heat-treatable, and more susceptible to corrosive attack than other groups of
Table
29
Wrought Aluminum Alloy Designations
Major Heat
Designation Alloying elements alloying elements treatable
1
xxx
99%
min. A1 None
No
2xxx
AI-Cu, Al-Cu-Mg-Si
(3-
Copper Yes
6%/Cu)
3xxx
Al-Si-Mn
Manganese
No
4xxx
Al-Si-Cu
Silicon
No
Sxxx
Al-Si-Cu-Mg Magnesium
No
AI-Mg
(1-2.5%
Mg)
Al-Mg-Mn
(34%
Mg)
6xxx
Al-Mg-Si
Magnesium-silicon Yes
7xxx
Al-Zn-Mg Zinc Yes
Al-Zn-Mg-Cu

- -
- -
Material Selection and Fabrication
769
Table
30
Common Wrought Alloys and Their Compositions
[171]
Composition
(%),
remainder A1 and impurities
Nominal Cu
Si
Mn Mg Zn Cr
1050
99.60%
minimum aluminurn
1060
99.60%
minimum aluminurn
1100
99.00%
minimum aluminum
-
3003
0.12
0.6
1.2
-
0.1
3004
-
1.2
1
.o
- -
0.4
-
-
3005
-
1.2
5005
-
-
0.8
5050
-
-
1.2
5052
-
-
2.5
-
0.20
5154
-
0.4
3.5
-
0.25
5454
-
0.75
2.7
-
0.12
5456
-
-
0.8
5.2
-
0.1
606 1
0.25
0.6
-
1
.o
-
0.20
-
0.7
-
-
6063
0.4
6151
-
1
.o
-
0.6
-
0.25
-
695 1
0.25 0.35
-
0.6
-
7005
-
0.45 1.4 4.5 0.13
- - -
1
.o
-
7072
wrought aluminum alloys. Heat-treated properties exceed those of mild steel. Fusion welding
is not recommended, but resistance welding is recommended.
3m
Series.
The major alloying element is manganese, up to about
1.5%.
These alloys are
non-heat-treatable. They have moderate strength, good corrosion resistance, and good work-
ability/formability, and are used in pressure vessels and brazed and soldered heat exchangers.
These are readily brazed and welded by all methods. Alloys 3003, 3004, and 3105 are widely
used as general-purpose alloys for moderate-strength applications requiring good formability
and workability. The strength of 3003 is higher than that of
1100
alloy, and 3004 is stronger
than 3003. Alloy 3004 is known for fine grain size and general freedom from critical grain
growth.
4xxx
Series.
The major alloying element is silicon. These are non-heat-treatable and are often
used for welding and brazing as filler metal with enough silicon to lower the melting point.
For example, 4343 containing 7.5% silicon and 4045 containing 10.5% silicon are used as the
clad brazing sheet. The core alloy is either 3xxx or 6xxx series.
5xxx
Series.
Alloys of the 5xxx series (AI-Mg) are the strongest non-heat-treatable aluminum
alloys. In most products, they are more economical than alloys of the lxxx and 3xxx series in
terms of strength per unit cost. Alloys of the 5xxx series have high resistance to general
corrosion and good resistance in marine atmospheres. The most widely specified weldable
grades are alloys
5083,
5052,
and 5454. Their applications are numerous in the pressure vessel
fields, including cryogenic applications. They are typically available as sheet and plate product
forms. These are most fusion welded
of
any series with a minimum loss of strength. Alloys
with more than
3.5%
Mg are essentially free from hot cracking. Because of their heavy oxide
films, extra care in surface preparation is necessary
[
1721. These alloys solder and braze poorly.
6xxx
Series.
These alloys contain both silicon and magnesium, which make the alloys heat-
treatable. Compared with other aluminum alloys, these have high resistance to rural atmos-

770
Chapter
I3
pheres, and good resistance to industrial and marine atmospheres. They generally possess ex-
cellent SCC resistance. These alloys possess good formability
,
machinablility
,
and weldability
.
The mechanical properties of the heat-treatable 6xxx series alloys are superior to those of
the 3xxx series alloys. Compared to 3xxx series, the yield strength of the 6xxx series is about
four to five times as high after heat treatment. The higher strength is desirable from the radiator
manufacturers’ point of view; this helps to minimize tube wall thickness in order to save weight
and material cost. But the disadvantages of 6xxx series are their lower melting temperature and
higher susceptibility to silicon penetration from the cladding to the core.
The 6xxx series alloys can be soldered, brazed, and resistance spot welded satisfactorily.
Alloy 6061 is the most widely specified weldable grade. The most popular temper for
6061
is
T6, although the T65
1,
T4, and
F
tempers are also popular.
The 6xxx series alloys are prone to hot cracking. However, this problem can be overcome
by correct choice of filler metal and proper joint design. Care in joint and component design
is also needed because of losses in strength in the heat-affected zones. The heat-affected zones
strength can be improved, however, through postweld heat treatment
[
1721.
7xx.r
Series.
These alloys contain zinc in the range of 1-8%, and smaller percentage of
Mg,
Cu, and Cr. They are heat-treatable alloys. 7072 is most widely used as the anodic coating of
the tubes and brazing sheet made of
3003, 3003,
5505,
6061, 6951, 7075, and 7178, and as
fin
stock.
Temper Designation System of Aluminum and Aluminum Alloys
A
temper designation system is used to indicate the condition of the product forms. The system
is based on the sequences of mechanical or thermal treatments, or both, used to produce the
various tempers. The temper designation follows the alloy designation and is separated from it
by a hyphen. Aluminum alloys come in four tempers:
F,
0,
H and T. Another,
W,
is sometimes
used to state the unstable condition following solution heat treatment. The digits designate the
sequence of basic treatments (e.g.,
1
100-Hl4 and 7075-T65
1).
The basic temper designations
are shown in Table 31.
Product Forms and Shapes
Aluminum
is
available
in
many forms and shapes, which include heat exchanger tubes (plain
and integral fin), plates and sheets, bars and rods, and extrusions. Some ASTM specifications
for tubes. plates and sheets, pipes, bar, rods, extrusions, and forgings are given in Tables
32
to 34.
Table
31
Basic Temper Designations for Aluminum Alloys
Designation Conditions
~~
F
As fabricated
Annealed, the softest temper
H
Strain hardened
HI
Strain hardened only
H2
Strain hardened and partially annealed
H3
Strain hardened and thermally stabilized
T
(T1
to
T12)
Heat-treated forms; heat treatment produces needed workability,
dimensional stability, and strength
W
Solution heat-treated
0
771
Material Selection and Fabrication
Table
32
Heat Exchanger Tubes
ASTM
spec.
Description Alloys
UNS
no.
B234
Aluminum and aluminum alloy drawn seamless tubes for
1060,
3003,
Alclad
3003,
condensers and heat exchangers
5052, 5454, 6061, 7072
B404
Aluminum and aluminum alloy drawn seamless condensers
1060, 3003,
Alclad
3003,
and heat exchanger tubes with integral fins
5052, 5454, 6061, 7072
Table
33
Aluminum Plates and Sheets
ASTM
spec.
Description Alloys
UNS
no.
B209
Aluminum and aluminum alloy plates and sheets
1060,
1100,
3003,
Alclad
3003, 3004,
Alclad
3004,
5050,
5052, 5083, 5086,
5154, 5254, 5454, 5456, 5652, 6061,
Alclad
6061
Table
34
Pipes, Bars, Rods, Extrusions, and Forgings
ASTM specification ASME spec.
Aluminum and aluminum alloy drawn seamless tubes
SB210
Aluminum and aluminum alloy bar, rod, and wire
SB211
Aluminum and aluminum alloy extruded bars, rods, wire, shapes, and tubes
SB22
1
Aluminum and aluminum alloy seamless pipe and seamless extruded tube
SB241
Aluminum and aluminum alloy die forging, hand forging, and rolled ring forging
SB247
Aluminum alloy
606
1
-T6
standard structural shapes
S
B 308
20.2
Corrosion Resistance
Surface Oxide Film on Aluminum
Aluminum owes its excellent corrosion resistance to the presence of a thin, compact, adherent
aluminum oxide film on its surface. In air, aluminum swiftly acquires a surface film that is
self-healing and self-limiting in thickness. The normal surface film thickness in air is about
50
A
[
1731.
Even if the surface film is damaged, it readily forms on the surface whenever a fresh
aluminum surface is created and exposed to either air or moisture/water. Generally the oxide
film is stable over the pH range of
4
to
9.0.
In strong acids and alkali, the oxide dissolves and
hence the metal corrodes rapidly by uniform dissolution, but there are exceptions, such as
stability in concentrated nitric acid (pH
1)
and concentrated ammonium hydroxide (pH
13).
Chemical Nature of Aluminum: Passivity
Aluminum is an active metal whose resistance to corrosion depends on the passivity exhibited
by the protective surface film. In aqueous solutions, the thermodynamic conditions under which
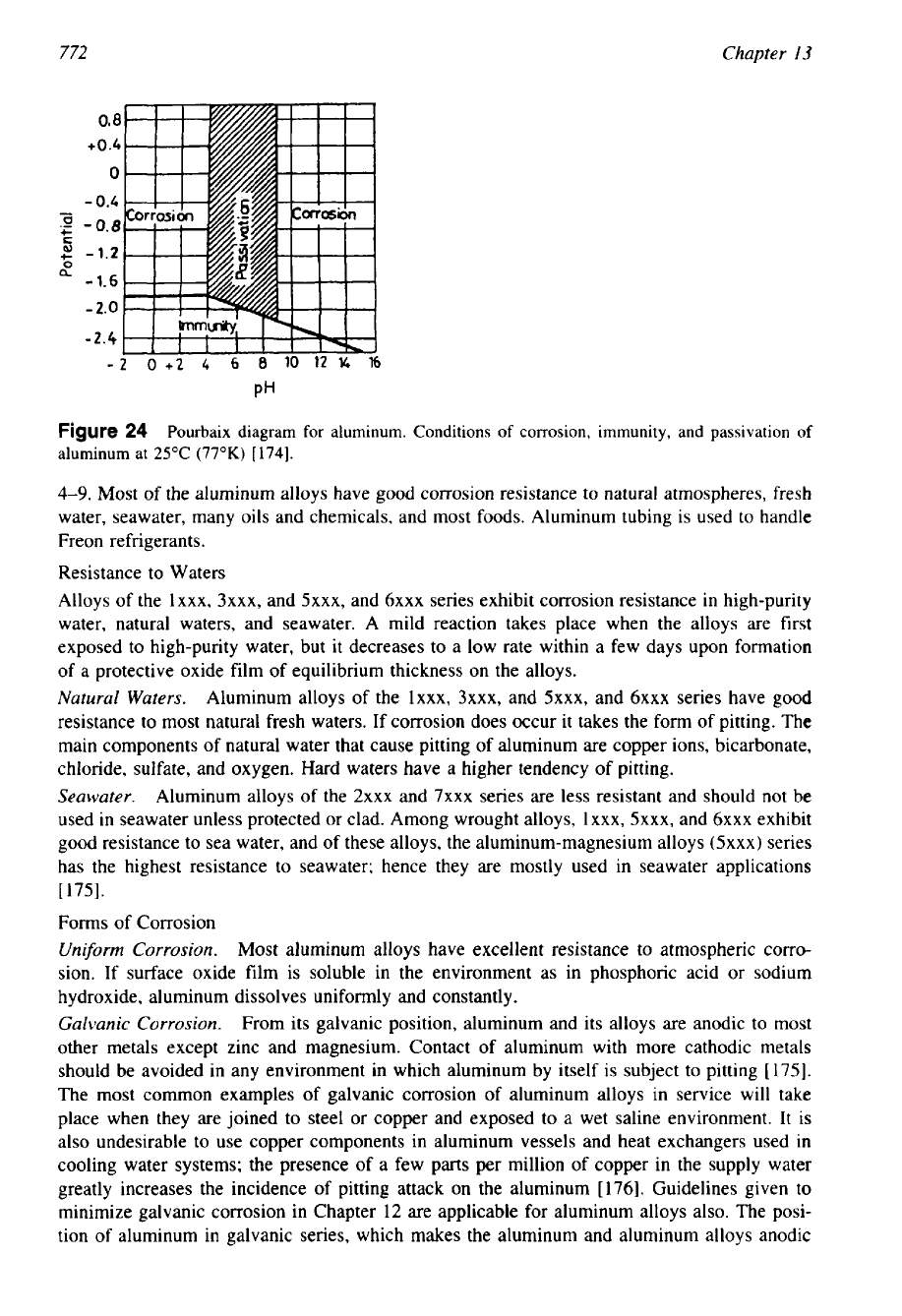
the film develops are expressed commonly by the potential-pH diagram (Fig.
24)
according
to
Pourbaix
[
1743.
This diagram shows that aluminum is passive only in the pH range of about
772
Chapter
I3
0.8
+0.4
0
-
-
0.4
-0.8
C
2
-1.2
0
a
-
1.6
-
2.0
-2.4
Figure
24
Pourbaix diagram for aluminum. Conditions of corrosion, immunity, and passivation
of
aluminum at
25°C
(77°K)
[174].
4-9.
Most of the aluminum alloys have good corrosion resistance
to
natural atmospheres, fresh
water, seawater, many oils and chemicals. and most foods. Aluminum tubing is used to handle
Freon refrigerants.
Resistance to Waters
Alloys of the lxxx,
~XXX,
and Sxxx, and 6xxx series exhibit corrosion resistance in high-purity
water, natural waters, and seawater. A mild reaction takes place when the alloys are first
exposed to high-purity water, but it decreases to a low rate within a few days upon formation
of a protective oxide film of equilibrium thickness on the alloys.
Natural Waters.
Aluminum alloys
of
the lxxx,
~XXX,
and Sxxx, and 6xxx series have good
resistance to most natural fresh waters. If corrosion does occur it takes the form of pitting. The
main components of natural water that cause pitting of aluminum are copper ions, bicarbonate,
chloride, sulfate, and oxygen. Hard waters have a higher tendency
of
pitting.
Seawater.
Aluminum alloys
of
the 2xxx and 7xxx series are less resistant and should not be
used in seawater unless protected or clad. Among wrought alloys, lxxx, Sxxx, and 6xxx exhibit
good resistance to sea water, and
of
these alloys, the aluminum-magnesium alloys (5xxx) series
has the highest resistance to seawater; hence they are mostly used in seawater applications
[
1751.
Forms of Corrosion
Uniform Corrosion.
Most aluminum alloys have excellent resistance to atmospheric corro-
sion. If surface oxide film is soluble in the environment as in phosphoric acid or sodium
hydroxide, aluminum dissolves uniformly and constantly.
Galvanic Corrosion.
From its galvanic position, aluminum and its alloys are anodic to most
other metals except zinc and magnesium. Contact of aluminum with more cathodic metals
should be avoided in any environment in which aluminum by itself is subject to pitting
[
1751.
The most common examples of galvanic corrosion of aluminum alloys in service will take
place when they are joined to steel or copper and exposed to a wet saline environment.
It
is
also undesirable to use copper components in aluminum vessels and heat exchangers used
in
cooling water systems; the presence of a few parts per million of copper in the supply water
greatly increases the incidence of pitting attack on the aluminum [176]. Guidelines given to
minimize galvanic corrosion in Chapter
12
are applicable for aluminum alloys also. The posi-
tion of aluminum in galvanic series, which makes the aluminum and aluminum alloys anodic

773
Material Selection and Fabrication
to most other metals and alloys, is utilized in Alclad products (sacrificial anode) to give ca-
thodic protection to the core alloys.
Pitting Corrosion.
Susceptible Alloys.
Of the commercial alloys, lxxx,
~XXX,
and Sxxx have excellent pit-
ting resistance. However, with copper content higher than
0.04%,
3xxx alloys are susceptible
to pitting. At 0.15% copper, they pit more extensively, especially in seawater. Alloys of the
~XXX,
~XXX,
and 7xxx series are normally clad to protect against pitting.
Factors Responsible for Pitting Corrosion.
Pitting corrosion is mostly due to copper,
mercury, and halide ions, of which chloride is the most damaging and often encountered in
service. Pitting corrosion can be prevented by removal of the reducible species required for a
cathodic reaction. In the absence of dissolved oxygen or other cathodic reactant, aluminum
will not corrode by pitting since it is not polarized to its pitting potential. Pitting in neutral
solutions is caused usually by oxygen.
Pitting Behavior.
The rate of pitting corrosion of aluminum depends on its polarization
behavior. As with other passive metals, corrosion of aluminum in the passive range, that is,
pH range
4-9,
may be of pitting corrosion. Pitting of aluminum diminishes beyond this range,
and corrosion proceeds uniformly.
Stress Corrosion Cracking.
SCC does not occur in commercial-purity alloys
(
1 xxx), alumi-
num-manganese alloys (~xxx), and aluminum-magnesium alloys containing less than 3% mag-
nesium (5xxx) and strengthened by cold work. On very rare occasions, SCC occurs in the
aluminum-magnesium-silicon
alloys (6xxx).
Susceptible Alloys.
SCC in wrought aluminum alloys is generally limited to those con-
taining appreciable amounts of soluble alloying elements of copper, magnesium, silicon, and
zinc [175]. Alloys that can be strengthened by cold work are relatively immune to SCC. The
wrought alloys that are susceptible to SCC include the following
[
175,1771:
The 2xxx series containing copper with smaller amounts of magnesium, manganese, and other
elements.
The 7xxx series containing zinc with small amounts of magnesium, manganese, copper, and
silicon, if any.
The Sxxx series containing more than
3%
magnesium with or without other alloying elements.
Factors Affecting Resistance to SCC.
In aluminum alloys, this cracking is characteristi-
cally intergranular. Factors that affect SCC of aluminum alloys are given next.
Directional Property
of
Aluminum.
SCC of an aluminum wrought alloy in a susceptible
temper is determined by the magnitude and the duration of a tensile stress acting on its surface,
and additionally, it is also determined by the direction of stressing. The effect of stressing in
different directions
is
caused by the highly directional grain structure that is typical of many
aluminum wrought products
[
173,1751. Resistance, which is measured by the magnitude of
tensile stress required to cause cracking,
is
highest when the stress is applied in the longitudinal
direction, lowest in the short transverse direction, and intermediate in the other direction. Resis-
tance in the short transverse direction usually controls application of products that are of thick
section or machined products with sustained tensile stresses in the short transverse direction.
Environment.
Water or water vapor is a requisite for the SCC of aluminum, and in its
absence, cracking does not occur. Among the species that accelerate cracking further, chlorides
have the greatest effect
[
1751.
Mitigation of SCC of Aluminum Alloys.
In addition to the usual procedures for prevent-
ing SCC of materials, there are several methods specifically applicable to aluminum alloys
[173]:
