Kelter P., Mosher M., Scott A. Chemistry. The Practical Science
Подождите немного. Документ загружается.


HM ChemSPACE
TM
Developed by teachers, for teachers,
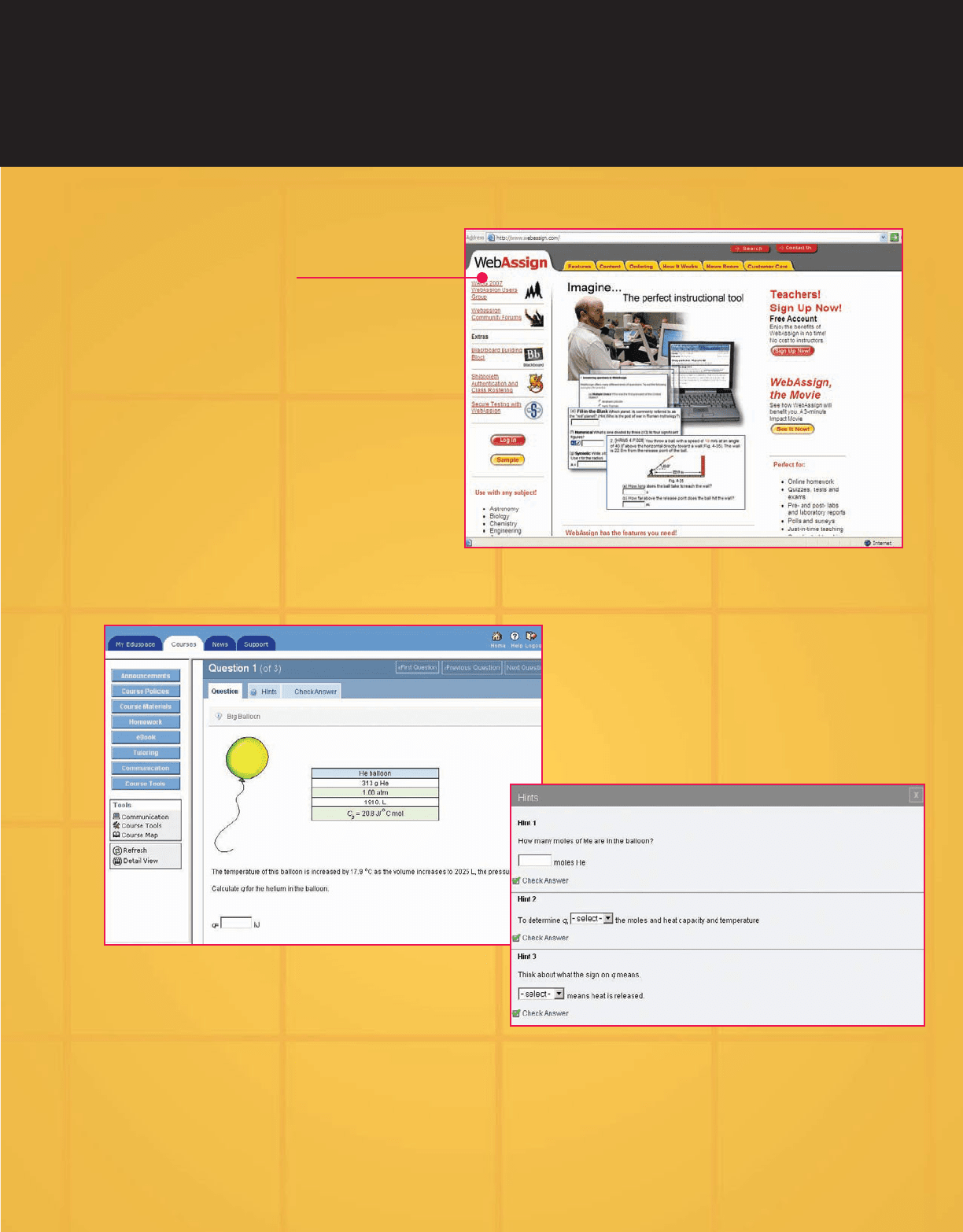
WebAssign
®
is known for offering the most flexible
and stable online homework system on
the market, allowing instructors to focus
on what really matters—teaching—rather
than grading. Create assignments from
our ready-to-use end-of-chapter ques-
tions, or write and customize your own
exercises. WebAssign transforms the
way your students learn!
TAKE A LOOK AT HOUGHTON MIFFLIN’S Best in Class Technology . . .
AN ONLINE HOMEWORK SYSTEM YOU CAN RELY ON . . .
A UNIQUE PROGRAM CRAFTED TO ENHANCE PROBLEM-SOLVING ABILITY . . .
When students need help, they ask for a hint and receive interac-
tive prompts and questions designed to advance their thinking,
without ever actually revealing the solution. These interactive hints
guide students through the problem-solving process, much like an
instructor would during office hours.
Refined over ten years of use by thousands of
students, ChemWork builds and enhances
students’ problem-solving skills. Online assign-
ments function as a “personal instructor” to
help students learn how to solve challenging
problems and learn how to think like chemists!
One of biggest challenges for students
is learning the process of successful
problem solving.
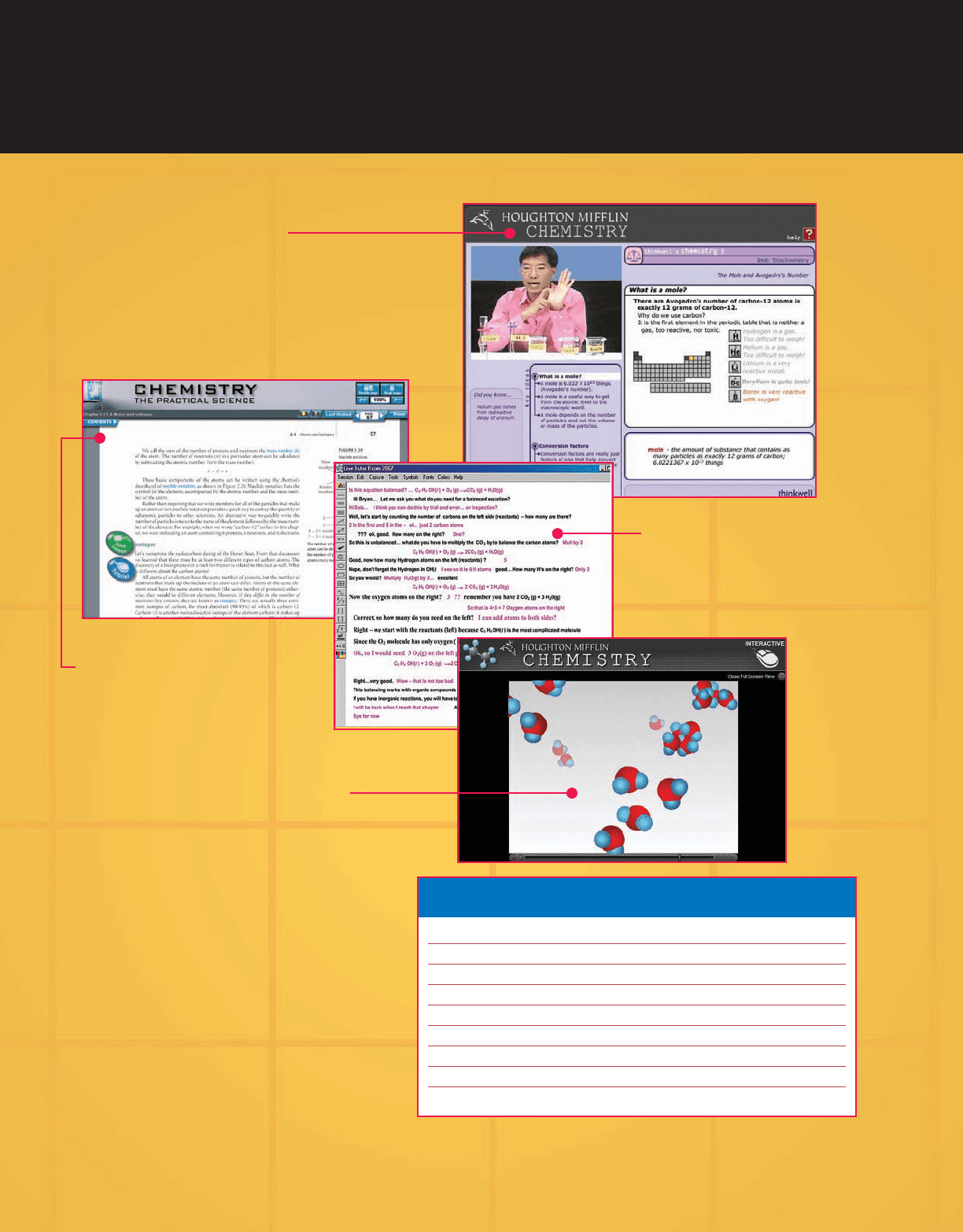
HM ChemSPACE
TM
Free with new texts, the text-specific
Online Multimedia eBook
integrates textbook content with
best in class interactive resources.
Interactive Tutorials and
Visualizations provide molecular animations
and lab demonstrations to help students
visualize and review key concepts.
Thinkwell
®
Video Lessons
offer an engaging and dynamic way for students to under-
stand core concepts. With over 45 hours of video, each mini-
lecture combines video, audio, and whiteboard examples to
address the various learning styles of today’s student.
SMARTHINKING
®
live, online
tutoring helps students compre-
hend challenging concepts and
problems. Contact your Houghton
Mifflin representative for details.
Best in Class Technology . . .
PREMIUM MEDIA RESOURCES REINFORCE KEY CONCEPTS . . .
HM ChemSPACE encompasses the
interactive online products and services
integrated with Houghton Mifflin chem-
istry textbook programs. HM ChemSPACE
is available through text-specific Student
and Instructor websites and via
Eduspace
®
, Houghton Mifflin’s online
course management system.
HM ChemSPACE
HM ChemSPACE with Eduspace
Online Homework in WebAssign ✔
ChemWork ✔
SMARTHINKING ✔
Online Multimedia eBook ✔✔
Thinkwell Video Lessons ✔✔
Interactive Tutorials ✔✔
Visualizations ✔✔
ACE Practice Tests ✔✔
Electronic Flashcards ✔✔
To learn more about HM ChemSPACE, contact your Houghton Mifflin sales representative or visit college.hmco.com/pic/kelterME E
MEDIA ENHANCED EDITION
Houghton Mifflin Company
Boston New York
Chemistry
Chemistry
The Practical Science
Paul Kelter
Northern Illinois University
Michael Mosher
University of Nebraska at Kearney
Andrew Scott
Perth College, UHI Millennium Institute
Contributing Writer
Charles William McLaughlin
University of Nebraska at Lincoln

Publisher: Charles Hartford
Senior Marketing Manager: Nicole Moore
Senior Development Editor: Rebecca Berardy Schwartz
Assistant Editor: Amy Galvin
Project Editor: Andrea Cava
Art and Design Manager: Jill Haber
Cover Design Manager: Anne S. Katzeff
Senior Photo Editor: Jennifer Meyer Dare
Senior Composition Buyer: Chuck Dutton
New Title Project Manager: James Lonergan
Editorial Associate: Henry Cheek
Marketing Associate: Kris Bishop
Cover photo © 2000 Richard Megna, Fundamental Photographs, NYC
Credits appear on pages A49–A52, which are considered an extension of the copyright page.
Copyright © 2009 by Houghton Mifflin Company. All rights reserved.
No part of this work may be reproduced or transmitted in any form or by any means, electronic or
mechanical, including photocopying and recording, or by any information storage or retrieval system
without the prior written permission of Houghton Mifflin Company unless such copying is expressly
permitted by federal copyright law. Address inquiries to College Permissions, Houghton Mifflin Company,
222 Berkeley Street, Boston, MA 02116-3764.
Printed in the U.S.A.
Library of Congress Control Number: 2007937002
ISBN-10: 0-547-05393-2
ISBN-13: 978-0-547-05393-6
123456789–CRK–11 10 09 08 07
To Barb, Kristie, and Margaret,
who are with us through it all, and to Seth, Aaron, Jamie, David, and
Alison, for whom we do these things
To Jim Carr,
master craftsman of the finest teachers

v
Develop effective
problem-solving skills
First Thoughts encourages
students to think about a
strategy before attempting
the solution.
Solution demonstrates the
detailed solution to the
problem and effectively
uses color to highlight key
information.
Further Insights continues
the discussion of the
problem, illustrating
applications of practical
importance and linking
multiple concepts
throughout the book.
Practice includes addi-
tional practice problems
for students to try on
their own, with answers
in the appendix.
EXERCISE 5.3 The First Law of Thermodynamics
Calculate the change in the energy of a system if 51.8 J of work is done by the system
with an associated heat loss of 12.3 J.
First Thoughts
We must pay particular attention to the sign conventions for heat and work in this
problem. In this case, work is done by the system. Is this work positive or negative?
A useful system to keep in mind is you! That is, when you do work—by running,
dancing, or even moving your textbooks from one class to the next—you are using
energy. After the process of moving your body or your books, you have less energy
than you had before, so the work has a “−” sign. Similarly, the 51.8 J of work done
by the system means that its energy change, w
system
,is−51.8 J. The energy loss as
heat by the system (such as that accompanying your run as your body tries to stay
cool) also has a “−” sign, so q
system
=−12.3 J.
Solution
From the standpoint of the system,
U = q + w
U =
−
12.3 J + (−51.8 J) =
−
64.1 J
Further Insights
We want to reinforce the point that there is no such thing as heat or work within a
system. In other words, a system does not contain heat or work. Rather, heat and work
exist only as types of energy transfer between the system and the surroundings. Work
is done by or on a system to move it through a distance. A system can transfer 64.1 J
of energy as heat and work. It does not contain 64.1 J of heat and work.
PRACTICE 5.3
Calculate the change in the energy of a system if 84.7 J of work is done on the sys-
tem, with an associated loss of energy as heat of 39.9 J.
See Problems 11–14, 19, 20, and 89.
The problem-solving approach is more about reason than rote. Before jumping
headfirst into an Exercise, students are asked to consider First Thoughts. These
remarks often ask students to apply their real-world experience to generate
ballpark answers. They also call on prior course content to help students see the
big picture and develop a plan of attack. A detailed solution is then followed by
Further Insights, which compel students to not only ask themselves if their an-
swer makes sense but to put the entire exercise in context and make connections
to biology and numerous other disciplines. In short, students are asked to think
like a scientist.—Ed O’Sullivan, Parkland College
This way of doing things gives students real insights into how “real chemists or
scientists” think.—Milton Johnston, University of South Florida
This approach to problem solving emphasizes problem strategies, and
then encourages students to look beyond the problem.

vi
Engage students through
integrated applications
A space shuttle launch is an awe-inspiring
demonstration of the ability of energy to trans-
port humans and material away from Earth’s surface
with an eye toward planetary exploration. The energy to
launch the craft and its crew comes from the explosive vio-
lence of chemical reactions. When these reactions are used in a
carefully controlled way, they can lift the massive shuttle (which
weighs in at a robust 2,000,000 kg), the booster rockets, and the fuel tank
and propel the ship into orbit hundreds of miles above Earth in a very brief
10-minute ride.
Two chemical reactions, indicated in Figure 5.1, power the launch of the space
shuttle. The combustion of hydrogen gas (the combination of hydrogen and oxygen
to form water) takes place in the main engines. At the same time, the solid rocket
boosters attached to the sides of the shuttle release a host of products resulting from
the oxidation of aluminum by ammonium perchlorate, though we show only the
primary equation here.
Main engines: 2H
2
(g) + O
2
(g) →2H
2
O(g)
Boosters: 10Al(s) + 6NH
4
ClO
4
(s) → 5Al
2
O
3
(s) + 6HCl(g) + 3N
2
(g) + 9H
2
O(g)
As the shuttle sits on the launch pad, it is hard to believe that the en-
ergy that will launch it with such a spectacular display of chemical mus-
cle is quietly present within the main fuel tank and the solid fuel of the
booster rockets. Chemicals can store huge amounts of energy in this
Application
C
HEMICAL ENCOUNTERS:
Setting the Stage with
the Space Shuttle
Interwoven throughout the chapter,
chemistry concepts are explained
through the use of real-world examples.
Application icons throughout the text
show at a glance where chemical
concepts are applied.
Applications are also found
in
How Do We Know? and
NanoWorld / MacroWorld
features, chapter openers,
photos, and end-of-chapter
exercises. Students will see how
chemistry connects to their lives,
their future careers, and their
world.
4. Diagram, using circles for atoms, a crystal of KCl versus the
same crystal of KCl dissolved in water.
Chemical Applications and Practices
5. Earth’s oceans contain tons of dissolved sodium chloride.Yet,
when a ship develops an oil leak, almost none of the oil dis-
solves in the ocean. Explain this phenomenon.
6. When an ion dissolves, it is surrounded by a hydration
sphere. If water molecules surrounded the ion so that the hy-
drogen portion of the water was closer to the ion, would the
ion most likely be a cation or an anion?
7. Pure water does not conduct an electric current. However,
aqueous solutions of some compounds do form solutions
that conduct electricity. Explain why the presence of some
solutes converts nonconducting water into a conducting
solution.
8. Glycerin can be produced as a by-product in soap making.
The compound dissolves so easily in water that it absorbs
water from the air. This latter characteristic is why glycerin
is often found in many skin lotions. As glycerin absorbs
the water, the skin can be kept moist. Glycerin’s structure is
shown below. What aspects of glycerin’s structure contribute
most to its ease of dissolving in water?
9. A conductivity-testing apparatus, such as the one shown in
this chapter, possesses a light bulb whose brightness is related
to how much current is flowing through it (and also through
the solution.) A small, but measurable, amount of current
must be present before the bulb becomes visibly brighter.
What effect would this characteristic of the apparatus have
12. Which of the following would best represen
t
water?
CHH
H
OH
C
H
OH
C
H
OH
Gl
y
cerin
(a) (b)
(c) (d)
Mg
2+
H
2
OCl
–
(a) (b)
(c) (d)
H
2
OH
“Smile, you’re on candid camera!” Everyone hopes never
to be ambushed with those words. In the nineteenth cen-
tury, when photography was in its infancy, candid pho-
tographs were not possible. In order to create a visual
memory of an event, you had to visit the photographer
and sit for a picture.“Sitting” meant holding a pose for
up to 30 seconds. Any movement would show as a blur
in the final picture. However, the idea that an inexpen-
sive, yet realistic, picture could be created in such a short
time brought people to the photographer’s studio in
droves.
Scientists in the early 1800s were intrigued by the
idea of painting portraits without the artist’s pen. In the
summer of 1827, Joseph Nicéphore Niépce, using mater-
ial that hardened on exposure to light exposed film for
eight hours in order to get a picture. Because no one
wanted to sit still that long, most of his pictures were
landscapes. In 1839, however, two other photographic
processes were developed, the daguerreotype (perfected
by Louis Daguerre) and the calotype (Henry Fox Talbot).
Although these techniques allowed pictures to be created
with much shorter exposures, they still produced a
black-and-white image (see Figure 9.30). This didn’t
matter much to the general public, and photographic
portrait studios seemed to pop up on every corner across
the globe. Despite some marginal success in producing
faint color images during the 1850s and 1890s, the ad-
vent of the color photograph as an inexpensive, repro-
ducible and stable process didn’t occur until the 1920s.
Researchers at Kodak Research Laboratories in 1935
finally hit upon the process that would bring the color
photograph to the world. Kodachrome®, their color
photography process, introduces color to a picture
by separating the three primary colors into specific
emulsion layers. How does it work? The emulsion layers
contain a compound that reacts when light strikes it. A
quick look at the chemistry behind the black-and-white
photograph will give us some insight.
In black-and-white photography, a piece of transpar-
ent plastic is coated with silver bromide crystals (see Fig-
ure 9.31) and a sensitizer. The plastic sheet is then placed
in a camera, and when the shutter is opened, the silver
bromide absorbs photons of light. In the presence of the
sensitizer, the silver cation is reduced by the sensitizer
(gains an electron) and becomes silver metal:
Ag
+
(s) + sensitizer n Ag°(s) + sensitizer
+
Then the film is wound back into its container and
sent to be developed. The technician rinses the plastic
film to remove unreacted silver bromide. The silver
metal remains in place on the plastic. The result is an in-
verted image called a negative. Next the technician shines
light through the negative onto another silver bromide/
sensitizer-coated support (typically paper this time) and
rinses off the unreacted silver bromide. Violà! We have a
positive image called a photograph.
Why is the film coated with silver bromide instead of
silver chloride? We can answer this by examining the
wavelength of light that is absorbed; see Figure 9.32.Com-
parison of the two shows that silver bromide absorbs a
large amount of the visible light that enters our camera.
To get the best picture, we want to absorb as much light
as we can.
NanoWorld / MacroWorld
Big effects of the very small:
Color photography and the tricolor process
FIGURE 9.30
A daguerreotype of Michael Faraday
(see Chapter 19) taken sometime
between 1844 and 1860 at Mathew
Brady’s studio in New York City.
Because the process required the
subject to remain completely
motionless for up to 10 seconds,
most daguerreotypes were taken
in well-lit studios. Even though
the early daguerreotypes cost one
month’s wages for the average
person, they were much cheaper
than a painted portrait. This made
them an instant success.
FIGURE 9.31
A magnified image of silver bromide crystals used in the photographic
industry. Note that the crystals are mostly hexagonal in shape and flat
so that more surface area is exposed to incoming photons.

vii
Dilution
How do municipal water treatment plant workers prepare water so that it contains
a fluoride ion concentration of about 1 part per million?
They use a concentrated
source of fluorine, hydrofluorosilicic acid, H
2
SiF
6
(“HFSA”), which they then di-
lute in the drinking water. HFSA reacts with water in a fairly complex way that re-
leases fluoride ion into the water. In Ireland, a company in the town of New Ross,
Application
Ask questions
Inquiry-based learning is integral to this book. Questions are asked throughout
the narrative to help students make the connection between what they are
reading, concepts throughout the text, and their everyday lives.
Highlighted questions
appear throughout the
chapter and prompt
students to learn how to
raise meaningful questions
about topics.
How do we know? essays
ask questions to probe
the use of key chemical
concepts in medicine,
research, and other
applications.
Students are also given
many opportunities to
reflect on what they have
learned throughout the
book.
Here’s What We
Know So Far in-chapter
summaries review
complex topics.
The moons of the outer planets make
most elegant chemical laboratories,
because conditions on these worlds are so very dif-
ferent from those here at home. When we look at
photos of Jupiter’s moons, Europa and Ganymede
(Figure 11.17), we see some vexing geological fea-
tures. How do we know what caused these features?
We can’t visit the moons, at least not directly. How-
ever, our space probes can gather various types of
electromagnetic radiation—including light, ultra-
violet radiation, and X-rays—that give us data
from which to draw conclusions.
The probes’ data seem to show that deep within
the surface of these moons, there are layers of ice,
each in a different phase, mixed with rocks. The phase
diagram of water that we showed as Figure 11.18
is inadequate to account for the low temperatures
and massive pressures found within these moons.
A more comprehensive phase diagram for water,
emphasizing its solid phases, is given as Figure 11.20.
The ice that is made in our kitchen freezer or found
in an ice storm—what we might call “normal ice”—
is technically called Ice Ih. Its structure is shown in
Figure 11.21. There are 11 other forms
of ice that have been made in the laboratory or
simulated by computer.
The phase diagram shows that as the
temperature and pressure inside the moons
change, different types of ice are formed.
Each ice has its own density. As layers
form versions of ice of different
densities, they form the geological
features, such as cracking and
How do we know?
The moons of Jupiter
1
10
3
10
6
10
9
10
12
0 100 200 300 400 500 600 700 800
Solid
IhIc
III
V
VI
VII
VIII
IX
XI
II
X
XI
Liquid
Vapor
Temperature (K)
Pressure (Pa)
FIGURE 11.20
This is an expanded phase diagram for water, taking into account
the phase changes among the various solid (ice) forms. These forms
of ice are thought to occur in layers beneath the surface of some
of the moons of the outer planets.
FIGURE 5.11
An endothermic process. Some chemical
reactions require the transfer of energy
from the surroundings to the system. This
is an endothermic process.
HERE’S WHAT WE KNOW SO FAR
■
There are three natural forces: the gravitational, electroweak, and strong
nuclear forces.
■
Potential energy is the energy stored within a system.
■
Kinetic energy is the energy associated with motion.
■
The law of conservation of energy states that energy can be neither created nor
destroyed. Instead, it just moves between the system and the surroundings
and can be transformed from one form to another.
■
Work and heat are two ways in which energy can move between the system
and the surroundings.
■
The flow of energy as heat from the system to the surroundings involves an
exothermic process. An endothermic process involves the flow of energy as
heat from the surroundings to the system.
■
Changes in the energy of a reaction can be studied by examining a reaction
profile diagram.
Cold packs use endothermic reactions.

viii
Dynamic, contemporary
art program
Let’s look at these concentration units in a slightly different way.As an example,
the EPA maximum level for hexachlorobenzene (C
6
Cl
6
), a pesticide used onwheat
in the United States until 1965, is 1 ppb. This means that the maximum allowable
level in drinking water should be 1 gram of C
6
Cl
6
per billion grams of solution.
1ppbC
6
Cl
6
=
1gC
6
Cl
6
10
9
g solution
If we use
1 g water
1 mL water
as the density of water and make the key assumption that the
solution is so dilute that its density is about equal to that of water, then
Density of very dilute aqueous solutions
=
1 g solution
1 mL solution
This means that we can express the concentration of hexachlorobenzene as the
number of grams of C
6
Cl
6
per liter of solution:
1gC
6
Cl
6
10
9
g solution
×
1 g solution
1 mL solution
×
10
3
mL solution
1 L solution
=
10
−6
gC
6
Cl
6
L solution
=
1 µgC
6
Cl
6
L solution
↑↑ ↑
(one ppb C
6
Cl
6
) (density of the solution) (convert mL to L)
The bottom line is that in dilute aqueous solutions, we can express parts per
billion as
136 Chapter 4 Solution Stoichiometry and Types of Reactions
Hexachlorobenzene
(a) (b) (c)
FIGURE 4.10
To visualize the idea of parts per million,
per billion, and per trillion, consider that
one drop of ink could be placed in these
quantities of water. (a) Placing that drop
of ink in a 12-gallon bucket results in
1 ppm. (b) Placing it in a tanker truck
results in 1 ppb. (c) Placing it in a
12-million-gallon reservoir results in
1 ppt.
This contemporary art and photo program appeals to tech-savvy students who
expect exciting and visually appealing graphics regardless of medium.
11.2 A Closer Look at Intermolecular Forces 445
Water boils and creates steam.
a mole of liquid methane, compared to 1650 kJ to break the four C—H bonds in
a mole of methane! We can extend this information to make the more general
statement that intermolecular interactions are much weaker than intramolecular
interactions. Less energy is required to boil a liquid than to break it into its
component elements.
11 2 ACl L k I l l F
Five molecules of liquid water interact
with each other. Note the positions of
the hydrogen atoms. They are being
shared with neighboring oxygen atoms.
Molecular-level illustrations
of key concepts help students
connect nanoscale activity to
macroscale phenomena.
In addition,
electrostatic
potential maps use vibrant
color to show how
electron density changes
across a molecule.
128 Chapter 4 Solution Stoichiometry and Types of Reactions
Ethanol
An electrostatic potential map of glucose and
ethanol. The electrostatic potential is plotted
on the surface of a computer-generated
model of the molecules. Note how the electron
density is distributed in each molecule, and
compare these models to that of water
(Figure 4.3).
D-glucose
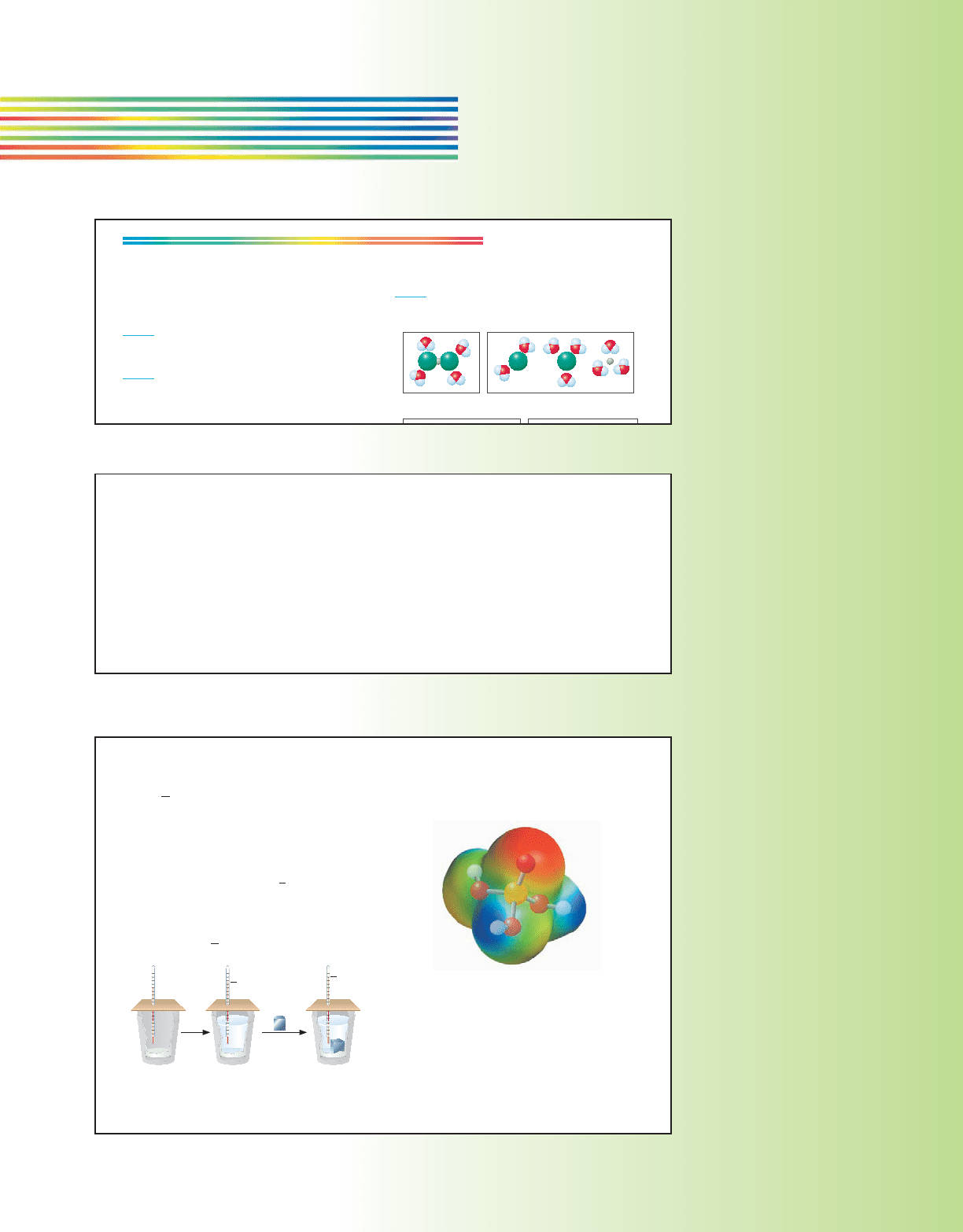
ix
A wealth of end-of-chapter
problems test students’
understanding of key
concepts and problem-
solving skills. They include
Skill Review and Chemical
Applications and Practices
,
organized by chapter
sections and in matched
pairs.
Comprehensive Problems
build on the students’
mastery of concepts in
the chapter and across
multiple chapters in the
textbook.
Thinking Beyond the
Calculation provides
rigorous, cumulative, and
conceptual problems based
on multiple concepts.
Focus Your Learning
The answers to the odd-numbered problems appear at the back of
the book.
Section 4.1 Water—A Most Versatile Solvent
Skill Review
1. Explain how water molecules can dissolve both cations and
anions.
2. Why doesn’t pure water conduct an electric current?
3. Explain what is meant by the term hydration sphere?
4. Diagram, using circles for atoms, a crystal of KCl versus the
same crystal of KCl dissolved in water.
Section 4.2 The Concentration of Solutions
Skill Review
11. Which of the following would best represent MgCl
2
dissolved
in water?
(a) (b)
Comprehensive Problems
85. Individual atoms and molecules are so small that they have
very low values of kinetic energy. However, given their mass
and velocity, it is possible to calculate the value. What is the
kinetic energy of an oxygen molecule (O
2
) in air that you are
breathing if its velocity is 460 m/s? Would you expect a
nitrogen molecule (N
2
) moving at the same speed to have
more or less kinetic energy than the oxygen molecule?
Explain.
86. Distinguish between the two terms in each of the following
pairs:
a. Heat and temperature
b. System and surroundings
c. Exothermic and endothermic
d. q and U
)pj
93. a. Suppose you are heating water (225 g) in a mug that you
have placed in a microwave oven.As you wait to add the in-
stant hot chocolate, please calculate, from the following
data, the amount of energy as heat that the water has ab-
sorbed: The original water temperature was 15.0°C. When
you remove the mug of hot water, you find that the tem-
perature has risen to 98°C.
b. What additional information would you need in order to
determine the heat absorbed by the mug?
94. You have just removed a hot cheese pizza from the oven, and
all of the ingredients are presumably at the same tempera-
ture. Without waiting for it to cool, you take a bite of the
pizza. As you bite the pizza, the bread is hot on your tongue
but does not burn. However, as you continue to bite, pizza
sauce (mostly tomatoes and water) squeezes out and burns
the roof of your mouth. Which has the higher specific heat,
a. If the value of H for the reaction is −453 kJ, what is the
value of H for the reverse of the reaction?
b. What is the value of H for this reaction if 10.0 g of
phosphoric acid is produced?
c. Is this reaction endothermic or exothermic?
d. If 1.50 g of P
4
O
10
(s) and 2.50 mL of water were mixed,how
many grams of phosphoric acid would result?
e. What is the enthalpy change for the process outlined in
part d?
f. If 10.0 g of P
4
O
10
were mixed with 1.00 kg of water at
25.0°C, what would be the final temperature of the water?
22.7°C
152.06 g =
Mass of
calorimeter
Water
added
Metal block
at 96.3°C
234.95 g =
Mass of
calorimeter
and water
257.88 g =
Mass of
calorimeter,
water, and
metal block
24.1°C
Phosphoric acid
96. A student’s coffee cup calorimeter, including the water it
contains, has been calibrated in a manner similar to that de-
scribed in Problem 46. The heat capacity was found to be
55.5
J
◦
C
. If a 65.8-g sample of an unknown metal, at 100.0°C,
was placed in the calorimeter initially at 25°C, and an equi-
librium temperature of 29.1°C was reached, what is the
specific heat of the metal?
97. The foods we eat provide fuel to keep us alive. Burning a
0.500-g sample of vegetable oil provides enough heat to raise
the temperature of a calorimeter by 2.5 K. Assuming the heat
capacity of the calorimeter to be 7.5
kJ
K
, determine the heat of
combustion for 1 g of the oil.
98. A student performs the experiment shown graphically here.
What is the specific heat of the block of metal used in the ex-
periment? (Assume that the heat capacity of the empty
calorimeter is 7.5
J
◦
C
.)
Thinking Beyond the Calculation
99. Phosphoric acid is used in many soft drinks to add tartness.
This acid can be prepared through the following reaction:
P
4
O
10
(s) + 6H
2
O(l) → 4H
3
PO
4
(aq)
Comprehensive
end-of-chapter materials
