Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.


422 CHAPTER 10
Table 10.1. Comparison of H
2
S and CO
2
solvents
Formula MEA DEA DGA DIPA SULFINOL SULFOLANE
Molecular wt. 61.1 105.1 105.14 133.19 120.17
Boiling point (
◦
F) 338.5 515.1 405.5 479.7 545
Boiling range,
5–95% (
◦
F)
336.7–
341.06
232–
336.7
205–230 – –
Freezing point
(
◦
F)
50.5 77.2 9.5 107.6 81.7
Sp. Gr., 77
◦
F
140
◦
F
1.0113
0.9844
1.0881
(86
◦
F)
1.0693
1.0572
1.022
–
0.981
(129
◦
F)
1.256
(86
◦
F)
1.235
Pounds per gallon,
77
◦
F
8.45 9.09
(86
◦
F)
8.82 8.3
(86
◦
F)
10.46
(86
◦
F)
Abs. visc., cps.,
77
◦
F
140
◦
F
18.95
5.03
351.9
(86
◦
F)
53.85
40
6.8
870
(86
◦
F)
86
(129
◦
F)
12.1
(86
◦
F)
4.9
Flash point (
◦
F) 200 295 260 255 350
Fire point (
◦
F) 205 330 285 275 380
Sp. ht.
Btu/lb—(
◦
F)
0.663 0.605 0.571 0.815 0.35
Critical-temp. (
◦
F) 646.3 827.8 765.6 – 982.4
Critical–press.
atm.
44.1 32.3 37.22 – 52.2
Ht. of vaporiz.
(Btu/lb)
357.94 267.00 219.14 202.72 225.7
Ht. of
reaction—CO
2
Btu/lb
(Approx.)
825 620 850 580
Ht. of
reaction—H
2
S,
Btu/lb
(Approx.)
650 550 674 500
MEA = HOC
2
H
4
NH
2
DIPA = (HOC
3
H
6
)
2
NH
DEA = (HOC
2
H
4
)
2
NH SULFOLANE = (CH
2
)
4
SO
2
DGA = HOCH
2
CH
2
OCH
2
C
2
H
4
NH
2
Diglycol amine
This process has been developed by the Fluor Company. It originally began as a
combination of 15% MEA, 80% triethylene glycol, 5% water. The system would
both sweeten and dehydrate (to the same level as 95% TEG) the gas in a single
step. The high vapor release during regeneration (both water vapors and acid gases)
causes severe erosion/corrosion problems in the amine/amine exchanger and in the
regeneration column. This system has generally been abandoned.
REFINERY GAS TREATING PROCESSES 423
The present system uses 2-(2-amono ethoxy) ethanol at a recommended solution
strength of 60 wt% in water. DGA has almost the same molecular weight as DEA
and reacts mole for mole with acid gases. DGA seams to tie up acid gases more
effectively so that the higher concentration of acid gas per gallon of solution does not
cause corrosion problems as experienced with the usual amine systems.
The system reactions are given below.
HOCH
2
CH
2
OCH
2
CH
2
NH
2
= RNH
2
= DGA
Low temp
2RNH
2
+ H
2
S
−−−−
−−−−
(RNH
3
)
2
S
High temp
Low temp
(RNH
3
)
2
S + H
2
S
−−−−
−−−−
2RNH
3
HS
High temp
DGA does react with COS and mercaptans similarly to MEA but forms N, N1, bis
(hydroxy, ethoxy ethyl) urea, BHEEU. BHEEU can only be detected using an infra-
red test rather than chromatography. Normal operating levels of 2–4% BHEEU are
carried in the DGA without corrosion problems. BHEEU is removed by the use of
a reclaimer identical to that for an MEA system but operated at 385
◦
F (196
◦
C).
Materials of construction are the same as those for MEA systems.
There has been a concern that DGA might be a good solvent for unsaturated hydro-
carbons. A survey of the DGA users indicates that many of the systems are operated
on gas containing concentrations of C
5
+ above 2% without any indication of hydro-
carbon loading of the system.
Those systems near their hydrocarbon dew point are usually installed with a flash tank
on the rich amine from the absorber. The flash tank is operated at a reduced pressure
just high enough to get into the plant fuel gas system. It reduces the vapor load on the
regenerator column. (A similar system is recommended on MEA systems operating
near the hydrocarbon dew point.)
DGA allows H
2
S removal to less than 1/4 grain per 100 scf and removes CO
2
to levels
of about 200 ppm using normal absorber design parameters.
Other gas treating processes
Hot potassium carbonate (Benfield)
The basic process concept has been known since the early 1900s. It was not an
economical, practically demonstrated process until the mid 1950s. Since that time the
424 CHAPTER 10
process has been used for bulk removal of acid gases where residual CO
2
content was
not needed in the ppm range.
The process is very similar to the amine processes. High temperatures favor high
solubility of potassium carbonate (PC) in water leading to high concentrations of PC.
High PC concentrations mean higher carrying capacity of acid gases in the system.
The system is ideal for streams having CO
2
partial pressures of 30–90 psi (205–620
kPa). It has a high affinity for H
2
S so that pipeline specification for H
2
S can easily be
reached at about 4 ppm. A stream with little or no CO
2
is not suited for the process
due to making regeneration of the lean PC extremely difficult.
This process is usually found as part of the hydrogen plant in those refineries that
need to produce hydrogen.
Sulfinol
This is a proprietary system developed by Shell Oil Company. The process uses
a solution containing both a chemically reactive component, Di-isopropanolamine
(DIPA) and a physical solvent, Tetra hydro thiophene 1-1 dioxide (sulfolane).
Sulfolane is a very active solvent for H
2
S, COS and the mercaptans. CO
2
is also
soluble in it, but not nearly as much as the S compounds are. Because of this, sulfinol
systems are most economically attractive (compared to amine systems) for H
2
S/CO
2
ratios greater than 1:1. If the bulk of the acid gas can be dissolved in the sulfolane
the system is much cheaper to operate than amine. The acid gases are picked up and
released with very little heat increase or heat required. The solubility of acid gases is
much higher in sulfinol than for the amines. Sulfinol loading are limited to 4–6 scf
acid gas/gal solvent versus 2.5 scf acid gas/gal amine solution. In addition the heat
capacity of sulfolane is about half that of the amines, further reducing the regeneration
heat required.
Other, sweetening liquid processes such as Vetracoke, Stretford, and Rectisol have
found high usage in the coal gasification and natural gas industries. They have not
reached the prominence of the amines or the PC processes in oil refining. Table 10.1
summarizes a comparison of the common solvents.
Calculating the amine circulation rate
The circulation rate for amine solvents is important to ensure effective treatment of
the sour gas. It is important also because it is a major contribution to the operating
cost of the plant. These costs are incurred by pumping cost, steam to reboiler and

REFINERY GAS TREATING PROCESSES 425
air cooler/condenser costs. This item provides a calculation method to establish this
circulating rate. It is based on some fixed parameters which have been accepted as
the optimum. These steps are:
Step 1. In DEA and MEA treating processes a ratio of the amine to H
2
S is about 3:1
mole.
Step 2. Obtain gas feed rate and its composition (from plant and lab tests). Determine
its mole weight and the volume percent H
2
S.
Step 3. Fix the weight percent of amine in the recirculating amine solution. This will
be between 15% and 20% weight of DEA or MEA in water.
Step 4. Calculate the H
2
S in moles/hr that is in the feed. This is done by resolving
volume flow of gas to moles and using lab data to provide moles/hr of H
2
S.
Step 5. Calculate the amount of H
2
S to be left in the lean gas. This is usually
10 grains/100 SCF with DEA as absorbent and 3 grains/100 set with MEA as
absorbent (1 grain = 0.0648 g or 0.0022857 ounces).
Step 6. Calculate H
2
S absorbed. This is the difference between Step 4 and Step 5.
Step 7. Using the ratio 3:1 fixed in Step 1 calculate the rate of DEA (or MEA) that
will be required.
Step 8. Mole weight of DEA is 105.1 and the mole weight of MEA is 61.1. Calculate
weight of the amine using percent weight of amine in solution calculate weight per
unit time of solution.
Step 9. Using the data from step 2 calculate the solution’s gallons per hour or per
minute.
Calculating the number of theoretical trays in an amine contactor
There are several accepted methods to calculate theoretical trays in amine contactors.
Among these are the McCabe Thiele—Graphical Method and the calculation method
described by the following steps. This calculation method is considered by many
to be the sounder and more accurate of the methods available. The following steps
describes this calculation procedure:
Step 1. The equation to determine the number of theoretical trays is:
N =
(Log 1/q (A − 1))
(Log A)
− (1)
where
N =number of theoretical trays
q = mole H
2
S in lean gas/mole H
2
S in feed gas
A = the absorption factor L/V · K
Step 2. Calculate H
2
S in lean gas in moles/hr and moles H
2
S in the rich gas. Divide
H
2
S in lean gas by that in the feed. This is q.
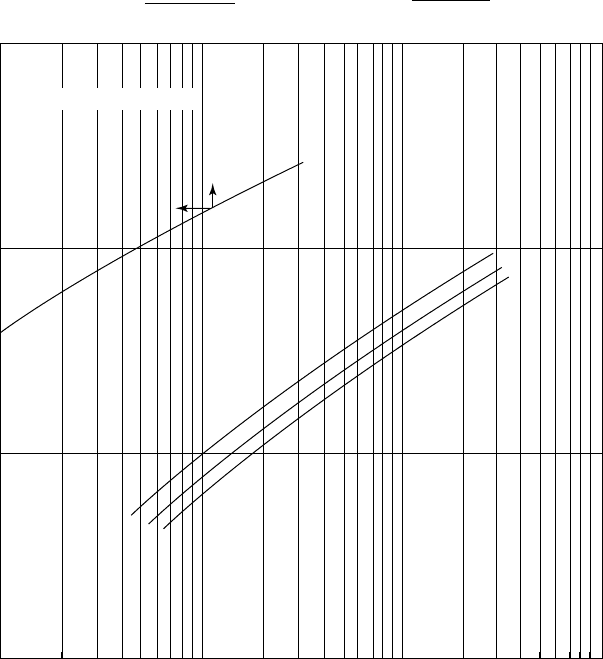
426 CHAPTER 10
120 °F
100 °F
140 °F
120 °F
20 wt % MEA solution
10000
1000
100
0.0001 0.001
H
2
S Partial Pressure psia
Grains H
2
S per US gal of Solution.
0.01 0.1
Note:
(Moles H
2
S)
325× wt % MEA×=
(Moles MEA)
Grains H
2
S
US gal
Figure 10.2. Partial pressure of H
2
S in amine solutions.
Step 3. Calculate the amine circulation rate in moles/hr amine, lbs/hr amine and lbs/hr
solution (about 20 vol%). Resolve to gals/hr of solution.
Step 4. Using the grains/hr of H
2
S leaving in the richer amine (that is grains in
feed gas less grain in product gas), calculate grains per gallon absorbed by amine
solution. Add to that value the H
2
S residual in the lean amine. The sum of these
is the grains of H
2
S per gallon of amine, and is the value used in Figure 10.2 to
determine the partial pressure of H
2
S in MEA.
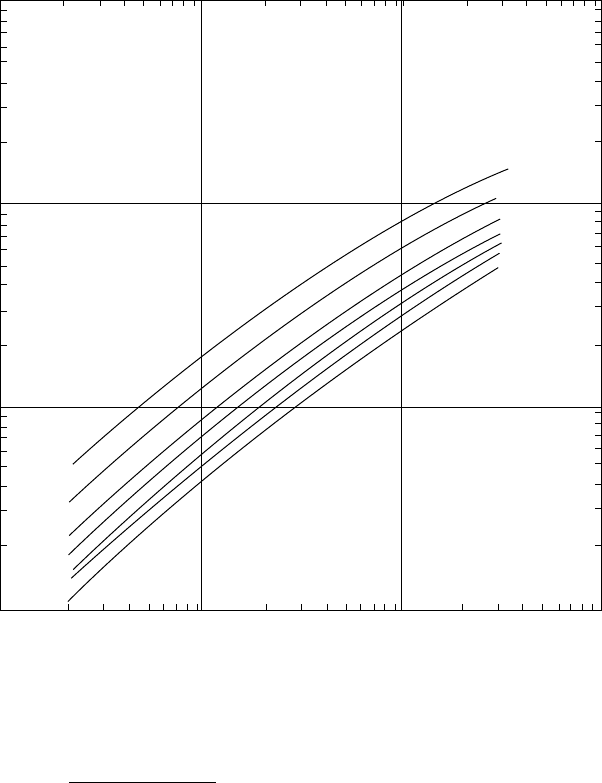
REFINERY GAS TREATING PROCESSES 427
15 wt % MEA solution
60 °F
80
100
110
120
130
140 °F
10000
1000
100
0.0001 0.001
H
2
S Partial Pressure psia
Grains H
2
S per US gal of Solution
0.01 0.1
Figure 10.2. (Cont.)
Step 5. The absorption factor is obtained from the equation:
A =
a(1 + Rr)(1− q)
pp/P
where
A = the absorption factor
a = mole fraction of H
2
S in gas feed
R = moles MEA/Moles H
2
S absorbed
r = residual H
2
S in lean MEA solution (mole H
2
S per mole MEA)
pp = partial pressure of H
2
S in rich solution PSIA
P = systems pressure (tower pressure) PSIA
428 CHAPTER 10
Step 6. Solve for A. Then solve equation given in Step 1 for N. Divide N by efficiency
factor (usually about 15% for absorbers) to determine actual number of trays.
An example calculation is given in Appendix 10.1 of this chapter.
Calculating absorber tray size and design
This procedure is similar to those described in other sections of this Handbook.
Emphasis is made here, however, to the foaming tendencies of MEA and DEA. Most of
these units contain facilities to inject anti-foaming chemicals. Under normal operation
these chemicals subdue foaming to a large extent particularly in the contactors. The
addition of a filter in the rich amine line also helps combat severe foaming.
It is believed that foaming is enhanced by the absorption of large quantities of hy-
drocarbons into the amine stream. This can occur quite easily if the temperature or
pressure change forces the hydrocarbon gas into a condition below its dew point. The
presence of large quantities of impurities such as COS (Carbonyl Sulfide) or the tarry
derivative of COS may also induce foaming. The addition of the filter and reclaimer
usually solves this problem to a large extent.
Loading of amine towers nevertheless is critical due to the foaming nature. Conse-
quently the towers are designed with more latitude than most other towers. In evalu-
ating its performance also the acceptable level of flooding is lower by 20–30% than
that for normal towers.
The calculation to evaluate the towers flooding follows the same steps as those de-
scribed in Chapter 4. In this case, however, the calculated value for V
L
is multiplied
by a system factor between 20% and 30%. If the unit is being used in service where
high concentration of impurities is present a figure of 30% should be used.
Calculating the heat transfer area for the lean/rich amine exchanger
The amine in the contactor picks up the heat of reaction which occurs with the
absorption of H
2
S. On leaving the amine contactor or absorber the rich amine is heat
exchanged against hot lean amine leaving the bottom of the stripper. The performance
of this exchanger is critical to the process as a whole. Usually the rich amine receives
no other heat before it enters the stripper. As in light end towers the feed condition
is vital to the proper operation of the tower. The purpose of this item is to provide a
calculation procedure to evaluate the heat transfer coefficient of this exchanger. The
following steps gives the procedure:
Step 1. Using lab data for operational units or the design specification for those units
to be designed, determine the quantity of H
2
S absorbed in the contactor.

REFINERY GAS TREATING PROCESSES 429
Table 10.2. The heat balance
Stream V or L lbs/hr
◦
F Btu/lb mmBtu/hr
IN
Rich product gas V 34,722 100 350 12.153
Lean amine soln L 194,747 105 98 19.085
Heat of reaction 650 4.696
∗
Total In 35.928
OUT
Lean Product gas V 34,722 105 355 12.326
Rich amine soln L 194,747 By Diff 23.602
Total out 35.928
∗
7,225 lbs of H
2
S absorbed/hr.
Step 2. From the data given in Table 10.1 calculate the total heat of reaction in Btu/hr.
Step 3. Set the temperature of the lean gas leaving the absorber to be the same as the
amine entering.
Step 4. Carry out a heat balance over the contactor with the rich amine temperature
being the unknown. Equate and solve for this.
Step 5. From plant data (or the Design Spec) apply the temperatures and flow in and
out of the exchanger to calculate its heat duty in Btu/hr.
Step 6. Calculate the LMTD (Log Mean Temperature Difference) and from Table 10.2
establish the overall heat transfer coefficient U in Btu/hr ft
2 ◦
F.
Step 7. Using the energy equation
Q = UAT
M
where
Q = the duty in Btu/hr
A = the area in ft
2
T
M
= the log mean temperature difference in
◦
F
U = the overall heat transfer coefficient in Btu/hr ft
2 ◦
F.
Calculate the value for A in sqft.
The stripper design and performance
This evaluation is based on reconciling the steam used for stripping the rich amine. The
quantity of steam is a major operating cost in this type of plant and therefore deserves
attention. An acceptable level of steam usage is about 0.8–1.1 lbs steam/gallon of
circulating solution for MEA and 1.1–1.3 lbs steam/gallon in the case of DEA. This
item describes a procedure to calculate this steam rate.
430 CHAPTER 10
Step 1. From plant data design specification ascertain the feed rate of rich amine
to the stripper, and its composition in terms of H
2
S, water, MEA (or DEA), and
hydrocarbons.
Step 2. Calculate recycle rate of lean amine leaving the bottom of the tower. Obtain
from the lab data or design specification its composition in terms of residual H
2
S,
water, MEA (or DEA) and hydrocarbon (if any).
Step 3. Develop the material balance over the tower.
Step 4. Using the data in Step 3 set the external tower top reflux ratio as 2:1, and
calculate the heat balance over the tower top to find the condenser duty.
Step 5. Calculate the heat in with the rich amine feed before the preheat exchangers
(see previous item).
Step 6. Using plant data for lean amine temperature in and out of the preheat ex-
changers calculate its duty. Add the duty to the enthalpy from Step 5 to give feed
enthalpy into the tower.
Step 7. Calculate the overall heat balance over the stripper to find the reboiler duty.
Remember to add in heat of dissociation which is equal to the heat of reaction in
the contactor (See previous item).
Step 8. Saturated 50 psig steam is usually used as the heating medium. Calculate the
amount of steam from its enthalpy data (steam tables) and the reboiler duty.
Step 9. If the steam usage is excessive check overhead stream and the lean amine
concentration. It is possible that a high volume of water is being evaporated. If this
is the case reduce the reboiler duty to maintain the amine concentration and H
2
S
concentration.
Removing degradation impurities from MEA
Although MEA (mono ethanol amine) is the most efficient absorbent in the amine
family. It has one major shortcoming. It is readily degraded by certain sulfur com-
pounds such as carbonyl sulfide (COS) and by carbon disulphides. Both these com-
pounds are found in significant quantities in gases from cracking processes such as
thermal crackers and catalytic crackers.
It is difficult if not impossible under normal refinery conditions to remove these
sulfides from the gas. What can be done and is the normal practice is to remove
the product of degradation and return the ‘clean’amine to the system. Two items of
equipment are added to the process to achieve this. These are a filter and a reclaimer.
The filter is a normal leaf type filter contained in two filter casings. These are piped
up in parallel with one on stream and the other shut down for cleaning and as spare.
Reclaimers are really kettle type reboilers. It takes as feed a portion of the lean amine
leaving the stripper. This stream is vaporized and the vapor returned to the stripping

REFINERY GAS TREATING PROCESSES 431
tower. The residue or sludge is the product of degradation and is dumped to waste.
Reclaimers can be designed to operate continuously or on a batch basis. Steam is
used as the heating medium. As in the case of the stripper reboilers the heating steam
temperature to the reclaimer is also carefully controlled. Thus the steam medium is
saturated 50 psig steam. It must be remembered that the duty to condense the vaporized
amine from the reclaimer must be added to the stripper overhead condenser duty. It
must also be included in any tower loading exercise that may follow.
Appendix 10.1 The process design of an amine gas treating unit
The following is an extract from a design specification and defines the parameters of
this example:
1.0 Unit required is a gas treating unit for the removal of H
2
S from a Hydrotreater
recycle gas stream. The feed to the hydrotreater consists of gas oil from straight
run source and streams from a thermal cracker and a catalytic cracker.
The recycle gas will therefore contain some COS.
2.0 The feed gas shall have the following properties:
r
The mole weight of the gas is 10.5
r
The H
2
S content of feed gas is 4,048 Grains/100 scf.
r
The gas rate is 30 mmscf/day
r
The pressure of the gas at the outlet of the contactor is 320 psig.
3.0 Product gas shall have a H
2
S content of no greater than 0.1 grain/100 scf.
4.0 The amine solvent to be used shall be mono ethanol amine.
r
Amine ratio to H
2
S shall be 3.0 moles amine to 1.0 mole H
2
S.
r
The amine solution shall be 20% by weight in water.
r
Residual H
2
S in the Lean Amine Solution shall be no greater than 0.09 mole
per mole of MEA.
r
Protection against degradation of the amine shall be included.
The contactor design
Calculating the amine solution circulation rate
Feed gas rate = 30 mmscf/day
= 3,306.9 moles/hr
= 34,722 lbs/hr
H
2
S in feed =
4048 × 0.0022857
16
= 0.578 lbs/100 scf
= 5,100 moles/day = 1.928 mmscf/day
= 212.5 moles/hr
