Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

9.2 Catalytic olefin condensation
Peter R. Pujad´o and Dennis J. Ward (retired)*
Introduction
Catalytic olefin condensation refers in general to the reaction of one molecule of an
olefin with one or more molecules of the same olefin or of other olefins to yield heavier
olefinic compounds. The term “condensation” reflects the fact that liquid products
are obtained from gaseous olefins.
Olefin condensation is in some ways similar to motor fuel alkylation, except that in
alkylation we react an olefin with an isoparaffin to yield a paraffinic compound. In
olefin condensation, on the other hand, the products that are made are olefinic, but
may be later hydrogenated in order to meet the requirements and specifications of the
end products.
Olefin condensation can be carried out in many ways and over a diversity of catalysts.
The common characteristic is that olefin condensation takes place over acid catalysts.
These catalysts may be solid phosphoric acid (SPA), liquid acids, organometallic
catalysts, zeolites, sulfonic acid resins, etc.
Although olefin condensation finds broad application in the petroleum refinery en-
vironment, it is also extensively used in petrochemical applications (often combined
with refinery operations) as, for instance, in the production of heptenes, nonenes,
dodecenes, etc. often used for the production of alkylaromatic derivatives. In fact,
some of the same catalysts (e.g., SPA) can also be used for the alkylation of olefins
with aromatics as in the production of cumene (isopropyl-benzene), ethylbenzene,
etc. This review will concentrate on refinery and olefin oligomer applications and
will not discuss aromatic alkylation applications.
*UOP LLC.
372
GASOLINE COMPONENTS 373
History
The discovery of acid catalysis, and in particular the development of solid phosphoric
acid (SPA) catalysts, is attributed to the pioneering work of Vladimir Ipatieff at
what was then called Universal Oil Products and is today known as UOP. The first
commercial unit for the production of octenes by the dimerization of butenes came
on-stream in 1935. By introducing this new ingredient in the formulation of gasolines,
it greatly contributed to the popularization of the automobile that had hitherto been
constrained by the limited availability of straight-run gasolines from the fractionation
of petroleum crudes. In addition, the octenes obtained by butene dimerization had a
higher octane number than naturally occurring straight-run gasolines. Several hundred
units were soon built to satisfy the growing demand for gasoline. Commercially,
the dimerization and oligomerization of olefins over SPA catalysts is known as the
Catalytic Condensation process. This is still the dominant process for the dimerization
and oligomerization of olefins. It is only recently that more work has been done on
the development of acid zeolite catalysts and, in some cases, of sulfonic acid resin
catalysts.
The other type of catalysts most often found in refinery applications for the produc-
tion of gasoline components are organometallic catalysts developed by the Institute
Fran¸cais du P´etrole (IFP) and used, for example, in the dimerization of propylene
to isohexenes (Dimersol G process), or in the oligomerization of light olefins from
cracked gases (Dimersol E), or in the production of heptenes and octenes from propy-
lene and/or butenes (Dimersol X).
Because the production of olefin dimers and oligomers almost invariably produces a
blend of isomers and often also a lighter and a heavier fraction, the production of these
olefins for petrochemical applications cannot be easily segregated from their appli-
cation in gasoline and diesel fuel uses. However, it is usually the case that catalysts
can be tailored within certain limits so as to yield more branched compounds (higher
octane) for gasoline applications and less branched products for petrochemical appli-
cations. Because the products from such processes always have some branching, the
quality of the products in the diesel range is somewhat poor (low cetane numbers)
and their use in diesel applications is not that extensive.
Catalytic condensation process
The catalytic condensation process was developed in the early 1930s as a means to
convert light gases produced by thermal cracking and thermal reforming into useful
products. In the early years of the 20th century, most operations in the petroleum
refining industry were limited to the distillation of crude oils into gasoline boiling
374 CHAPTER 9
range materials (straight-run gasolines), kerosenes, and lamp fuels. The yields in the
gasoline fraction were low (sometimes as low as 5%, depending on the crude) and
did not meet the requirements of the growing automobile industry.
One of the early ways to increase gasoline yields was by thermal cracking. The
dominant thermal cracking technology was the Dubbs Process. In fact, out of the de-
velopment of the Dubbs Process emerged the Universal Oil Products company (now
UOP). A by-product from the Dubbs Process was a light gas stream that contained
saturated and olefinic components that ranged from methane to butenes. Although
there was no immediate use for those gases, refiners knew that by circulating these
gases through a strong mineral acid it was possible to condense the olefins into heav-
ier liquid products, and actually this was the principle applied in the Orsat apparatus
used to measure the olefin content of those gases. Use of strong mineral acids (e.g.,
sulfuric acid) poses significant corrosion problems and presents considerable opera-
tional difficulties. Ideally, it was desired to operate with a single carbon steel vessel
in which the strong acid could be contained. R. E. Schaad and V. N. Ipatieff dis-
covered that phosphoric acid mixed with kieselguhr (an abundant naturally occurring
siliceous diatomite mineral) solidified upon heating. When the solid mass was cooled,
broken up, and placed inside a reactor, the propylene and/or butenes condensed into
a liquid in the gasoline boiling range. This discovery was scaled up and eventually
commercialized (1).
Shortly after the introduction of the Catalytic Condensation process came the catalytic
cracking process, initially as a fixed process and later as the fluidized-bed catalytic
cracking process, or FCC. These catalytic cracking processes, and in particular FCC,
yielded even more olefins than thermal cracking, principally propylene and butenes,
which at that time had very little commercial value. Thus, further application of the
Catalytic Condensation process ensued.
The early operations were, by today’s standards, primitive. To manufacture the catalyst,
phosphoric acid and kieselguhr were mixed together, formed into a cake that was
heated and then cooled, and broken up with a sledge hammer. The pieces were screened
into the proper size range and loaded into the reactor. The reactor operated at very
low pressures and produced such amounts of carbon that the catalyst had to be burnt
off every other week, and be completely replaced every four to five weeks. The
technology evolved by first improving the catalyst both in terms of manufacture (e.g.,
by producing an extrudate) and of formulation and also by optimizing the operating
conditions.
Early units operated at about 10 atmospheres, since that was the pressure level of many
liquefied petroleum gas (LPG) recovery systems. However, it was soon discovered
that, if the reactor was operated at 30 or more atmospheres, catalyst lives could be
extended by a factor of four to six times. Thus, began the evolution of higher pressure
GASOLINE COMPONENTS 375
units. Some units that employed tubular reactors at 60 atmospheres were constructed
and were found to be even more successful.
SPA catalyst was also tried for other reactions. In the late 1930s, it was discovered
that SPA would catalyze the reaction of olefins with aromatic compounds to yield
mono-olefinic alkyl aromatics. This fortunate discovery was widely used to make
cumene (isopropyl-benzene) for aviation fuel used in World War II. Cumene has a
very high performance number necessary for the aviation gasoline used in internal
combustion engines in aircraft. After the war, use of cumene in aviation fuel declined,
but cumene found an even more important outlet as feedstock for the production
of phenol and acetone—to this date, this petrochemical use of cumene continues
to dominate this industry, even though in recent years there has been a significant
shift from SPA to acid zeolite catalysts for this aromatic alkylation. During World
War II there was also a high demand for ethylbenzene and styrene. Some units were
built to produce ethylbenzene by reacting ethylene with benzene over SPA catalyst.
The reaction was slow and fairly inefficient, so SPA never gained relevance for this
application; most ethylbenzene units at the time made use of aluminum chloride
catalysts. Today, practically all existing capacity makes use of acid zeolite catalysts.
World War II also led to increased demand and production of polyvinylchloride (PVC).
PVC is an excellent plastic material but lacks the flexibility required for many ap-
plications. It was discovered, however, that octyl phthalates and the esters of higher
alcohols could be used to “plasticize” PVC; that is, to make it more flexible and supple.
Octyl to decyl alcohols could be obtained by the “oxo” reaction (hydroformylation
reaction) of olefins with carbon monoxide and hydrogen. The corresponding olefins,
with one carbon less than the desired alcohols, could be recovered as by-products
from the gasoline operation simply by fractionating the desired olefins out of the
olefinic gasoline blend obtained by the Catalytic Condensation process. To this date,
this is still the way that these olefins, from heptenes to nonenes, are produced, except
that now these olefins are the main products from this operation, and the remaining
lighter and heavier fractions are blended back into the gasoline pool.
A further application that derived from World War II was the advent of synthetic deter-
gents to overcome the shortage of soap caused by the diversion of glycerin supplies to
the manufacture of explosives. Though not readily biodegradable, one of the earliest
and still one of the most active detergent ingredients is dodecylbenzene sulfonate.
This was obtained by first alkylating dodecene with benzene according to technology
developed independently by UOP and Standard Oil of California (now Chevron).
Dodecene, or propylene tetramer, was readily produced by the oligomerization of
propylene over SPA catalyst.
Still, in the apex of its success, the Catalytic Condensation process was used mostly for
the production of gasoline fractions. During World War II, an important component in
376 CHAPTER 9
the aviation fuels used in fighter airplanes of that time was hydrocodimer, described
more fully below. This together with cumene, also derived from phosphoric acid
catalyst operations, was credited with being a significant factor in Britain’s success
in the Battle of Britain. The typical refinery product was known as polymer gasoline
or “poly gasoline” even though in reality it consisted mostly of fairly simple dimers
and trimers. At one time there were as many as 300–400 Catalytic Condensation units
producing gasoline alone. Today, production of polymer gasoline is less significant,
but the Catalytic Condensation process is still the preferred route to olefin dimers and
oligomers and, despite the inroads made by zeolitic catalysts, is still widely used for
the production of cumene. Based on the current demand for SPA catalyst, it may be
estimated that about 40% is for gasoline, 40% for higher olefins (plasticizer olefins),
and the balance 20% for cumene applications.
SPA catalyst may contain as much as 65 wt% phosphoric acid, but not all is active. Only
the so-called “free phosphoric acid” provides catalytic activity, and this is typically
around 16–20 wt% of the catalyst as manufactured. The activity of SPA is dictated
by its equilibrium with water. Anhydrous phosphoric acid, P
2
O
5
, is not catalytic
for it lacks the hydroxyl groups that are necessary for activity. Too much water, on
the other hand, may cause the catalyst to get “soupy” and lose mechanical strength
or even dissolve and come out of the unit. The successful operation of a Catalytic
Condensation unit with SPA catalyst requires a close control on the water balance
going into the reactor, both in order to obtain the desired level of activity and also in
order to achieve long catalyst lives. Use of SPA catalyst in aromatic alkylation units
may present additional constraints owing to the partial solubility of the phosphoric
acid in aromatic streams; a small purge stream of phosphoric acid is usually maintained
in these units.
Catalytic condensation process for gasoline production
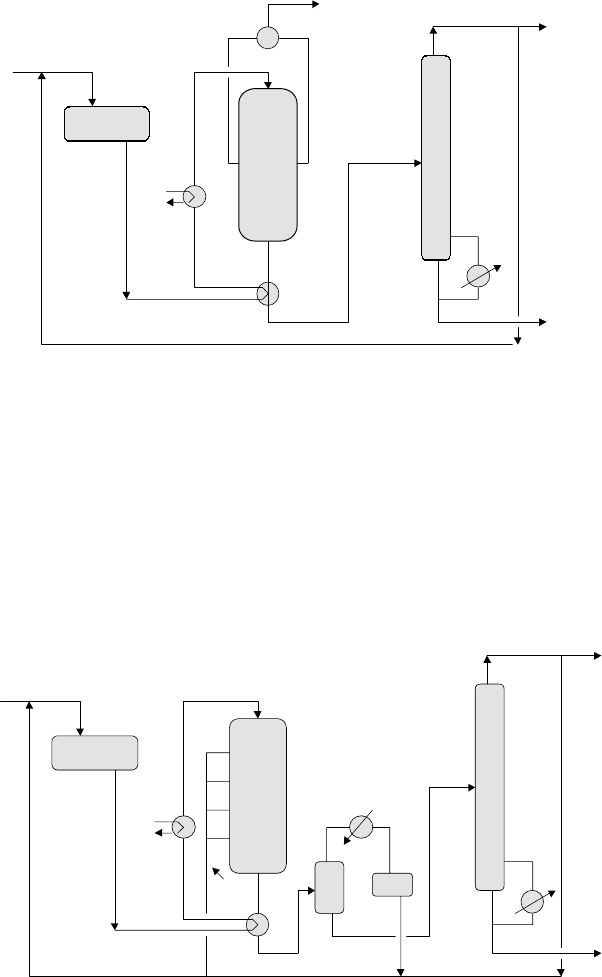
Figure 9.2.1 illustrates a simplified process flow diagram for gasoline production
using a tubular reactor. Figure 9.2.2 illustrates a similar diagram using an adiabatic
plug flow reactor. Either type can be used, except that smaller size catalyst particles
are normally used with a tubular reactor so as to minimize wall effects.
The tubular reactor system has the catalyst in the tubes and condensate or boiler
feed water is used to remove the exothermic heat of reaction and to raise steam on
the shell side. The reactants are circulated through the catalyst and the effluent is
cooled and passed to a stabilizer column. An LPG (propane–butane) recycle may be
maintained to control the concentration of olefins in the feed and the heat release in
the system. Usually, the olefin concentration in the feed to the reactor is maintained
at not more than about 50%. The gasoline produced is removed via the bottoms of the
stabilizer.
GASOLINE COMPONENTS 377
surge drum
reactorsteam
LPG recycle
steam LPG product
gasoline
olefinic feed
stabilizer column
Figure 9.2.1. Catalytic condensation with tubular reactor.
Adiabatic plug flow reactors do not have provisions for steam generation within the
reactor. Instead, an LPG interbed quench is used to control the temperature rise within
the reactor with maybe as many as five catalyst beds being arranged within the vessel.
The product is sent to a flash drum in which partial condensation occurs, and the
liquid is sent to a stabilizer column. The bottom from this column is the final gasoline
product. With this type of reactor, the concentration of olefins in the feed is usually
limited to about 30%. From an energy point of view, it is less efficient than a tubular
surge drum
reactor
steam
LPG recycle
LPG product
olefinic feed
flash system,
stabilizer column
gasoline
quench
Figure 9.2.2. Catalytic condensation with plug flow reactor and quench.

378 CHAPTER 9
Table 9.2.1. Oligomerization reactions
Propylene/butene feed: FCC–C
3
/C
4
fraction
Operation: without polymer recycle
Wt% yield based on feed olefins
Unreacted propylene 1.3 (97.3% conv.)
Unreacted butenes 3.7 (93.1% conv.)
C
6
olefins 4.4
C
7
olefins 41.3
C
8
olefins 24.0
C
9
olefins 15.8
C
10
+ olefins 9.5
reactor, but it is more economical to build. For practical purposes, the products made
by either system are almost identical.
Table 9.2.1 illustrates a typical product composition distribution based on a mixed
C
3
-C
4
feed from an FCC unit expressed as weight percent of the olefins in the charge
stock. Feed olefin conversion is about 97% for propylene and 93% for butenes, but
this may vary somewhat depending on the state of the catalyst, the feed composition,
and the operating conditions. Table 9.2.2 provides typical distillation properties of
depentanized gasoline; actual composition will vary depending on what vapor pres-
sure adjustments are desired to adapt the product to the gasoline pool requirements.
This means that the end product may also contain some pentenes and butenes if the
higher vapor pressure is acceptable for blending into the gasoline pool.
Table 9.2.2 also serves to illustrate three different typical operations of these units:
(1) polymer gasoline made from propylene only with no attempt made to produce
higher olefins beyond the gasoline range; (2) polymer gasoline from a mixed C
3
–C
4
feed; and (3) polymer gasoline from a unit operating with butenes only. The specific
Table 9.2.2. Typical polymer gasoline properties
C6+ basis
Polymer type: C3 poly C3/C4 poly C4 poly
Specific gravity 0.739 0.738 0.738
Engler distillation (
◦
C)
IBP 57 93 62
10 114 101 103
30 132 109 113
50 141 119 119
70 151 137 127
90 191 168 181
EP 218 211 216
GASOLINE COMPONENTS 379
gravities of all three products are virtually identical. Although these data are from
actual commercial operations, some differences arise from unit to unit. Also, it may
be appreciated that the mid-point (50%) and the end point (100%) for a propylene feed
are higher than for mixed feeds or butene feeds. In actual practice, these end points can
be adjusted in order to meet product specifications. Often too the amount of polymer
gasoline blended into the pool is small, so that it does not significantly alter the overall
properties of the pool; only seldom is the product rerun in order to meet more stringent
specifications. Interestingly, the product from a mixed C
3
-C
4
feed, although having
a higher molecular weight, has a lighter boiling range than that of the propylene
product. The butene product in Table 9.2.2 is typical of a codimer operation. This
type of operation was rare in the past, but because the octane number from this mode
of operating is exceptionally high, it is currently generating considerable interest as a
replacement for the production of methyl-tert-butyl ether (MTBE) in existing MTBE
units, and will be discussed later in more detail.
Hydrogenated versus nonhydrogenated polymer gasolines
from the catalytic condensation process
Polymer gasoline as obtained is almost totally olefinic. Polymer olefins, however, can
be easily hydrogenated to yield a very interesting range of products. Most polymer
gasolines blended in the gasoline pools have traditionally been olefinic, without any
attempt of hydrogenation. When the Catalytic Condensation process was first de-
veloped, polymer gasoline was far better than the natural straight-run materials then
available. Gasoline characteristics have changed dramatically since the 1930s, and
while polymer gasoline used to be the best material in the leaded gasoline pool be-
cause of its excellent lead susceptibility, it is now one of the poorest in today’s unleaded
gasolines. This perception, however, can be altered significantly if, instead of con-
sidering untreated polymer gasoline, one looks at the properties of the hydrogenated
products.
Olefinic products have a high Research (R) octane number (RON), but a low Mo-
tor (M) octane number (MON). As a result, the average octane index, (R + M)/2,
of unsaturated polymer gasolines is low. The difference (RON–MON) is called the
octane sensitivity; saturated or paraffinic products usually do not have a high octane
sensitivity and in general, but not always, have much higher octane indices.
Hydrogenation is frequently performed at conditions that lead to the skeletal isomer-
ization of the product. Polymer gasolines from SPA catalysts are highly branched,
much more than predicted from thermodynamic equilibrium. Therefore, skeletal iso-
merization of these products would tend to move in the direction to equilibrium and,
thus, to less branched products and to lower octane numbers. Thus, in general, hydro-
genation catalysts that avoid isomerization should be selected.
380 CHAPTER 9
Table 9.2.3. Octane numbers of hydrogenated polygasolines
Polymer type
C4 poly
C3 poly C3/C4 poly (codimer)
Olefinic polygasoline
RON 93 97 99
MON 82 83 84
Hydrogenated polygasoline
RON 49 70 99
MON 59 76 94
Table 9.2.3 illustrates typical octane numbers of clear olefinic and hydrogenated
products. These values were measured on actual commercial samples and correspond
to the trends that can be observed with these products. It is clear that hydrogenation
does not enhance the octane numbers in all cases, so polymer gasolines from propylene
feeds are best used without hydrogenation. It is interesting to note though that the MON
of the hydrogenated material is much higher than the RON—an octane sensitivity of
minus 10 is quite unusual in gasoline components of any kind.
Polymer gasoline from propylene–butene mixtures has higher RON typically in the
96–97 range, and a MON of about 83. In the past, when tetra ethyl lead (TEL)
was added, both of these numbers increased by about 3, so leaded polymer gasoline
from propylene–butylene had excellent characteristics with (R + M)/2 of about 93.
When this gasoline is hydrogenated, both the RON and MON drop significantly, again
rendering this product unattractive for motor fuel usage, but here too we encounter a
reverse octane sensitivity of minus six. We may add that, when used in its leaded form,
the RON and MON of the hydrogenated product increased to 86 and 90, respectively,
thus making it an acceptable blending component for the older leaded pools.
Of more interest is perhaps the operation with butene feed components. The RON
and MON of the product, sometimes called “codimer,” are up to 99 and 84, re-
spectively. Contrary to the negative experience with propylene and propylene–butene
feedstocks, the hydrogenated product from an all-butene feed operation, sometimes
called “hydrocodimer,” has increased RON and MON of 99 and 94, respectively,
which is excellent for gasoline blending in clear pools. This response was even more
striking in the past when lead was added. With the addition of 0.5 g/L TEL, the RON
and MON increased to 110–112 and to about 103, respectively. However, even in the
absence of lead, both the olefinic and the saturated products have excellent blending
characteristics.
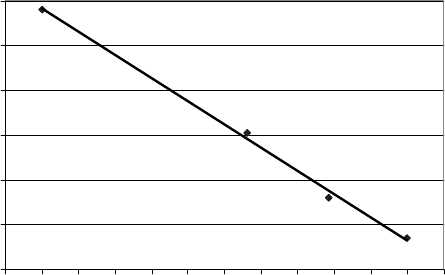
Figure 9.2.3 illustrates an approximate linear correlation between the butene content
in the feed and the RON of the hydrogenated product. The more butene there is in
GASOLINE COMPONENTS 381
40
50
60
70
80
90
100
-10
0
10
20 30 40 50 60 70 80 90 100 110
wt % propylene reacted relative to total gasoline
RON of the hydrogenated product
Figure 9.2.3. Effect of propylene content on RON of the hydrogenated polygasoline.
the feed to the catalytic olefin condensation unit, the higher will be the RON of the
hydrogenated product and the better the product will be as a paraffinic component of
the gasoline pool. Because of the absence of olefins and aromatics, this product meets
or exceeds all current environmental regulations.
It is seldom the case that the various components that integrate the composition of a
gasoline pool are used straight without blending. Normally, a motor fuel gasoline is
a complex blend of hydrocarbons generated from a number of sources, for example:
straight-run gasoline (mostly paraffinic with some aromatics, depending on the cut),
alkylate (paraffinic), isomerate (paraffinic), reformate (aromatic), FCC gasoline (aro-
matic), polymer gasoline (olefinic or paraffinic), and sometimes also hydrocracked
naphtha (mostly paraffinic). The octane numbers of these various components do not
blend as the octane numbers of the pure fractions; a “blending octane” has to be used
instead. Thus, for example, if two gasoline materials both with an octane number of
90 are blended together, there is no assurance that the mixture will have on octane
of 90; the octane of the blend may be the same, lower, or higher, depending on the
synergism among the various components as parametrized by the blending octane.
Table 9.2.4 illustrates the blending octanes of various polymer gasoline fractions
when blended with different base stocks. The reference base stock was a mixture of
approximately 60% FCC gasoline and 40% reformate, having a clear octane of 93
RON and 80.8 MON. Blend stocks consisting of the various polymer gasolines were
added at 10 or 20 vol% levels and the blending octanes were back calculated from
the octanes measured in the resulting mixtures. It must be noted, however, that small
errors in the determination of the octane values can lead to significant fluctuations in
the estimation of the blending octanes.
