King M.R., Mody N.A. Numerical and Statistical Methods for Bioengineering: Applications in MATLAB
Подождите немного. Документ загружается.


that the transport characteristics of the drug (drug dissolution rate, diffusion of drug in three-
dimensional aqueous channels within the degrading polymeric matrix, diffusion of drug in the hydro-
dynamic layer surrounding the particle, polymer degradation kinetics, and kinetics of cleavage of
covalently attached drug from polymer) can be conveniently represented by a time-invariant mass
transfer coefficient k.
A mass balance on a drug-loaded PLGA microparticle when placed in an aqueous medium is given
by the first-order ODE
dM
dt
¼ kA C
s
C
m
ðÞ;
where M is the mass of the drug remaining in the particle, k is the mass transfer coefficient that
characterizes the flux of solute per unit surface area per unit concentration gradient, A is the
instantaneous surface area of the sphere, C
s
is the solubility of the drug in water, and C
m
is the
concentration of drug in the medium. If the particle is settling under gravity in the quiescent medium,
the sedimentation rate of the particle is assumed to be slow. The concentration of drug immediately
surrounding the particle is assumed to be equal to the maximum solubility of drug at body
temperature.
If C
drug
is the uniform concentration of drug within the microsphere that does not change with time
during the polymer degradation process, and a is the instantaneous particle radius, we have
M ¼ C
drug
4
3
πa
3
:
The mass balance equation can be rearranged to obtain
da
dt
¼
k
C
drug
C
s
C
m
ðÞ: (7:62)
The size a of the microsphere shrinks with time.
For a spherical particle settling in Stokes flow under the effect of gravity, the momentum or force
balance that yields a first-order ODE in velocity was introduced in Example 7.2. However, the changing
particle size produces another term in the equation (adapted from Pozrikidis (2008)). The original
momentum balance for a settling sphere in Stokes flow,
4
3
πρ
p
dða
3
vÞ
dt
þ 6μπav
4
3
πa
3
ðρ
p
ρ
m
Þg ¼ 0;
now becomes
a
3
dv
dt
þ 3a
2
v
da
dt
¼ a
3
ðρ
p
ρ
m
Þ
ρ
p
g
9μa
2ρ
p
v: (7:63)
Substituting Equation 7.62 into Equation 7.63, we get
dv
dt
¼
ðρ
p
ρ
f
Þ
ρ
p
g
9μ
2a
2
ρ
p
v þ
3vk
aC
drug
C
s
C
m
ðÞ: (7:64)
The initial condition of Equation (7.64) is vð0Þ¼
dz
dt
t¼0
¼ 0. Equation (7.64) is nonlinear in a.
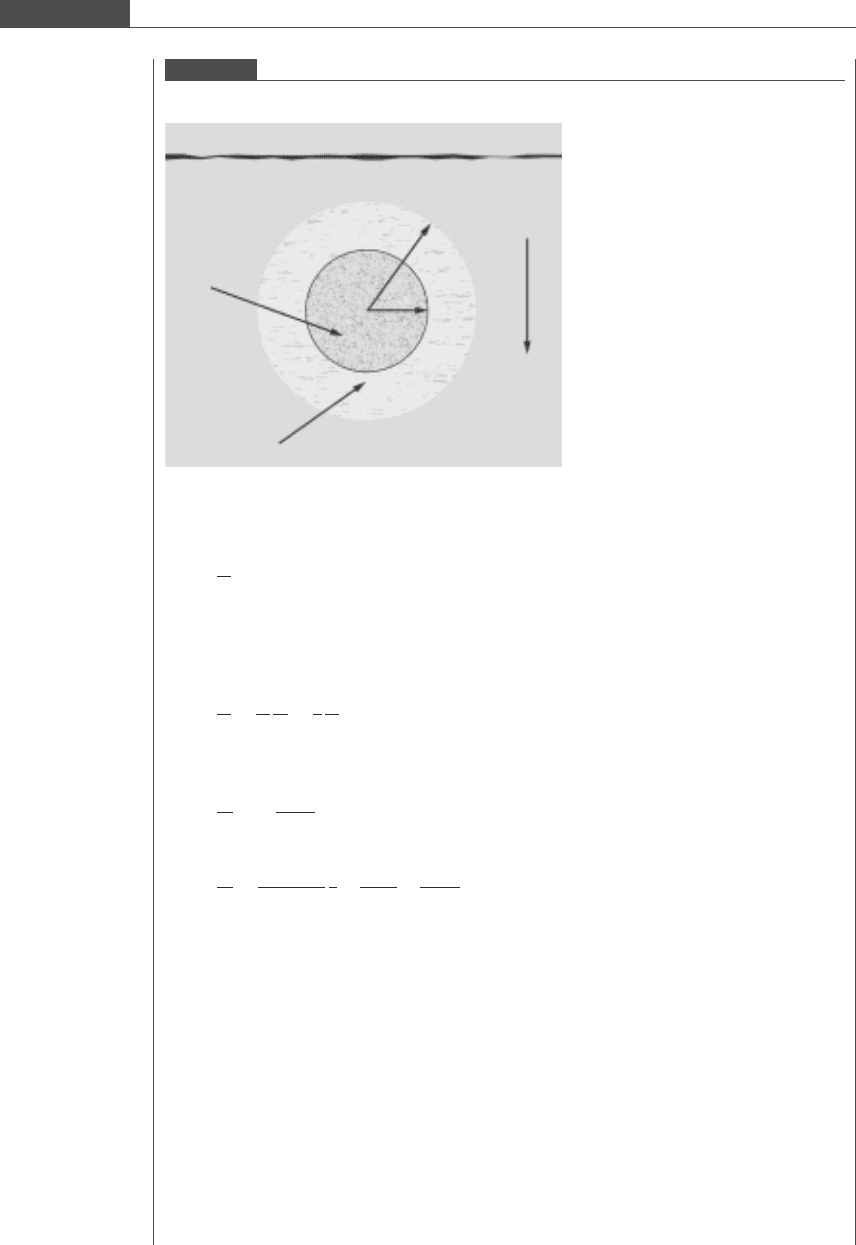
We are asked to determine the size, a
0
, of the largest drug-loaded microparticle that, when added to
the top of a chamber, will dissolve by the end of its travel down some vertical distance through a body of
fluid. Figure 7.15 illustrates the geometry of the system. Under gravity, the drug-loaded particle sinks in
low Reynolds number flow. As the particle falls through the medium, the drug is released in a controlled
and continuous manner.
To calculate the size of the particle that, starting from rest, will shrink to zero radius after falling a
distance L, we simultaneously integrate Equations (7.62) and (7.64). However, the time interval of
integration is unknown. Integration of the system of ODEs must include the first-order equation
467
7.7 Shooting method for boundary-value problems
dz
dt
¼ v
to calculate the distance traveled, so we can stop the integration when z ¼ L. It is more convenient to
integrate for a known interval length. Therefore, we change the independent variable of Equations (7.62)
and (7.64)fromt to z using the rule
df
dz
¼
df
dt
dt
dz
¼
1
v
df
dt
:
Equations (7.62) and (7.64) become
da
dz
¼
k
vC
drug
C
s
C
m
ðÞ; 0 z L; (7:65)
dv
dz
¼
ðρ
p
ρ
m
Þ
ρ
p
g
v
9μ
2a
2
ρ
p
þ
3k
aC
drug
C
s
C
m
ðÞ; 0 z L: (7:66)
The boundary conditions for Equations (7.65) and (7.66) are
v 0ðÞ¼0; aLðÞ¼0:
We use the secant method to solve for a
0
. We try different values of a
0
as suggested by Equation (7.61)
to satisfy the boundary condition aLðÞ¼0.
The drug loading in a single microparticle is 20% w/w. The initial distribution of drug is uniform
within the particle. The parameters are assigned the following values at T ¼ 37 °C:
C
s
¼ 0:1 mg/ml (Sugano, 2008),
C
m
¼ 0:0 mg/ml,
C
drug
¼ 0:256 g/cm
3
,
k ¼ 1 10
6
cm/s (Siepmann et al., 2005),
ρ
p
¼ 1:28 g/cm
3
(Vauthier et al., 1999),
ρ
f
¼ 1:0 g/cm
3
,
Figure 7.15
Schematic diagram of the process of controlled drug release from a microparticle settling in aqueous fluid.
Solid core
Water
Air
z
a
a
0
Degraded hollow shell
ρ
m
ρ
s
468
Numerical integration of ODEs

μ ¼ 0:7cP,
L ¼ 10 cm.
The CGS system of units is used. However, the equations will be designed such that a
0
can be entered in
units of μ m. We make the following assumptions.
(1) As the polymer matrix-loaded drug dissolves into the aqueous surroundings, concomitantly the
outer particle shell devoid of drug completely disintegrates, or is, at the least, sufficiently hollow
that for all practical purposes it does not exert a hydrodynamic drag. The radius a therefore refers to
the particle core that has a density of ρ
s
¼ 1:28 g/cm
3
.
(2) The density, ρ
p
, of the particle core that contains the bulk of the drug does not change. In other
words, hydrolysis of polymer and dissolution of drug occur primarily at the shell surface and
penetrate inwards as a wavefront.
(3) The velocity is small enough that mass transfer is dominated by diffusional processes, and the
mass transfer coefficient is independent of particle velocity (Sugano, 2008).
On making the following substitutions
y
1
¼ a; y
2
¼ v; x ¼ z
into Equations (7.65) and (7.66), we obtain
dy
1
dx
¼
k
y
2
C
drug
C
s
C
m
ðÞ; (7:67)
dy
2
dx
¼
ðρ
p
ρ
m
Þ
ρ
p
g
y
2
9μ
2y
2
1
ρ
p
þ
3k
y
1
C
drug
C
s
C
m
ðÞ: (7:68)
We will not be able to solve Equations (7.67) and (7.68) using the boundary conditions stated above.
Can you guess why? The solution becomes singular (blows up) at a = 0 and at v = 0. We must thus
make the boundary conditions more realistic.
(1) If we want 90% of the drug contained in the microsphere to be released, i.e. 90% of the sphere
volume to vanish during particle descent in the fluid, the boundary condition at x ¼ L becomes
y
1
x ¼ LðÞ¼0:464a
0
:
In other words, when the radius of the particle drops to below 46.4% of its original size, at least 90%
of the drug bound in the polymer matrix will have been released.
(2) We assume that the microparticle is introduced into the fluid volume from a height such that, when
the particle enters the aqueous medium, it has attained terminal Stokes velocity in air at T =20°C
(N
RE
(in air) ~ O 10
5
). Accordingly, the initial velocity at x = 0 is 0:016a
2
0
cm/s, where a
0
is
measured microns.
We also want to know the time taken for drug release. Let y
3
¼ t. We add a third equation to the ODE
set,
dy
3
dx
¼
1
v
(7:69)
The secant equation (Equation (7.61)) is rewritten for this problem as
a
0;iþ1
¼ a
0;i
a
0;i
a
0;i1
y
1;i
LðÞ0:464a
0;i
y
1;i
L
ðÞ
0:464a
0;i
y
1;i1
L
ðÞ
0:464a
0;i1
(7:70)
Example 7.2 demonstrated that the problem of time-varying settling velocity is stiff. We use ode15s
to solve the IVP defined by Equations (7.67) – (7.69) and the initial conditions
469
7.7 Shooting method for boundary-value problems
y
1
x ¼ 0ðÞ¼a
0
; y
2
x ¼ 0ðÞ¼0:016a
2
0
; y
3
x ¼ 0ðÞ¼0:
Care must be taken to start with two guessed values for a
0
that are close to the correct initial condition
that satisfies the boundary conditions at x ¼ L . We choose the two guessed values for the initial
particle size to be a
0;1
¼ 1:2 μm and a
0;2
¼ 1:3 μm. MATLAB programs 7.16 and 7.17 contain the code
used to obtain the result.
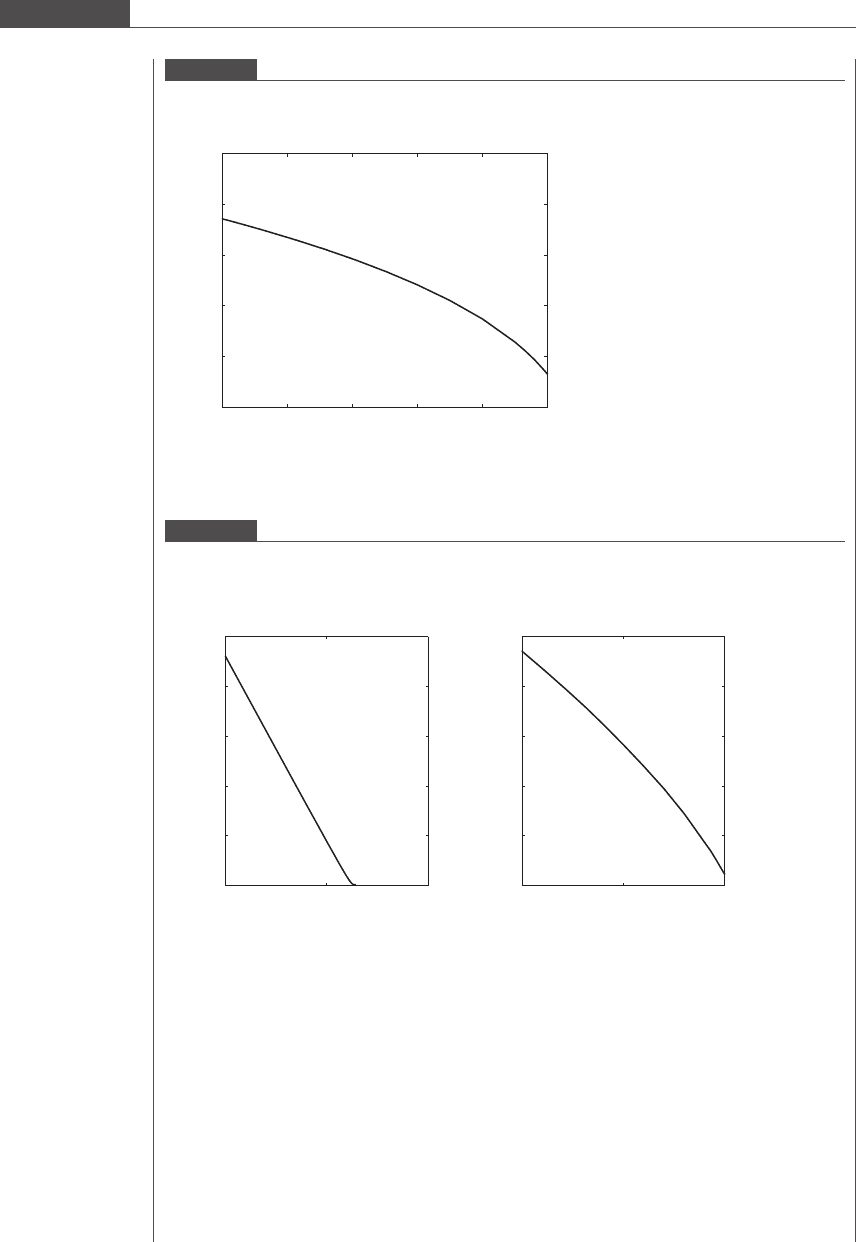
After seven iterations of Equation (7.70), we obtain a solution that satisfies our tolerance condition:
a
0
¼ 1:14 μm. The time taken for 90% of the particle volume to dissolve is 43.6 hours. Figures 7.16 and
7.17 depict the change in radius and velocity of the particle as it traverses downward. Note that initial
velocity retardation is immediate and occurs over a very short time of O 10
6
s. This region of
integration is very stiff.
Figure 7.16
Change in microparticle size with distance traversed as the drug–polymer matrix erodes and dissolves into the
medium.
0 2 4 6 8 10
0.4
0.6
0.8
1
1.2
1.4
x (cm)
a (μm)
Figure 7.17
Change in microparticle settling velocity with distance traversed as the drug–polymer matrix erodes and dissolves
into the medium. (a) Large retardation in velocity of the particle over the first 0.00013 μm of distance traveled.
(b) Gradual velocity decrease taking place as the particle falls from 0.00013 μmto10μm.
0
(a) (b)
10
–8
2 × 10
−8
0
50
100
150
200
250
x (cm)
v (μm/s)
1.3e−008 5 10
0.2
0.4
0.6
0.8
1
1.2
x (cm)
v (μm/s)
470
Numerical integration of ODEs

MATLAB program 7.16
% Use the shooting method to solve the controlled drug delivery
problem.
clear all
% Variables
maxloops = 50; % maximum number of iterations allowed
L = 10; % length of interval (cm)
y0(3) = 0; % initial time (s)
% First guess for initial condition
a0_1 = 1.2; % radius of particle (um)
y0(1)= a0_1;
y0(2) = 0.016*a0_1^2; % velocity (cm/s)
% Call ODE Solver: First guess
[x, y] = ode15s(‘drugdelivery’, L, y0);
y1(1) = y(end,1); % End-point value for y1
% Second guess for initial condition
a0_2 = 1.3; % radius of particle (um)
y0(1) = a0_2;
y0(2) = 0.016*a0_2^2; % velocity (cm/s)
% Call ODE Solver: Second guess
[x, y] = ode15s(‘drugdelivery’, L, y0);
y1(2) = y(end,1); % End-point value for y1
% Use secant method to find correct initial condition
for i = 1:maxloops
% Secant Equation
a0_3 = a0_2 - (a0_2 - a0_1)*(y1(2)-a0_2*0.464)/ ...
((y1(2) - a0_2*0.464) - (y1(1) - a0_1*0.464));
% New guess for initial condition
y0(1) = a0_3;
y0(2) = 0.016*a0_3^2; % velocity (cm/s)
% Solving system of ODEs for improved value of initial condition
[x, y] = ode15s(‘drugdelivery’, L, y0);
y1(3) = y(end,1);
% Check if final value satisfies boundary condition
if abs(y1(3) - a0_3*0.464) < a0_3*0.001 % tolerance criteria
break
end
a0_1 = a0_2;
a0_2 = a0_3;
y1(1) = y1(2);
y1(2) = y1(3);
end
MATLAB program 7.17
function f = drugdelivery(x, y)
% Evaluate f(t,y) for controlled-release drug delivery problem
% Constants
471
7.7 Shooting method for boundary-value problems

7.8 End of Chapter 7: key points to consider
(1) An ordinary differential equation (ODE) contains derivatives with respect to only one
independent variable.
(2) An ODE of order n requires n constraints or boundary values to be specified before a
unique solution can be determined. An ODE is classified based on where the con-
straints are specified. An initial-value problem has all n constraints specified at the
starting point of the interval and a boundary-value problem has some conditions
specified at the beginning of the interval and the rest specified at the end of the interval.
(3) To integrate an ODE numerically, one must divide the integration interval into
many subintervals. The number of subintervals depends on the step size and the size
of the integration interval. The endpoints of the subintervals are called nodes or time
points. The solution to the ODE or system of ODEs is determined at the nodes.
(4) A numerical integration method that determines the solution at the next time point,
t
kþ1
, using slopes and/or solutions calculated at the current time point t
k
, and/or
prior time points (e.g. t
k1
; t
k2
) is called an explicit method, e.g. Euler’s forward
method. A numerical integration method whose difference equation requires a slope
evaluation at t
kþ1
in order to find the solution at the next time point, t
kþ1
, is called an
implicit method, e.g. Euler’s back ward method. The unknown terms in an implicit
formula are located on both sides of the difference equation.
(5) The local truncation error at any time point in the integration interval is the numerical
error due to the truncation of terms, if one assumes that the previous solution(s) used to
calculate the solution at the next time point is exact. The global truncation error at any
time point in the integration interval is the error between the numerical approximation
and the exact solution. The global error is the sum of (i) accumulated errors from
previous time steps, and (ii) the local truncation error at the current integration step.
(6) The order of a numerical ODE integration method is determined by the order of the
global truncation error. An nth-order method has a global truncation error of Oh
n
ðÞ.
(7) A one-s tep integration method uses the solution at the current time point to predict
the solution at the next time point. A multistep integration method evaluates the slope
function at previous time steps and collectively uses the knowledge of slope behavior
at multiple time points to determine a solution at t
kþ1
.
(8) An ODE or system of ODEs is said to be stable if its solution is bounded at all time t.
This is possible if the real parts of all eigenvalues of the Jacobian matrix are negative.
If even one eigenvalue has a positive real part, the solut ion will grow in time without
Cs = 0.1e-3; % solubility of drug (g/m1)
Cm = 0.0; % concentration of drug in medium (g/m1)
Cdrug = 0.256; % concentration of drug in particle (g/cm^3)
rhop = 1.28; % density of particle (g/cm^3)
rhof = 1.0; % density of medium (g/cm^3)
mu = 0.7e-2; % viscosity of medium (Poise)
k = 1e-6; % mass transfer coefficient (cm/s)
g = 981; % acceleration due to gravity (cm/s^2)
f = [-k/y(2)/Cdrug*(Cs - Cm)*1e4; ...
(rhop-rhof)/rhop*g/y(2) - 9*mu/2/(y(1)*1e-4)^2/rhop + ...
3*k/(y(1)*1e-4)/Cdrug*(Cs - Cm); ...
1/y(2)];
472
Numerical integration of ODEs

bound. A numerical solution is said to be stable when the error remains constant or
decays with time, and is unstable if the error grows exponentially with time. The
stability of a numerical solution is dependent on the na ture of the ODE system, the
properties of the ODE solver, and the step size chosen. Implicit methods have better
stability properties than explicit methods.
(9) A stiff differential equation is difficult to integrate since it has one or more rapidly
vanishing or accelerating transients contained in the solution. To integrate a stiff
ODE, one must use a numerical scheme that has superior stability properties. An
integration method with poor stability properties must necessarily use vanishingly
small step sizes to ensure that the numerical solution remains stable. When the step
size is too small, round-off error will preclude a stable solution.
(10) The shooting method is used to solve boundary-value ODE problems. This method
converts the boundary-value problem into an initial-val ue problem by guessing
values for the unspecified initial conditions. The numerical estimates for the boun-
dary endpoint values at the end of the integration are compared to the target values.
One can use a nonlinear root-finding algorithm to improve iterations of the guessed
initial condition(s).
(11) Table 7.5 lists the MATLAB ODE functions that are discussed in this chapter.
7.9 Problems
7.1. Monovalent binding of ligand to cell surface receptors Consider a monovalent
ligand L binding reversibly to a monovalent receptor R to form a receptor/ligand
complex C:
R þ L
k
f
!
k
r
C;
where C represents the concentration of receptor (R) – ligand (L) complex, and k
f
and k
r
are the forward and reverse reaction rates, respectively. If we assume that the
Table 7.5. MATLAB ODE functions discussed in the chapter
ODE
function Algorithm Type of problem
ode45 RKF method: RK−4 and RK−5 pair
using the Dormand and Prince
algorithm
non-stiff ODEs
ode23 RKF method: RK−2 and RK−3 pair
using the Bogacki and Shampine
algorithm
non-stiff ODEs when high
accuracy is not required
ode113 Adams–Bashforth–Moulton method non-stiff ODEs when high
accuracy is required
ode15s multistep method stiff problems; use on the first try
ode23s one-step method stiff problems at low accuracy
ode23t trapezoidal rule stiff equations
ode23tb implicit RK formula stiff equations at low accuracy
473
7.9 Problems

ligand co ncentration remains constant at L
0
, the following differential equation
governs the dynamics of C (Lauffenburger and Linderman, 1993):
dC
dt
¼ k
f
R
T
C½L
0
k
r
C;
where R
T
is the total number of receptor molecules on the cell surface.
(a) Determine the stability criterion of this differential equation by calculating the
Jacobian. Supposing that we are interested in studying the binding of fibronectin
receptor to fibronectin on fibroblast cells (R
T
=5× 10
5
sites/cell, k
f
=7× 10
5
/
(M min), k
r
= 0.6/min). What is the range of ligand concentrations L
0
for which
this differential equation is stable?
(b) Set up the numerical integration of the differential equation using an impl icit
second-order rule, i.e. where the error term scales as h
3
and h = 0.1 min is the
step size. Use L
0
¼ 1 μM. Perform the integration until equilibrium is reached.
How long does it take to reach equilibrium (99% of the final concentration C is
achieved)? (You will need to calculate the equilibrium concentration to perform
this check.)
7.2. ODE model of convection–diffusion–reaction The following equation is a differ-
ential model for convection, diffusion, and reaction in a tubular reactor, assuming
first-order, reversible reaction kinetics:
1
Pe
d
2
C
dx
2
dC
dx
¼ Da C;
where Pe is the Peclet number, which measures the relative importance of convection
to diffusion, and Da is the Damkohl er number, which compares the reaction time
scale with the characteristic time for convective transport.
Perform a stability analysis for this equation.
(a) Convert the second-order system of equations into a set of coupled first-order
ODEs. Calculate the Jacobian J for this system.
(b) Find the eigenvalues of J. (Find the values of λ such that A λI
jj
¼ 0.)
(c) Assess the stability of the ODE system.
7.3. Chlorine loading of the stratosphere Chlorofluorocarbons (CFCs), hydrofluor-
ocarbons (HCFCs), and other haloalkanes are well known for their ozone-depleting
characteristics. Accordingly, their widespread use in industries for refrigeration, fire
extinguishing, and solvents has been gradually phased out. CFCs have a long life-
span in the atmosphere because of their relative inertness. These compounds enter
the stratosphere, where UV radiation splits the molecules to produce free chlorine
radicals (Cl·) that catalyze the destruction of ozone (O
3
) located within the strato-
sphere. As a result, holes in the ozone have formed that allow harmful UV rays to
reach the Earth’s surface.
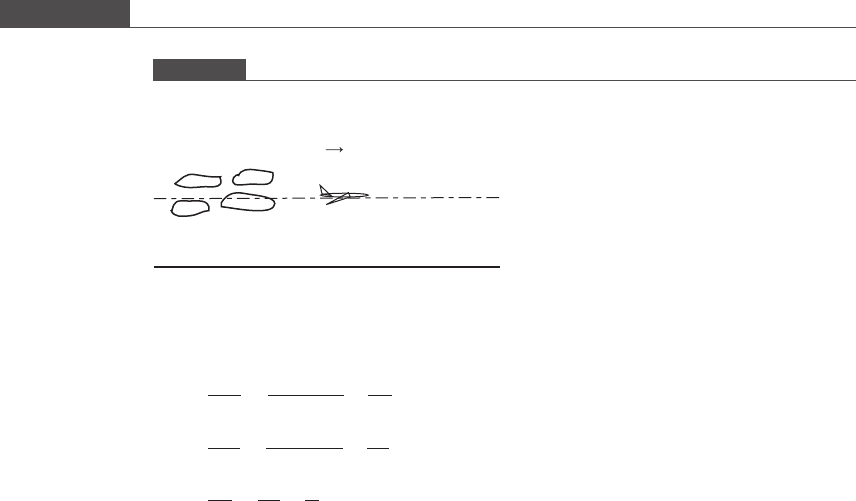
CFC molecules released into the atmosphere cycle every three years or so between
the stratosphere and the troposphere. Halogen radicals are almost exclusively pro-
duced in the stratosphere. Once transp orted back to the troposphere, these radicals
are usually washed out by rain and return to the soil (see Figure P7.1).
The HCFCs are not as long-lived as CFCs since they are more reactive; they break
down in the troposphere and are eliminated after a few years. The concentrations of
halocarbons in the stratosphere and troposphere as well as chlorine loading in the
stratosphere can be modeled using a set of ordinary differential equations (Ko et al.,
1994). If B
S
is the concentration of undissociated halocarbons in the stratosphere, B
T
is the concentration of undissociated halocarbons in the troposphere, and C is the
474
Numerical integration of ODEs
quantity of chlorine in the stratosphere, then we can formulate the following
equations:
dB
T
dt
¼
B
S
fB
T
τ
B
T
L
T
;
dB
S
dt
¼
fB
T
B
S
τ
B
S
L
S
;
dC
dt
¼
B
S
L
S
C
τ
where
τ is the characteristic time for replacing stratospheric air with tropospheric air,
f = 0.15/0.85 is the scaled fraction of tropospher ic air that enters the stratosphere
(15% of atmospheric mass lies in the stratosphere),
L
T
is the halocarbon lifetime in the troposphere that characterizes the time until
chemical degradation,
L
S
is the halocarbon lifetime in the stratosphere that characterize s the time until
chemical degradation.
It is assumed that chlorine is quickly lost from the troposphere. The parameter
values are set as τ ¼ 3 yr, L
T
= 1000 yr, and L
S
= 5 yr for the halocar bon CFCl
3
(Ko
et al., 1994).
(a) What is the Jacobian for this ODE system? Use the MATLAB function eig to
calculate the eigenvalues for J. Study the magnitude of the three eigenvalues,
and decide if the system is stiff. Accordingly, choo se the appropriate ODE
solver.
(b) If 100 kg of CFC is released at time t = 0, what is the CFC loading in the
troposphere and chlorine loading within the stratosphere after 10 years? And
after 100 years?
7.4. Population dynamics among hydrocarbon-consuming bacterial species Pseudomonas
is a class of bacteria that converts methane in the gas phase into methanol.
Secreted methanol participates in a feedback inhibition control process inhibiting
further growth. When grown with another bacterial species, Hyphomicrobium , both
species benefit from an advantageous interdependent interaction (mutualism).
Hyphomicrobium utilizes methanol as a substrate and thereby lowers the methanol
concentration in the surrounding medium promoting growth of Pseudomonas.
Wilkinson et al.(1974) investigated the dynamic behavior of bacterial growth of
these two species in a chemostat. Their developments are reproduced here based on
the discussion of Bailey and Ollis (1986). Let x
1
denote the Pseudomonas population
mass and let x
2
denote the Hyphomicrobium population mass. Dissolved oxygen is a
Figure P7.1
First two atmospheric layers.
Troposphere
Stratosphere
Cl
2
2Cl·
UV
Ozone layer
Sea level
475
7.9 Problems
requirement for Pseudomonas growth, and its growth rate can be described by the
following kinetic rate equation:
r
x
1
¼
μ
x
1
;max
c
O
2
K
x
1
þ c
O
2
1
1 þ S=K
i
x
1
:
This rate equation takes into account the inhibitory action of methanol (S), where K
i
is the inhibition constant. The growth of Pseudomonas exhibits a monod dependence
on dissolved oxygen concentration, c
O
2
, and S is the methanol concentration.
The growth of Hyphomicrobium is not oxygen-limited, but is substrate (methanol)-
dependent, and its growth kinetics are described by the following rate equation:
r
x
2
¼
μ
x
2
;max
S
K
x
2
þ S
x
2
:
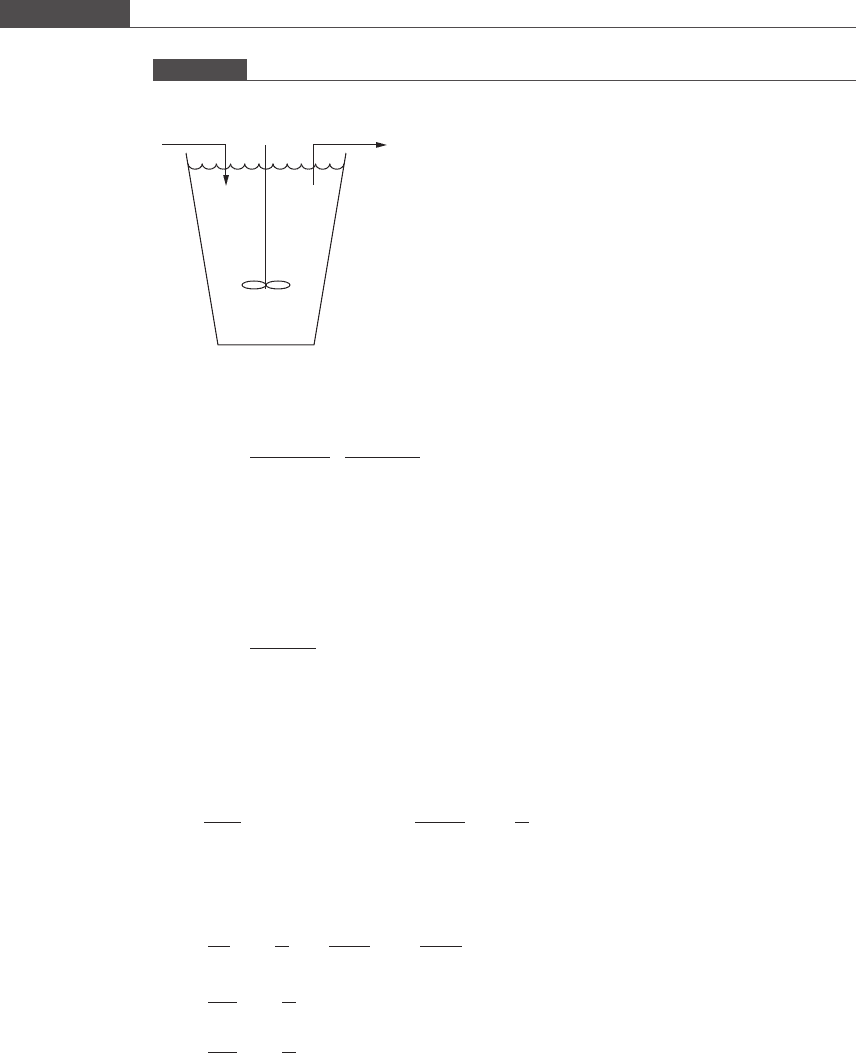
The reactions take place in a chemostat (see Figure P7.2, and see Box 7.4A for an
explanation of this term).
The following mass balance equations describe the time-dependent concentra-
tions of the substrates and two bacterial populations:
dc
O
2
dt
¼ k
l
ac
O
2
s
c
O
2
ðÞ
1
Y
x
1
jO
2
r
x
1
F
V
c
O
2
;
where k
l
ac
O
2
s
c
O
2
ðÞis the mass transfer rate of oxygen at the gas–liquid interface.
We have
dS
dt
¼
F
V
S þ
1
Y
x
1
jS
r
x
1
1
Y
x
2
jS
r
x
2
;
dx
1
dt
¼
F
V
x
1
þ r
x
1
;
dx
2
dt
¼
F
V
x
2
þ r
x
2
:
The reaction parameters estimated by Wilkinson et al.(1974) are listed below.
μ
x
1
;max
¼ 0:185/hr; μ
x
2
;max
¼ 0:185/hr,
K
x
1
¼ 1 10
5
g/l; K
x
2
¼ 5 10
6
g/l,
K
i
¼ 1 10
4
g/l,
Y
x
1
jO
2
¼ 0:2gpseudomonas bacteria/g O
2
consumed,
Y
x
1
jS
¼ 5:0gpseudomonas bacteria produced/g methanol produced,
Figure P7.2
Flow into and out of a chemostat.
V
FS
0
FS
Stirrer
476
Numerical integration of ODEs
