Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.


402 CHAPTER 9
Table 9.3.1. Hydrocarbon octane values
Research octane Motor octane
by ASTM 2699 by ASTM 2700 (R + M )/2
n-Butane 93.8 89.6 91.7
i-Butane 100.4 97.6 99.0
n-Pentane 61.7 62.6 62.2
i-Pentane 92.3 90.3 91.3
n-Hexane 24.8 26.0 25.4
2-Methylpentane 73.4 73.5 73.4
3-Methylpentane 74.5 74.3 74.4
2,2-Dimethylbutane 91.8 93.4 92.6
2,3-Dimethylbutane 101.0 94.3 97.6
at two severity levels. Often, the average of these two values, or (R + M)/2, is used
to express the overall engine performance of a gasoline component or blend. Ta-
ble 3.7.1 shows that isopentane has an octane value nearly 30 numbers higher than
n-pentane (1).
Similarly, the hexane isomer, 2,2-dimethylbutane (2,2-DMB) has an octane about 67
numbers higher than n-hexane. Clearly, highly branched isoparaffins are the desired
isomers for motor fuel production.
Thermodynamic equilibria for the branched paraffin isomers are generally favored
by low temperatures. Figures 9.3.1, 9.3.2, and 9.3.3 illustrate this trend (2). The
most active catalyst, when all other variables are equal, is capable of producing the
highest-octane products.
Normal Butane
Isobutane
100
80
60
40
20
0
40 150 260 370 480
Temperature, °C
mole%
Figure 9.3.1. Butane equilibrium.

GASOLINE COMPONENTS 403
Normal Pentane
Isopentane
100
80
60
40
20
0
40 150 260 370 480
Temperature, °C
mole%
Neopentane
Figure 9.3.2. Pentane equilibrium.
Primary reaction pathways
Paraffin isomerization is most effectively catalyzed by a dual-function catalyst con-
taining a noble metal and an acid function. The reaction is believed to proceed through
an olefin intermediate, which is formed by paraffin dehydrogenation on the metal site
(reaction (1)):
CH
3
-CH
2
-CH
2
-CH
3
↔
Pt
CH
3
-CH
2
-CH = CH
2
+ H
2
. (1)
Although the equilibrium conversion of the paraffin in reaction (1) is low at paraffin
isomerization conditions, sufficient olefins are present to be converted to a carbonium
ion by the strong acid site (reaction (2)):
CH
3
-CH
2
-CH=CH
2
+ [H
+
][A
−
] → CH
3
-CH
2
-C
+
H-CH
3
+ A
−
. (2)
Temperature, °C
MCP / (MCP + CH)
100
80
60
40
20
0
93 149 204 260 316
mole%
2-MP + 3-MP /
∑
C
6
Paraffins
2, 2-DMB /
∑
C
6
Paraffins
n-Hexane /
∑
C
6
Paraffins
2, 3-DMB /
∑
C
6
Paraffins
Figure 9.3.3. C
6
fraction equilibrium.
404 CHAPTER 9
The formation of the carbonium ion removes product olefins from reaction (1) and
allows the equilibrium in reaction (1) to proceed. The carbonium ion in reaction (2)
undergoes a skeletal isomerization, probably through a cycloalkyl intermediate as
shown in reaction (3):
CH
3
-CH
2
-C
+
H-CH
3
→ CH
2
/
CH
2
——
\
C-CH
3
+
→ CH
3
-C
|
+
-CH
3
.
CH
3
cyclopropyl
cation
(3)
Reaction (3) proceeds with difficulty because it requires the formation of a primary
carbonium ion at some point in the reaction. Nevertheless, the strong acidity of iso-
merization catalysts provides enough driving force for the reaction to proceed at high
rates. The isoparaffinic carbonium ion is then converted to an olefin through loss of
a proton to the catalyst site (reaction (4)):
CH
3
-CH
|
CH
3
+
-CH
3
+ A
−
→ CH
3
-C
|
CH
3
= CH
2
+ [H
+
][A
−
]. (4)
In the last step, the isoolefin intermediate is hydrogenated rapidly back to the analogous
isoparaffin (reaction (5)):
CH
3
-C
|
CH
3
= CH
2
+ H
2
→
pt
CH
3
-CH
|
CH
3
-CH
3
. (5)
In addition to these primary reaction pathways, some evidence indicates the exis-
tence of a bimolecular reaction mechanism, in which olefinic intermediates dimerize,
internal carbon atoms are protonated, skeletal isomerization occurs, and the dimer
undergoes beta scission that results in the product isoparaffin. In addition to the C
13
labeling experiments that support this mechanism, a relatively small amount of hy-
drocarbons containing carbon numbers higher than the feed are always found in the
reaction products. The bimolecular mechanism has a minor impact on commercial
isomerization processing.
Isomerization catalysts
Operation at the lowest temperature results in the formation of the highest-octane
product (Table 9.3.1 and Figures 9.3.1, 9.3.2, and 9.3.3). Catalyst activity and the
lowest operating temperature are important to achieve an economic operation. In the
previous discussion of the primary reaction pathway, the primary functionality of a
paraffin isomerization catalyst is to protonate a secondary carbon atom. All known
paraffin isomerization catalysts have a combination of strong Lewis and Br¨onsted
acid sites, which result in varying levels of protonation activity.
GASOLINE COMPONENTS 405
In addition to a strong acid function, isomerization catalysts must also be capable
of hydrogenolysis, which not only assists in the protonation step, but also serves
to saturate olefin intermediates and aromatic hydrocarbons and to assist in the ring
opening of cycloparaffins. This function also gives activity stability to isomerization
catalysts, thereby improving the process economics.
An aluminum chloride catalyst for alkane isomerization was first developed in the
1930s (3). The original application was for the conversion of n-butane to isobutane,
which was, and still is, reacted with C
3
,C
4
, and C
5
olefins to produce motor fuel
alkylate. The first application of this high-octane product was in the production of
high-octane aviation gasoline. Subsequent developments of this predominantly Lewis-
acid catalyst resulted in the current alumina-supported, bifunctional catalyst. UOP’s
I-8 catalyst is one commercial example of this catalyst system, which has seen wide
commercial application since 1981.
Chlorided alumina, the highest-activity paraffin isomerization catalyst available, in-
creases the octane of a typical light-naphtha stream from about 70 to as high as 85
RONC in a once-through paraffin isomerization unit. Higher product octanes, up to
93 RONC, can be obtained by recycling low-octane hydrocarbons. The C
+
5
yield from
chlorided alumina catalysts is the highest from any commercial catalyst because of
high catalyst selectivity and low operating temperature. Because chlorided alumina
systems are not economically regenerable, eventual reloading of the isomerization
catalyst must be considered. Nevertheless, the chlorided alumina system is often the
most-economic choice because of its inherent high activity. In addition, only chlorided
alumina catalysts have enough activity to economically isomerize butanes.
As a result of ongoing intensive research and development in paraffin isomeriza-
tion technology, UOP’s I-80 catalyst is one of the highest-activity chlorided alumina
catalyst currently available. The I-80 catalyst, is significantly more active than the
I-8 catalyst, and is based on a unique formulation and manufacturing technique. By
simply reloading the I-80 catalyst in existing reactors, a gain of 0.5–1.0 RON can be
realized compared with the product RON when I-8 catalyst is used.
Zeolitic isomerization catalysts, such as UOP’s HS-10™ or I-7™ catalysts, operate at
higher temperatures than chlorided alumina catalysts. The maximum product octane
that can be achieved is limited by the unfavorable equilibrium at these conditions.
Yields are also lower as a result of the higher operating temperature and the less-
selective characteristics of zeolitic catalysts. A typical octane upgrade for a once-
through zeolitic isomerization unit is from 70 to about 79 RONC. Higher product
octanes (86–88) can be obtained in a recycle operation, such as a TIP™ unit.
The most-attractive benefit of zeolitic isomerization catalysts is that they are not
permanently deactivated by water or other oxygenates and are fully regenerable.
406 CHAPTER 9
Consequently, zeolitic catalysts have often been used when revamping other process
units, such as hydrotreaters or reformers to isomerization service. Sulfur can be present
in the feedstock with catalysts such as the HS-10 catalyst, but performance is always
affected to some degree. Sulfur suppresses the platinum function as it does in any
platinum-containing catalyst. Although isomerization activity is maintained, a net C
+
5
product yield loss results. Hydrotreating is not an absolute requirement with zeolitic
catalysts, but it is necessary to get the optimum performance from the catalyst.
Sulfated metal oxide catalysts, which have been described as solid super acids, exhibit
high activity for paraffin isomerization reactions. These metal oxides form the basis
of the new generation of isomerization catalysts that have been actively discussed
in the scientific literature in recent years. These catalysts are most commonly tin
oxide (SnO
2
), zirconium oxide (ZrO
2
), titanium oxide (TiO
2
), or ferric oxide (Fe
2
O
3
)
that has been sulfated by reaction with sulfuric acid or ammonium sulfate. Sulfated
alumina is not an active catalyst for hydrocarbon reactions.
A typical metal oxide catalyst, UOP’s LPI-100 catalyst (4), has a considerably higher
activity than that of traditional zeolitic catalysts, and this activity advantage is equiva-
lent to about 80
◦
C lower reaction temperature. The lower reaction temperature allows
for a product with a significantly higher product octane, about 82 RONC for a typ-
ical feed or three numbers higher than that of a zeolitic catalyst. Similar to zeolitic
catalysts, the LPI-100 catalyst is not permanently deactivated by water or oxygenates
in the feedstock. These catalysts are also fully regenerable using a simple oxidation
procedure that is comparable to the one used for zeolitic catalysts. The high activ-
ity of the LPI-100 catalyst makes it an ideal choice for revamping existing zeolitic
isomerization units for higher capacity and higher octane isomerate or for new units
where the full performance advantage of chlorided alumina catalysts is not required
or where the refiner is concerned about contaminants in the feedstock.
The isomerization performance of the previously discussed I-8, I-80, HS-10, and LPI-
100 catalysts is compared in Figure 9.3.4, which plots the i-C
5
to total C
5
product ratio
for all the catalysts as a function of reactor temperature. This ranking clearly illustrates
the striking activity advantage for the LPI-100 catalyst over that of the zeolitic HS-10
catalyst, an advantage that translates directly to octane improvement. Likewise, this
ranking shows the performance benefit of the I-80 catalyst over the predecessor I-8
catalyst. The development and benefits of the I-80 and LPI-100 catalysts, are discussed
in the following sections.
I-80 catalyst development and applications
Chlorided alumina represents the industry standard for isomerization catalysts. UOP
introduced the second-generation, high-activity I-8 chlorided alumina catalyst in
GASOLINE COMPONENTS 407
I-80
90
80
70
60
50
50
40
100 152
200
Relative Temperature, °C
iC
5
/Total C
5
Ratio, wt%
I-8
LPI-100
HS-10
0
Figure 9.3.4. Light paraffin isomerization catalyst performance.
1981. The I-8 catalyst has been successfully used in more than 50 operating Bu-
tamer™ butane isomerization units and 90 operating Penex™ light-naphtha isomer-
ization units. The flow schemes range from simple once-through hydrocarbon pro-
cesses to complex recycle operations to achieve high product octane that have been
described in detail elsewhere (5). In 1998, UOP introduced the I-80 catalyst, a new
generation, high-activity chlorided alumina catalyst. The I-80 catalyst has stability
and selectivity identical to that of the I-8 catalyst.
Higher catalyst activity can be used in a number of ways in existing or new isomeriza-
tion units. The best application depends on site-specific factors and whether the unit
operates in a once-through or recycle mode. Typical means to exploit higher activity
are:
r
Higher throughput at constant octane (Case A)
r
Reduced catalyst volume at constant throughput and octane (Case B)
r
Higher octane at the same throughput (Case C)
r
Longer catalyst life (Case D)
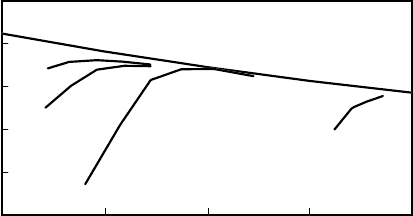
Case A operates at higher throughput but maintains a constant product octane. The per-
formance of the I-8 and I-80 catalysts is compared in Figure 9.3.5 as a plot of product
octane against relative reactor temperature. The newer I-80 catalyst has a substantial
30% activity advantage over the previous catalyst. This advantage is equivalent to
about a 12
◦
C reduction in reactor temperature. A 30% activity increase corresponds
directly to an increase in space velocity, or throughput.
If the I-80 catalyst is reloaded in place of the existing I-8 catalyst, the charge rate to
the reactor can be increased by up to 30% with no decrease in product octane. This
throughput advantage is a major benefit in equipment cost savings if a higher-capacity
revamp is being considered because additional reactor volume is not required. Another

408 CHAPTER 9
Equilibrium
85
82
81
80
79
78
-20
20
40
60
Relative Reactor Temperature,
°C
C
5
+
RONC, Calculated
83
84
80
I-80
Catalyst
I-8
Catalyst
0
Figure 9.3.5. Chlorided alumina catalyst performance.
benefit of a higher-activity catalyst is the increased flexibility to respond to seasonal
fluctuations in product demand.
The ability to operate at higher space velocities with the I-80 catalyst can be used in
existing units by reducing the volume of catalyst in the reactor of existing units by
up to 30% and using a smaller overall reactor size in new units (Case B). These cost
savings are obvious. The refiner also realizes a savings in platinum inventory.
As mentioned previously, isomerization reactions are equilibrium limited and the
extent of the reaction improves at lower temperatures. The 12
◦
C activity advantage
of the I-80 catalyst can be exploited by operating at a lower temperature to achieve
higher product octanes (Case C). This advantage is illustrated in Figure 9.3.6 as a
plot of product octane against C
+
5
yield. Achieving a 0.5–1.0 RON higher product
I-8 Catalyst
83
82
81
80
79
78
96 97 98 99 100
C
5
+
Yield, wt%
iC
5
RONC
I-80 Catalyst
Figure 9.3.6. Chlorided alumina catalyst yields.
GASOLINE COMPONENTS 409
quality at similar yields is possible with the I-80 catalyst in once-through units. This
improvement in quality is equivalent to an additional revenue of about $600,000 U.S.
per year at an octane value of $0.25 per octane-barrel. Thus, the I-80 catalyst is an
obvious choice when catalyst replacement is required. The benefit for recycle units is
lower because the savings are related to the cost reduction associated with maintaining
higher conversion per pass.
The higher activity of the I-80 catalyst is achieved by increasing the number of
catalytically active sites on the catalyst surface. Because the deactivation of chlorided
alumina catalysts is mostly due to ingress of moisture or oxygenates, this higher
acid-site density can translate into longer catalyst life at a constant catalyst loading
(Case D). If all other factors are equal, catalyst life may be extended by about 30%.
Increased catalyst life has the obvious benefit of reducing operating costs. However,
the benefit is relatively small in well-run commercial units that typically achieve a
catalyst life in excess of 5 years.
LPI-100 catalyst development and applications
The development of the LPI-100 catalyst represents the first successful application
of sulfated metal oxide in industrial catalysis. This new catalyst is the culmination of
a joint development effort between Cosmo Research Institute (CRI) and Mitsubishi
Heavy Industries (MHI) in Japan and UOP.
The basic formulation for this sulfated metal oxide catalyst was developed by CRI
and MHI researchers in the late 1980s. Several years of intensive development were
required to translate the CRI–MHI laboratory formulation into a commercially vi-
able extruded catalyst that met all the original performance targets. The commercial
product is now the UOP LPI-100 catalyst.
The first commercial loading of the LPI-100 catalyst was completed at the Flying J
Refinery in North Salt Lake City, Utah. The catalyst was loaded in a zeolitic isomer-
ization reactor that had previously been operated with UOP’s I-7 catalyst. The loading
was completed and the reactor placed into operation in December 1996. Performance
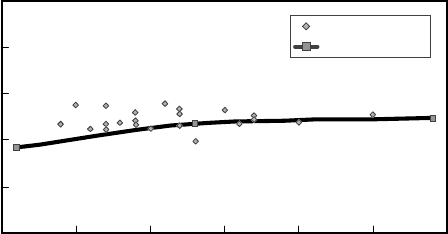
data for the LPI-100 catalyst in the Flying J unit are compared to pilot plant predictions
in Figure 9.3.7 as a plot of the i -C
5
to total C
5
product ratio against reactor temperature.
The performance at Flying J was in line with UOP’s expectations. Since the initial op-
eration at Flying J, the LPI-100 catalyst has been used in another once-through zeolitic
isomerization unit in Canada and in other units in Canada and the Middle East.
Contaminant-sensitivity studies with the LPI-100 catalyst have shown responses
comparable to those experienced with conventional zeolitic catalysts. Water and
oxygenates at typical concentrations are not detrimental. Sulfur suppresses activity, as
410 CHAPTER 9
85
80
75
70
65
60
170 175 180 185 190
Reactor Temperature,
°C
iC
5
/C
5
Product Ratio, wt%
Pilot Plant Data
Commercial Data
195 200
Figure 9.3.7. LPI-100 performance at Flying J refinery.
expected for any catalyst containing noble metals. The suppression effect of sulfur is
reversed by subsequent processing with clean feedstocks. The effects of other common
contaminants are similar to those experienced with conventional zeolitic catalysts.
In pilot plant evaluations, the stability of the LPI-100 catalyst is comparable that of
the HS-10 zeolitic catalyst. This stability translates to a commercial process cycle of
at least 18 months before regeneration is required. Multiple-cycle regeneration testing
was completed and showed that the catalyst was fully regenerable through more than
three regenerations. Regeneration consists of a simple carbon-burn step at conditions
comparable to those used for regenerating zeolitic catalysts.
New isomerization process technologies
The introduction of new catalyst systems, such as the LPI-100 catalyst, allows the de-
velopment of new process technologies that fully use the characteristics of the catalyst.
The process technology developed for the LPI-100 catalyst is the Par-Isom™ pro-
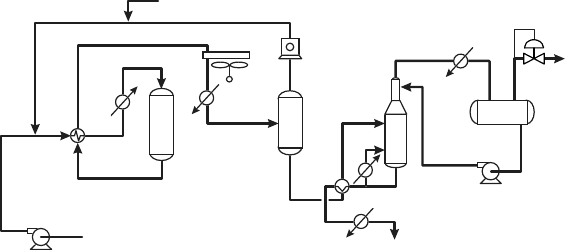
cess. A simplified flow scheme for the Par-Isom process operating in a once-through
configuration is shown in Figure 9.3.8. For simplicity, a once-through hydrocarbon
flow scheme is discussed in this chapter. Recycle flow schemes previously developed
for naphtha isomerization use either molecular sieve separation, such as in the TIP
process, or fractionation, such as a deisohexanizer column.
The process flow scheme shown in Figure 9.3.8 is similar to that used for conventional
once-through Penex and zeolitic isomerization units. The fresh C
5
–C
6
feed is com-
bined with makeup and recycle hydrogen and directed to the charge heat exchanger,
where the reactants are heated to reaction temperature. A fired heater is not required
in the Par-Isom process, because of the much lower reaction temperature needed with
GASOLINE COMPONENTS 411
Stabilizer
Stabilizer
Bottoms
Reactor
Feed
Offgas
Reactor
Product
Separator
Makeup Gas
Figure 9.3.8. Par-Isom process flow scheme.
the LPI-100 catalyst than with zeolitic catalysts. Hot oil or high-pressure steam can
be used as the heat source in this exchanger. The heated combined feed is then sent
to the isomerization reactor.
Either one or two reactors can be used in series, depending on the specific application.
Two reactors obtain the best performance from the process but at a higher capital and
additional catalyst inventory costs. In a two-reactor system, the first reactor is operated
at higher temperature (200–220
◦
C) to improve the reaction rate, and the second reactor
is operated at a lower temperature to take advantage of the more-favorable equilibrium
distribution of higher octane isomers. One reactor is generally specified for the Par-
Isom process to reduce capital and operating cost of the unit, but a small debit in
performance can result.
The reactor effluent is cooled and sent to a product separator, where the recycle hy-
drogen is separated from the other products and returned to the reactor section. The
liquid product is sent to a stabilizer column, where the light ends and dissolved hy-
drogen are removed. The stabilized isomerate product can be sent directly to gasoline
blending. In recycle flow schemes, either molecular sieve or fractionation options are
used to separate the lower-octane isomers for recycle to the reactor. The selection of
the separation scheme depends on the feed composition, availability of utilities, and
the product octane desired.
The higher activity of the LPI-100 catalyst makes it an ideal candidate for revamps
of existing zeolitic isomerization units to achieve higher capacity or for revamps
of idle process units, such as reformers or hydrotreaters to isomerization service.
The integration of a Par-Isom reactor into an existing semiregeneratative reforming
unit can be a particularly attractive revamp opportunity for economically adding
isomerization capacity to a refinery. A simple flow scheme illustrating the integrated
