King M.R., Mody N.A. Numerical and Statistical Methods for Bioengineering: Applications in MATLAB
Подождите немного. Документ загружается.


produced by the numerical method will grow increasingly inaccurate with time.
5
The
ODE solver is said to be numerically unstable for the problem at hand. An ODE
solver that successfully solves one ODE problem may not necessarily produce a
convergent solution for another ODE problem, when using the same step size. The
numerical stability of an ODE solver is defined by a range of step sizes within which a
non-divergent solution (solution does not blow up at long times) is guaranteed. This
step size range is not only ODE solver specific, but also problem specific, and thus
varies from problem to problem for the same solver. Some numerical integration
schemes have be tter stability properties than others (i.e. wider step size range for
producing a stable solution), and selection of the appropriate ODE solver will
depend on the nature of the ODE problem.
Some ODE problems are more “difficult” to solve. Solutions that have rapidly
changing slopes can cause difficulties during numerical integration. A solution that
has a fast decaying component, i.e. an exponential term, e
αt
, where α 0, will
change rapidly over a short period. The solution is said to have a large transient.
ODEs that have large transients are called stiff equations. In certain processes, often
multiple phenomena with different time scales will contribute to the behavior of a
system. Some of the contributing factors will rapidly decay to zero, while others will
evolve slowly with time. The transient parts of the solution eventually disappear and
give way to a steady state solution, which is unchanging with time. The single ODE
initial-value problem given by
dy
dt
¼ ft; yðÞ; y 0ðÞ¼y
0
;
can be linearized to the form dy=dt ¼ αy, such that α ¼ df=dy. Note that αðtÞ varies
in magnitude as a function of time. If at any point α becomes large and negative, the
ODE is considered stiff.
Unless the time step is chosen to be vanishingly small throughout the entire
integration interval, the decay of a transient part of the solution will not occur
when using certain ODE solvers. Large errors can accumulate due to the magnifi-
cation of errors at each time step when, at any time during the integration, the step
size jumps outside the narrow range that guarantees a stable, convergent solution.
Solvers that vary the step size solely based on local truncation error are particularly
prone to this problem when solving stiff equations.
Ordinary ODE solvers must use prohibitively small step sizes to solve stiff prob-
lems. Therefore, we turn to special solution techniques that cater to this category of
differential equations. Stiff ODE solvers have better stability properties and can
handle stiff problems using a step size that is practical. Very small step sizes increase
the solution time considerably, and may reach the limit of round-off error. In the
latter case, an accurate solution cannot be found. Stiffness is mo re commonly
observed in systems of ODEs rather than in single ODE problems.
For a system of linearized, first-order ordinary differential equations, the set of
equations can be compactly represented as
dy
dt
¼ Jy; y 0ðÞ¼y
0
;
5
Although we have referred to time as the independent variable in an initial-value problem, the methods
developed for solving initial-value problems apply equally well to any continuous independent variable,
such as distance x, speed s, or pressure P.
457
7.6 Stability and stiff equations
where
J ¼
∂f
1
∂y
1
∂f
1
∂y
2
...
∂f
1
∂y
n
∂f
2
∂y
1
∂f
2
∂y
2
...
∂f
2
∂y
n
:
:
:
∂f
n
∂y
1
∂f
n
∂y
2
...
∂f
n
∂y
n
2
6
6
6
6
6
6
6
6
6
6
6
6
4
3
7
7
7
7
7
7
7
7
7
7
7
7
5
:
J is called the Jacobian, and was introduced in Chapter 5. The exact solution to this
linear problem is
y ¼ e
Jt
y
0
: (7:52)
Before we can simplify Equat ion (7.52), we need to introduce some important linear
algebra concepts. The equation Ax ¼ λx comprises an eigenvalue problem. This
linear matrix problem represents a linear transformation of x that simply changes
the size of x (i.e. elongation or shrinkage) but not its direction. To obtain non-zero
solutions of x, A λIjjis set as equal to zero, and the values of λ for which the
determinant A λI
jj
equals zero are called the eigenvalues of A. The solution x for
each of the n eigenvalues are called the eigenvectors of A.
If J (a square matrix of dimensions n × n)hasn distinct eigenvalues λ
i
and n distinct
eigenvectors x
i
, then it is called a perfect matrix, and the above exponential equation
can be simplified by expressing J in terms of its eigenvectors and eigenvalues. How this
can be done is not shown here since it requires the development of linear algebra
concepts that are beyond the scope of this book. (The proof is explained in Chapter 5
of Davis and Thomson (2000).) The above equation simplifies to
y ¼
X
n
i¼1
c
i
e
λ
i
t
x
i
: (7:53)
If an eigenva lue is complex, then the real part needs to be negative for stability. If the
real part of any of the n eigenvalues λ
i
is positive, the exact solution will blow up. If
all λ
i
are negative, the exact solution is bounded for all time t. Thus, the sign of the
eigenvalues of the Jacobian reveal the stabi lity of the true solution, i.e. whether the
solution remains stable for all times, or becomes singular at some point in time. If the
eigenvalues of the Jacobian are all negative and differ by several orders of magni-
tude, then the ODE problem is termed stiff. The smallest step size for integration is
set by the eigenvalue of largest magnitude.
Using MATLAB
MATLAB offers four ODE solvers for stiff equations, ode15s, ode23s, ode23t,
and ode23tb .
*
ode15s is a mu ltistep solver. It is suggested that ode15s be tried when ode45 or
ode113 fails and the problem appears to be sti ff.
*
ode23s is a one-step solver and can solve some stiff problems for which ode15s fails.
*
ode23t and ode23tb both use the modified Euler method, also call ed the trape-
zoidal rule or the Adams–Moulton second-order method; ode23tb also imple-
ments an implicit RK formula.
458
Numerical integration of ODEs
For stiff ODE solvers, you can supply a function that evaluates the Jacobian matrix
to the solver (using options) to speed up the calculations. If a Jacobian matr ix is
not provided for the function, the components of the Jacobian are estimated by the
stiff ODE solver using finite difference formulas. See MATLAB help for further
details.
Box 7.4C Microbial population dynamics
We re-solve the coupled ODEs presented below that describe predator–prey population dynamics in a
mixed culture microbial system:
dy
1
dt
¼
F
V
y
1;0
y
1
1
Y
N
1
jS
μ
N
1
;max
y
1
y
2
K
N
1
þ y
1
;
dy
2
dt
¼
F
V
y
2
þ
μ
N
1
;max
y
1
y
2
K
N
1
þ y
1
1
Y
N
2
jN
1
μ
N
2
;max
y
2
y
3
K
N
2
þ y
2
;
dy
3
dt
¼
F
V
y
3
þ
μ
N
2
;max
y
2
y
3
K
N
2
þ y
2
;
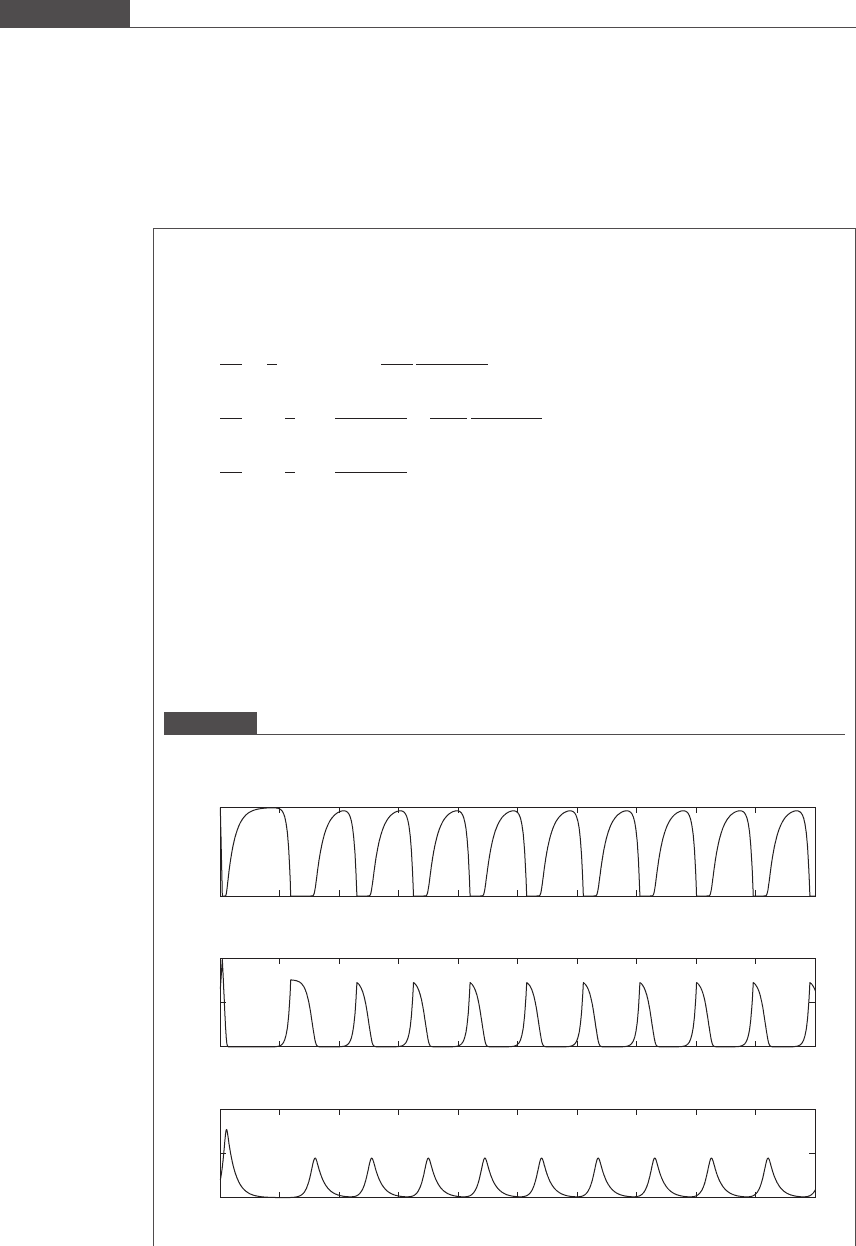
using the stiff ODE solver ode15s. For this parameter set, we get an oscillatory response of the
system, shown in Figure 7.13. Changes in the initial values of the variables N
1
and N
2
do not affect the
long-term oscillatory properties of the system. On the other hand, the nature of the oscillations that are
characteristic of the population dynamics predicted by the Lotka–Volterra predator–prey model are
affected by changes in initial predator or prey numbers.
There are a number of operating variables that can be changed, such as the feed-to-volume ratio and
the substrate concentration feed. At time t = 50 days, we introduce a step change in the feed glucose
Figure 7.13
Evolution of number densities of E.coli and Dictyostelium discoideum, and substrate concentration with time in a
chemostat for S
0
= 0.5 mg/ml.
0 5 10 15 20 25 30 35 40 45 50
0
0.5
S
(mg/ml)
0 5 10 15 20 25 30 35 40 45 50
0
1
2
× 10
9
0 5 10 15 20 25 30 35 40 45 50
0
1
2
× 10
6
t (days)
t (days)
t (days)
N
2
(cells/ml)
N
1
(cells/ml)
459
7.6 Stability and stiff equations
MATLAB program 7.14
% Use ode15s to solve the microbial population dynamics problem.
clear all
% Variables
S0 = 0.5; % glucose concentration in feed mg/ml
% Initial conditions
y0(1) = 0.5; % mg glucose/ml
y0(2) = 13e8; % bacteria/ml
y0(3) = 4e5; % amoeba/ml
% Time interval
tf = 1200; % hr
% Call ODE Solver
[t, y] = ode15s(‘popdynamics’, tf, y0, [], S0);
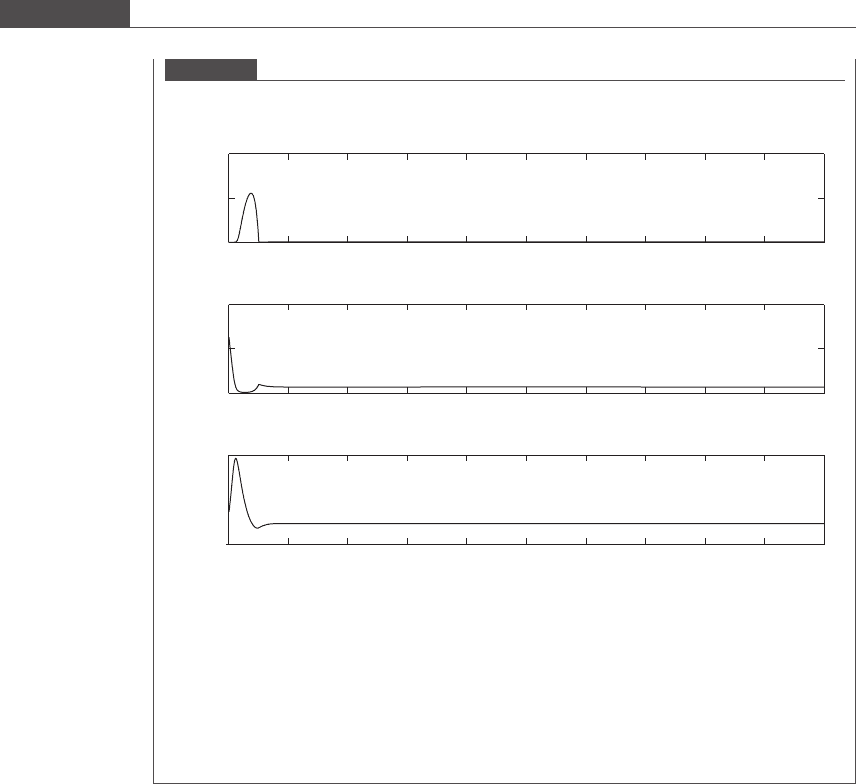
concentration from 0.5 mg/ml to 0.1 mg/ml. The behavior of the system for the next 50 days is shown in
Figure 7.14.
The system quickly stabilizes to steady state values. The long-term system behavior is a function of
the F/V ratio and the S
0
concentration. Operating at F=V ¼ð1=5Þ/s and S
0
¼ 0:5 mg/ml leads to
washout of amoebae (predator).
In the program listing, the plotting commands have been removed for brevity.
Figure 7.14
Evolution of number densities of E.coli and Dictyostelium discoideum amoebae, and substrate concentration with
time, in a chemostat for S
0
= 0.1 mg/ml.
0 5 10 15 20 25 30 35 40 45 50
0
0.05
0.1
S
(mg/ml)
0 5 10 15 20 25 30 35 40 45 50
0
1
2
× 10
9
N
1
(cells/ml)
0 5 10 15 20 25 30 35 40 45 50
0
5
× 10
5
t (days)
t (days)
t (days)
N
2
(cells/ml)
460
Numerical integration of ODEs
% Step change in substrate concentration in feed
S0 = 0.1; %mg glucose/ml
y0 = y(end, :); % Values of variables at last time point
% Call ODE Solver
[t, y] = ode15s(‘popdynamics’, tf, y0, [], S0);
MATLAB program 7.15
function f = popdynamics(t, y, flag, S0)
% Evaluate slope f(t,y) of coupled ODEs for microbial population dynamics
% Constants
FbyV = 1/16; % feed to chemostat volume ratio (/hr)
muN1max = 0.25; % max specific growth rate for E coli (/hr)
muN2max = 0.24; % max specific growth rate for amoeba (/hr)
KN1 = 5e-4; % saturation constant (mg glucose/ml)
KN2 = 4e8; % saturation constant (bacteria/ml)
invYN1S = 3.3e-10; % reciprocal yield factor (mg glucose/bacteria)
invYN2N1 = 1.4e3; % reciprocal yield factor (bacteria/amoeba)
f = [FbyV*(S0 - y(1)) - invYN1S*muN1max*y(1)*y(2)/(KN1 + y(1)); ...
-FbyV*y(2) + muN1max*y(1)*y(2)/(KN1 + y(1)) - ...
invYN2N1*muN2max*y(2)*y(3)/(KN2 + y(2)); ...
-FbyV*y(3) + muN2max*y(2)*y(3)/(KN2 + y(2))];
7.7 Shooting method for boundary-value problems
A unique solution for an ordinary differential equation of order n is pos sible only
when n constraints are specified along with the equation. This is because, if a solution
exists for the ODE, the constraints are needed to assign values to the n integrati on
constants of the general solution, thus making the solution unique. For an initial-
value problem, the n constraints are located at the initial point of integration. Such
problems are often encountered when integration is performed over the time
domain. When the interval of integration lies in the physical space domain, it is
likely that some of the n constraints are avail able at the starting point of integration,
i.e. at one end of the boundary, while the remaining constraints are specified at the
endpoint of integration at the other end of the boundary. Constraints that are
specified at two or more boundary points of the interval of integration are called
boundary conditions. ODEs of second order and higher that are supplied with
boundary conditions are called boundary-val ue problems (BVPs).
Consider the second-order ODE
d
2
y
dx
2
dy
dx
þ y ¼ ρ:
To solve this non-homogeneous ODE problem, y is integrated with respect to x. The
interval of integration is a x b, and ρ is some constant.
There are several types of boundary conditions that can be specified for a second-
order ODE. At any boundary endpoint, three types of boundary conditions are
possible.
461
7.7 Shooting method for boundary-value problems

(1) y ¼ α A boundary condition that specifies the value of the dependent variable at an
endpoint is called a Dirichlet boundary condition.
(2) y
0
¼ α A boundary condition that specifies the value of the derivative of the
dependent variable at an endpoint is called a Neum ann boundary condition.
(3) c
1
y
0
þ c
2
y ¼ c
3
A boundary condition that linearly combines the dependent vari-
able and its derivative at an endpoint is called a mixed boundary condition.
For any second-order boundary-value ODE problem, one boundary condition is
specified at x ¼ a and another condition at x ¼ b. The boundary-value problem
y
00
y
0
þ y ¼ ρ; yaðÞ¼α; ybðÞ¼β
is said to be have two Dirichlet boundary conditions.
Several numerical methods have been designed specifically to solve boundary-
value problems. In one popular technique, called the finite difference method, the
integration interval is divided into many equally spaced discrete points called
nodes. The derivatives of y in the equation are substituted by finite difference
approximations. This creates one algebraic equation for each node in the inter val.
If the ODE system is linear, the numerically equivalent system of algebraic equa-
tions is linear, and the methods discussed in Chapter 2 for solving linear equations
are used to solve the problem. If the ODE system is nonlinear in y, the resulting
algebraic equations are also nonlinear, and iterative methods such as Newton’s
method must be used.
Another method for solving boundary-value problems is the shooting method.
This method usually provides better accuracy than finite difference methods.
However, this method can only be applied to solve ODEs and not partial differential
equations (PDEs). On the other hand, finite difference methods are easier to use for
the higher-order ODEs and are commonly used to solve PDEs. In the shooting
method, the nth-order ODE is first converted to a system of first-order ODEs. Any
unspecified initial conditions for the set of first-order equations are guessed, and the
ODE system is treated as an initial-value pro blem. The ODE integration schemes
described earlier in this chapter are used to solve the problem for the set of known
and assumed initial conditions. The final endpoint values of the variables obtained
from the solution are compared with the actual boundary conditions supplied. The
magnitude of deviation of the numerical solution at x ¼ b from the desired solution
is used to alter the assumed initial conditions, and the system of ODEs is solved
again. Thus, we “shoot” from x ¼ a (by assuming values for unspecified boundary
conditions at the first endpoint) for a desired solution at x ¼ b, our fixed target.
Every time we shoot from the starting point, we refine our approximation of the
unknown boundary conditions at x ¼ a by taking into account the difference
between the numerically obtained and the known boundary conditions at x ¼ b.
The shooting method can be combined with a method of nonlinear root finding (see
Chapter 5) for improving the future iterations of the initial boundary condition.
Boundary-value problems for ODEs can be classified as linear or nonlinear, based
on the dependence of the differential equations on y and its derivatives. Solution
techniques for linear boundary-value problems are different from the methods used
for nonlinear problems. Linear ODEs are solved in a straightforward manner with-
out iteration, while nonlinear BVPs require an iterative sequence to converge upon a
solution within the tolerance provided. In this section, we discuss shooting methods
for second-order ODE boundary-value problems. Higher-order boundary-value
problems can be solved by extending the methods discussed here.
462
Numerical integration of ODEs

7.7.1 Linear ODEs
A linear second-order boundary value problem is of the form
y
00
¼ pxðÞy
0
þ qxðÞy þ rxðÞ; x 2 a; b½; (7:54a)
where the independent variable is x. It is assumed that a unique solution exists for the
boundary conditions supplied. Suppos e the ODE is subjected to the following
boundary conditions:
yaðÞ¼α; ybðÞ¼β: (7:54b)
A linear problem can be reformulated as the sum of two simpler linear problems,
each of which can be determined separately. The superposition of the solutions of
the two simpler linear problems yields the solution to the original problem . This is
the strategy sought for solving a linear boundary-value problem. We express
Equation (7.54) as the sum of two linear initial-value ODE problems. The first linear
problem has the same ODE as given in Equation (7.54a):
u
00
¼ pxðÞu
0
þ qxðÞu þ rxðÞ; uaðÞ¼α; u
0
aðÞ¼0: (7:55)
One of the two initial conditions is taken from the actual boundary conditions
specified in Equation (7.54b). The second boundary condition assumes that the first
derivative is zero at the left boundary of the interval. The second linear ODE is the
homogeneous form of Equat ion (7.54a):
v
00
¼ pxðÞv
0
þ qxðÞv; vaðÞ¼0; u
0
aðÞ¼1: (7:56)
The initial conditions for the second linear problem are chosen so that the solution of
the origina l ODE (Equation (7.54)) is
yxðÞ¼uxðÞþcv xðÞ; (7:57)
where c is a constant that needs to be determined. Before we proceed further, let us
confirm that Equation (7.57) is indeed the solution to Equation (7.54a).
Differentiating Equation (7.57) twice, we obtain
y
00
¼ u
00
þ cv
00
¼ pxðÞu
0
þ qxðÞu þ rxðÞþcpxðÞv
0
þ qxðÞvðÞ:
Rearranging,
y
00
¼ pu
0
þ cv
0
ðÞ
þ quþ cv
ðÞ
þ r:
We recover Equation (7.54a),
y
00
¼ pxðÞy
0
þ qxðÞy þ rxðÞ:
Thus, the solution given by Equation (7.57) satisfies Equation (7.54a).
Equation (7.57) must also satisfy the boundary conditions given by Equation
(7.54b) at x ¼ a:
uaðÞþcv aðÞ¼α þ c 0 ¼ α:
We can use the boundary condition at x ¼ b to determine c. Since
ub
ðÞ
þ cv b
ðÞ
¼ β;
c ¼
β u ðbÞ
vðbÞ
:
463
7.7 Shooting method for boundary-value problems

If vbðÞ¼0, the solution of the homogeneous equation can be either v ¼ 0 (in which
case y ¼ u is the solution) or a non-trivial solut ion that satisfies homogeneous
boundary conditions. When vbðÞ¼0, the solution to Equation (7.54) may not be
unique.
For vbðÞ6¼ 0, the solution to the second-order linear ODE with boundary con-
ditions given by Equation (7.54b) is given by
yxðÞ¼uxðÞþ
β uðbÞ
vðbÞ
vxðÞ: (7:58)
To obtain a solution for a linear second-order boundary-value problem subject
to two Dirichlet boundary conditions, we solve the initial-value problems
given by Equations (7.55) and ( 7.56), and then combine the solutions using
Equation (7.58).
If the boundary condition at x ¼ b is of Neumann type ( y
0
bðÞ¼β) or of mixed
type, and the boundary condition at x ¼ a is of Dirichlet type, then the two initial-
value problems (Equations (7.55) and (7.56)) that must be solved to obtain u and v
remain the same. The method to calculate c is, for a Neumann boundary condition at
x ¼ b , given by
y
0
bðÞ¼u
0
bðÞþcv
0
bðÞ¼β
or
c ¼
β u
0
bðÞ
v
0
bðÞ
:
The initial conditions of the two initial-value ODEs in Equations (7.55) and (7.56)
will depend on the type of boundary condition specified at x ¼ a for the original
boundary-value problem.
7.7.2 Non-linear ODEs
Our goal is to find a solution to the nonlinear second-order boundary-value problem
y
00
¼ fx; y; y
0
ðÞ; a x b; yaðÞ¼α; ybðÞ¼β: (7:59)
Because the ODE has a nonlinear dependence on y and/or y
0
, we cannot simplify the
problem by converting Equation (7.59) into a linear combination of simpler ODEs,
i.e. two initial-value problems. Equation (7.59) must be solved iteratively by starting
with a guessed value s for the unspecified initial condition, y
0
aðÞ, and improving our
estimate of s until the error in our numerical estimat e of yðb Þ is reduced to below the
tolerance limit E. The deviation of yðbÞ from its actual value β is a nonlinear function
of the guessed slope s at x ¼ a. We wish to minimize the error, ybðÞβ, which can be
written as a function of s:
ybðÞβ ¼ zsðÞ: (7:60)
We seek a value of s such that zsðÞ¼0. To solve Equation (7.60), we use a nonlinear
root-finding algorithm. We begin by solving the initial-value problem,
y
00
¼ fx; y; y
0
ðÞ; a x b; yaðÞ¼α; y
0
aðÞ¼s
1
;
464
Numerical integration of ODEs

using the methods discussed in Sections 7.2– 7.6, to obtain a solution y
1
ðxÞ.If
z
1
¼ y
1
bðÞβ E, the solution y
1
ðxÞ is accepted.
Otherwise, the initial-value problem is solved for another guess value, s
2
, for the
slope at x ¼ a,
y
00
¼ fx; y; y
0
ðÞ; a x b; yaðÞ¼α; y
0
aðÞ¼s
2
;
to yield another solution y
2
ðxÞ corresponding to the guessed value y
0
aðÞ¼s
2
.If
z
2
¼ y
2
bðÞβ E, then the solution y
2
ðxÞ is accepted. If neither guess for the
slope s
1
or s
2
produce a solution that meets the boundary condition criterion at
x ¼ b , we must generate an impr oved guess s
3
. The secant method, which approx-
imates the function z within an interval Δs as a straight line, can be used to
produce a next guess for y
0
aðÞ. If the two points on the functi on curve, zsðÞ, that
define the interval, Δs, are s
1
; z
1
ðÞand s
2
; z
2
ðÞ, then the slope of the straight line
joining these two points is
m ¼
z
2
bðÞz
1
bðÞ
s
2
s
1
:
A third point lies on the straight line with slope m such that it crosses the x-axis
at the point s
3
; 0ðÞ, where s
3
is our approximation for the actual slope that
satisfies zsðÞ¼0. The straight-line equation passing through these three points is
given by
0 z
2
bðÞ¼ms
3
s
2
ðÞ
or
s
3
¼ s
2
s
2
s
1
ðÞz
2
ðbÞ
z
2
bðÞz
1
bðÞ
:
We can generalize the formula above as
s
iþ1
¼ s
i
s
i
s
i1
ðÞz
i
ðbÞ
z
i
bðÞz
i1
bðÞ
; (7:61)
which is solved iteratively until zs
iþ1
ðÞE. A nonlinear equation usually admits
several solutions. Therefore, the choice of the starting guess value is critical for
finding the correct solution.
Newton’s method can also be used to iteratively solve for s. This root-finding
algorithm requires an analytical expression for the derivative of yðbÞ with respect
to s. Let’s write the function y at x ¼ b as y ðb; sÞ, since it is a function of the initial
slope s. Since the analytical form of zðsÞ is unknown, we cannot directly take the
derivative of yðb; sÞ to obtain dy=dsðb; sÞ. Instead, we create another second-order
initial-value problem whose solution gives us ∂y=∂s ðx; sÞ. Construction of this
companion second-order ODE requires obtaining the analytical form of the deriv-
atives of fx; y; y
0
ðÞwith respect to y and y
0
. While Newton’s formula results in
faster convergence, i.e. fewer iterations to obtain the desired solution, two second-
order initial-value problems must be solved simultaneously for the slope s
i
to
obtain an improved guess value s
iþ1
. An explanation of the derivation and appli-
cation of the shooting method using Newton’s formula can be found in Burden
and Faires (2005).
465
7.7 Shooting method for boundary-value problems

Box 7.5 Controlled-release drug delivery using biodegradable polymeric microspheres
Conventional dosage methods, such as oral delivery and injection, are not ideally suited for sustained
drug delivery to diseased tissues or organs of the body. Ingesting a tablet or injecting a bolus of drug
dissolved in aqueous solution causes the drug to be rapidly released into the body. Drug concentrations
in blood or tissue can rise to nearly toxic levels followed by a drop to ineffective levels. The duration over
which an optimum drug concentration range is maintained in the body, i.e. the period of deriving
maximum therapeutic benefit, may be too short. To maintain an effective drug concentration in the
blood, high drug dosages, as well as frequent administration, become necessary.
When a drug is orally administered, in order to be available to different tissues, the drug must first
enter the bloodstream after absorption in the gastrointestinal tract. The rate and extent of absorption may
vary greatly, depending on the physical and chemical form of the drug, presence or absence of food,
posture, pH of gastrointestinal fluids, duration of time spent in the esophagus and stomach, and drug
interactions. Uncontrolled rapid release of drug can cause local gastrointestinal toxicity, while slow or
incomplete absorption may prevent realization of therapeutic benefit.
In recent years, more sophisticated drugs in the form of protein-based and DNA-based com-
pounds have been introduced. However, oral delivery of proteins to the systemic circulation is
particularly challenging. For the drug to be of any use, the proteins must be able to pass through the
gastrointestinal tract without being enzymatically degraded. Many of these drugs have a small
therapeutic concentration range, with the toxic concentration range close to the therapeutic range.
Research interest is focused on the development of controlled-release drug-delivery systems that can
maintain the therapeutic efficacy of such drugs. Controlling the precise level of drug in the body
reduces side-effects, lowers dosage requirements and frequency, and enables a predictable and
extended duration of action. Current controlled-release drug-delivery technologies include trans-
dermal patches, implants, microencapsulation, and inhaled and injectable sustained-release peptide/
protein drugs.
An example of a controlled-release drug-delivery system is the polymer microsphere. Injection of
particulate suspensions of drug-loaded biodegradable polymeric spheres is a convenient method to
deliver hormonal proteins or peptides in a controlled manner. The drug dissolves into the
surrounding medium at a pre-determined rate governed by the diffusion of drug out of the polymer
and/or by degradation of the polymer. Examples of commercially available devices for sustained
drug release are Gliadel (implantable polyanhydride wafers that release drug at a constant rate as
the polymer degrades) for treating brain cancer, Lupron Depot (injectable polymer microspheres) for
endometriosis and prostate cancer, and Nutropin Depot for pituitary dwarfism. Lupron Depot
(leuprolide) is a suspension of microspheres made of poly-lactic-glycolic acid (PLGA) polymer
containing leuprolide acetate, and is administered once a month as an intramuscular injection. The
drug is slowly released into the blood to maintain a steady plasma concentration of leuprolide for
one month.
Since controlled-release drug-loaded microparticles are usually injected into the body, thereby
bypassing intestinal digestion and first-pass liver metabolism, one must ensure that the polymeric
drug carrier material is non-toxic, degrades within a reasonable time frame, and is excreted from the
body without any accumulation in the tissues. Biodegradable polymers gradually dissolve in body
fluids either due to enzymatic cleavage of polymer chains or hydrolytic breakdown (hydrolysis) of the
polymer. PLGA is an example of a biocompatible polymer (polyester) that is completely biodegrad-
able. As sections of the polymer chains in the outer layers of the drug–polymeric system are cleaved
and undergo dissolution into the surrounding medium, drug located in the interior becomes exposed
to the medium. The surrounding fluid may penetrate into the polymer mass, thereby expediting drug
diffusion out of the particle. Transport of drug molecules from within the polymeric mass to the
surrounding tissues or fluid is governed by several mechanisms that occur either serially or in
parallel. Mathematical formulation of the diffusional processes that govern controlled release is far
from trivial.
Here, we avoid dealing with the par tial differential equations used to model the diffusion of drug in
time and space within the particle and in the surrounding medium. We make the simplifying assumption
466
Numerical integration of ODEs
