Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

432 CHAPTER 10
H
2
S in product gas = 0.1 grain/100 scf
= 0.126 moles/day
= 0.00521 moles/hr
H
2
S absorbed in amine = 212.5 − 0.00521
= 212.49 moles/hr
amine ratio is 3.0 moles amine per mole H
2
S
Moles amine circulating = 637.47 moles/hr. = 38,949 lbs/hr (mol wt. MEA =
61.1) 20% wt solution = 194,747 lbs /hr made up of 155,798 lbs water and 38,949
amine.
lbs/gal MEA = 8.45
lbs/gal water = 8.328
MEA gals/hr = 4,609
Water gals/hr = 18,708
Amine solution circulation rate = 23,317 gals/hr.
Calculating the number of trays and the overall dimensions of the contactor
The number of theoretical stages required in the contactor will be calculated using
the equation:
N =
(Log 1/q (A − 1))
(Log A)
− 1 (1)
where
N = number of theoretical trays
q = mole H
2
S in lean gas/mole H
2
S in feed gas
A = the absorption factor L/V·K
‘q’ in this case =
moles H
2
S in lean gas
moles H
2
S in feed gas
=
0.00521
212.5
= 2.45 × 10
−5
REFINERY GAS TREATING PROCESSES 433
Total moles of acid gas absorbed = 212.49 moles/hr
Acid gas residual in the lean amine = 57.37 moles/hr
Total acid gas in grains/hr =
269.87 × 34 × 16
0.0023
= 63.83 × 10
6
grains/hr
Total MEA solution = 23,317 gals/hr
Then grains acid gas per gal of amine solution = 2,738 grains H
2
S/gal MEA.
From Figure 10.2 for 20% MEA solution H2S partial pressure is 0.33 psia.
Using the following equation the absorption factor A is calculated as follows:
A =
a(1 + Rr)(1 − q)
pp/P
‘a’ is the mole fraction of H
2
S in feed gas =
212.5
3,306.9
= 0.0643
‘R’ = moles MEA per mole acid gas absorbed = 3.0
‘r’ = 0.09 moles H
2
S per mole lean MEA.
Then
A =
0.0643 ×{1 + (3.0 × 0.09)}×{1 − (2.45 ×10
−5
)}
0.33 ÷ 335
= 82.1
Therefore
N =
log{(1 ÷ 2.45 × 10
−5
) × (82.1 − 1)}
log 82.1
=
log(3.283 × 10
6
)
log 82.1
=
6.5
1.91
= 3.4 theoretical trays.
Set tray efficiency at 15% then actual number of trays = 23
MEA has a tendency to foam therefore set tray spacing at 30 inches.
Then trayed section will have a height of 22 × 1.5 ft = 33 ft.
Calculating the contactor diameter
Use foaming factor of 60%.
Feed gas to the contactor is 30 mmscf/day

434 CHAPTER 10
Temperature of gas is 100
◦
F and its pressure is 335 psia (these are average conditions).
Then actual cubic feet per second (ACFS) =
30 × 10
6
× 14.7 × 580
24 × 3600 × 520 × 335
= 16.41 cfs
Feed gas in lbs/hr is 34,722 = 9.645 lbs/sec
ρ
v
=
9.645
16.41
= 0.588lbs/cuft
lbs/hr of MEA solution is 194,747
Gals/hr is 23,317
And cubic ft /hr is 3,117
Then lbs/cuft = 62.48 and at 120
◦
F (MEA inlet temperature) = 62.1 lbs/cuft.
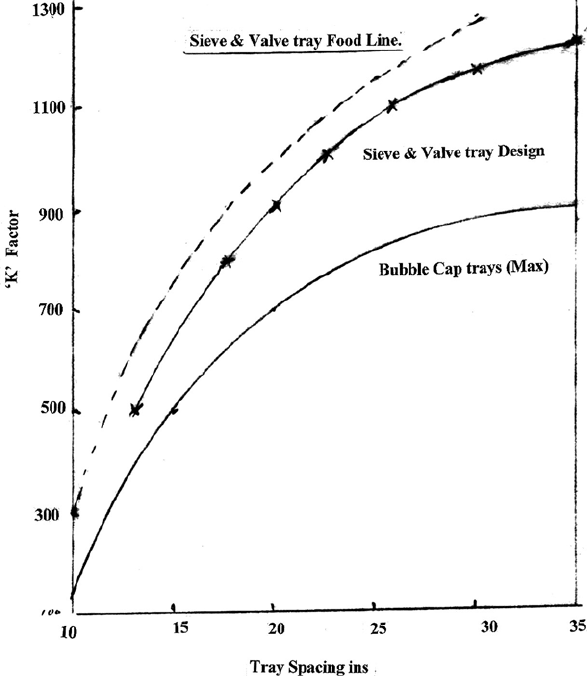
Loading at flood: K
f
√
{ρ
v
× (ρ
l
− ρ
v
)}
K
f
is 1,280 from Figure 10.3 and inserting the 60% foam factor K
f
= 768.
Loading = 768 ×
√
36.17 = 4,619 lbs/hr·sqft.
Let design load be 70% of flood = 3,233 lbs/hr·sqft
Cross sectional area of tray (and tower I/D) =
34,722
3233
= 10.74 sqft.
Internal diameter of tower (calculated) = 3.7 ft
Call it 4 ft = 12.6 sqft cross sectional area.
The actual tray design will be done by others (tray manufacturer) to protect the
guarantee requirements.
Calculate the amine hold up in the bottom of the tower
The liquid hold up will have be 1 min to NLL.
The volume of amine in 10 min =
3,117
60
= 51.95 cuft
Then NLL will be
51.95
12.6
= 4.1ftsay4ft
Then HLL will be set at 8 ft and LLL at 4 ft above Tan.
Add a further 4 ft from HLL to bottom tray.
REFINERY GAS TREATING PROCESSES 435
Figure 10.3. The Brown and Souder flood constant.
The overall dimensions of the contactor
The height of the contactor is as follows:
From Bottom Tan line
To bottom tray = 12 ft
To top absorbing Tray = 45 ft
To wash water draw off = 48 ft (bottom of chimney tray)
To top of wash section = 54 ft
To top tan = 58 ft
Overall dimensions for the contactor is 4 ft i.d × 58 ft Tan-Tan.

436 CHAPTER 10
Calculating the heat balance over the contactor
The rich gas flow will enter at 100
◦
F and 320 psig and will have an enthalpy of 350
Btu/lb. Its flow rate is 34,722 lbs/hr.
The heat of reaction is calculated at 650 Btu/lb of H
2
S absorbed.
The lean amine solution enters at a temperature of 105
◦
F which will be set by heat
transfer. The lean product gas will leave at about the same temperature.
The rich amine leaving the contactor is determined by difference
Temperature of rich amine out =
23.602 × 10
6
(38,949 × 0.663) + 155,798
= 129.95 say 130
◦
F
The heat exchanger design
The hot lean amine stream to the contactor will be cooled from the stripper bottom
temperature first by heat exchange against the rich amine leaving the contactor. It
will then be trim cooled to the contactor inlet temperature by either water or air.
The following is the calculation to determine the size of the lean/rich amine ex-
changer.
The lean amine from the stripper will be cooled to 175
◦
F in the heat exchange with
the rich amine leaving the contactor. The duty of this exchanger is:
{38,949 × 0.663 × (249 − 175)}+(155,798 × 74) = 13.44 mmBtu/hr
The figure 240
◦
F is a value for the stripper bottom temperature estimated by a quick
bubble point calculation of the lean amine at the stripper bottom conditions of tem-
perature and pressure. This will be checked later.
Temperature of the rich amine feed to the stripper (T ) is as follows:
(23.602 + 13.44) = (38,949 × 0.663 × T ) + 155,798T
T = 204
◦
F
The overall heat transfer coefficient for the amine exchanger can be taken as 100
Btu/hr sqft
◦
F (this will be checked by the exchanger manufacturer). The exchanger
size is:
LMTD 249 −→ 175
204 ←− 130 = 45
◦
F
REFINERY GAS TREATING PROCESSES 437
Table 10.3.
Com MW lbs/Gal Feed O/heads Lean amine
Mole/hr lbs/hr GPH Mole/hr lbs/hr GPH Mols/hr lbs/hr GPH
H
2
S 34 6.55 270 9,180 1,401 212.5 7,225 1,103 57.5 1,955 298
H
2
O 18 8.33 8,655 155,790 18,702 8.43 151 18 8,646.6 155,639 18,684
HC 72 5.25 1.8 130 25 1.8 130 25 Nil Nil Nil
MEA 61 8.45 637.5 38,888 4,602 Nil Nil Nil 637.5 38,888 4,602
Total 9,564.3 203,988 24,730 222.73 7,506 1,146 9,341.6 196,482 23,484
Area =
13,440,000
45 × 100
= 2,987 sq ft.
The stripper design
Total moles of acid gas in feed = moles absorbed = 212.49 moles/hr
Residual acid gas = 57.37 moles/hr
= 269.86 moles/hr
Moles amine = 637.47 moles/hr
(This assumes no losses)
Moles water = 8,655 moles/hr
Moles hydrocarbon dissolved = 1.8 moles/hr as C5
The material balance
The material balance over the stripper is given in Table 10.3.
The following calculation establishes the composition of the overhead product and
the composition of the liquid reflux stream thus:
Let x be the moles per hour of water in the overhead product. The H
2
S content is
established by the total in the feed less the residual H
2
S in the bottom product—the
lean amine. It is assumed that all the hydrocarbon will leave with the overhead vapor
product. The value of x is found by the following Dew point calculation of the o/head
product at the reflux drum conditions of temperature and pressure (Table 10.4).
The reflux drum conditions were set at 23 psia pressure and 100
◦
F and the dew point
calculation at these conditions gave a reflux stream composition of:
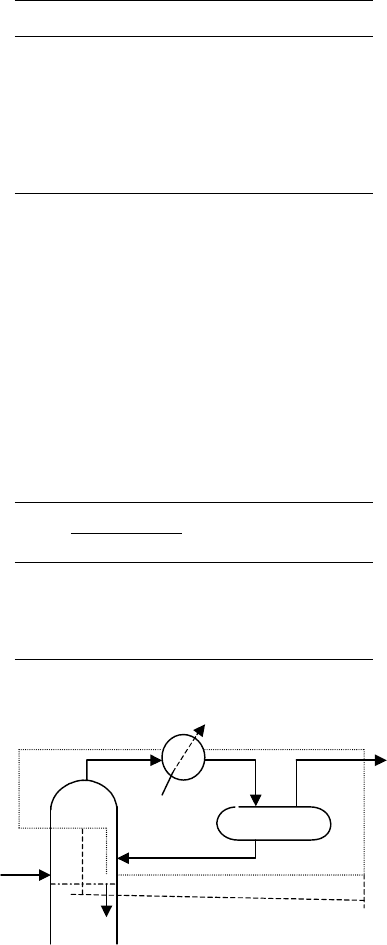
438 CHAPTER 10
Table 10.4.
Comp Moles/hr K100 x = y/k
H
2
S 212.5 14.75 14.40678
H
2
O x 0.041 x/.041
C5’s 1.8 0.67 2.686567
214.3 17.09335
214.3 +x 17.09 + x/0.41
197.21 x/.041 + x
x = 8.43
Moles/hr lbs/hr
H
2
S 0.065 mole fraction = 29.5 1,003
H
2
O 0.923 = 419.6 7,553
HC 0.012 = 5.5 396
= 454.6 8,952
Moles reflux is 2 × product vapor.
Tower top conditions and condenser duty
The tower top pressure shall be the reflux drum pressure plus say 3 psi pressure drop
over the condenser and about 0.7 psi for piping etc. Then tower top pressure will be
26.7 psia. The total overhead vapor will be product plus reflux thus:
Product Reflux
Moles/hr Total mole Fraction
H
2
S 212.5 29.5 242 0.357
H
2
O 8.43 419.6 428.03 0.632
HC 1.8 5.5 7.3 0.011
222.73 454.6 677.33 1.000
The dew point calculation gave the tower top temperature of 218
◦
F.
Product
Feed
Envelope 1
Reflux
Envelope 2
x
The condenser duty is calculated from the following heat balance over the tower top.
The (Refer to envelop 1) heat balance is shown in Table 10.5.

REFINERY GAS TREATING PROCESSES 439
Table 10.5.
Stream V or L
◦
F lbs/hr Btu/lb mmBtu/hr
IN
Total O/head V 218 16,458 615 10.122
Total in 10.122
OUT
Prod V 100 7,506 155.7 1.169
Reflux L 100 8,952 92.5 0.828
Condenser By Diff 8.125
Total out 16458 10.122
Condenser duty to strip vapors from the feed is 8.125 mmBtu/hr. To this will be added
the vapor from the reclaimer that has to be condensed. This will be done later.
To calculate the internal reflux from the top tray
Knowing the condenser duty, the internal reflux x lbs/hr can be calculated from the
heat balance over the tower top as shown in envelope 2 of diagram 1 (Table 10.6).
Solving for x:
9.294 + 222x = 0.923 + 1162x
x = 8,905 lbs/hr
Mole weight of reflux = 18.5 (from the dew point calculation)
Moles/hr reflux = 481
The moles of vapor from the reclaimer will be added to this figure when calculating
the vapor loading over the top tray. Two trays above the feed tray will be provided as
wash trays.
Table 10.6.
Stream Vor L
◦
F lbs/hr Btu/lb mmBtu/hr
IN
Int ref V 222 x 1,162 1,162x
Product V 222 7506 123 0.923
Total in 7,506 + x 0.923 + 1,162x
OUT
Prod V 100 7,506 155.7 1.169
Reflux L 222 x 222 222x
Condenser 8.125
Total out 7,506 + x 9.294 + 222x
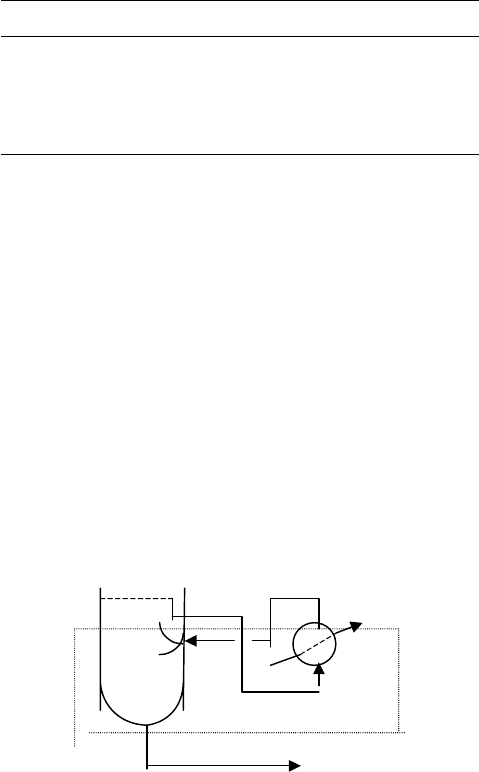
440 CHAPTER 10
Table 10.7.
Temperature 249
◦
F
Comp Moles/hr Mole frac K@ 34 psia y = xk
H
2
S 57.5 .0062 45.4 0.2815
Water 8646.6 .9256 0.79 0.7312
MEA 637.5 .0682 0.09 0.0057
Total 9,341.6 1.0000 1.0184
The stripper bottom conditions and reboiler duty
The pressure at the bottom of the tower is fixed at 34 psia. This allows a pressure drop
of about 0.35 psi per tray which is estimated as a total of 20 trays. The tower bottom
temperature is calculated by a bubble point calculation of the bottom product at this
pressure of 34 psia (see Table 10.7).
Enthalpy of bottom product = 230 Btu/lb as liquid.
Calculating the reboiler duty
This is determined from the overall tower heat balance as shown in Table 10.8.
Vapor/liquid on bottom tray
The bottom tray will have a temperature of 240
◦
F. (See calculation diagram 2 and
Table 10.9). In the following heat balance let the lbs/hr of the stripout vapors to the
tray be x,
V + L
Bot Tray
V
steam
L
Lean Amine (l)
x = 25,748 lbs/hr Mole wt = 23.08
Moles/hr = 1,115.6
V/L at bottom tray = 1,115.6/10,457
= 0.107
To calculate the number of theoretical trays in the stripper
The Kremser equation which is shown graphically by Figure 10.4 will be used for
this calculation.

REFINERY GAS TREATING PROCESSES 441
Table 10.8.
Stream V or L
◦
F lbs/hr Btu/lb mmBtu/hr
IN
Feed V + L 204 203,988 181.7 37.06
Reboiler By Diff 22.049
Total in 203,988 59.109
OUT
O/head prod V 100 7,506 155.7 1.169
Bottoms L 249 196,482 230 45.179
Ht reaction 4.696
Condenser 8.125
Total out 203,988 59.109
The V/L factor calculated above will be used for this equation. A Tower average K
value for each component in the feed will also be used. The equation is shown by
Table 10.10.
Three theoretical trays will achieve the stripping required. Stripping trays have poor ef-
ficiency between 12% and 18%. Use 15% in this case, then actual trays will be 3/.15 =
20 actual trays.
The anomaly for the amount of HC stripped in the above calculation stems from the
assumption that the HC is pentane. It is probably a heavier hydrocarbon.
Calculating the reclaimer duty and size
A slip stream of 2 wt% of lean amine solution will be routed through the reclaimer. It
will be vaporized to leave a sludge stream of 2% of the reclaimer feed. The operation
will be continuous and the vapor will be routed back to the tower entering below the
bottom tray. The material balance over the reclaimer is shown in Table 10.11.
Table 10.9.
Stream V or L
◦
F lbs/hr Btu/lb mmBtu/hr
IN
Bot prod L 240 196,482 224 44.012
Stripout L 240 x 224 224x
Reboiler 22.049
Total in 196,482 + x 66.061 + 224x
OUT
Strip out V 249 x 1,035 1,035x
Bottoms L 249 196,482 230 45.179
Total out 196,482 + x 45.179 + 1,035x
