Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

352 CHAPTER 8
catalyst. Therefore, in many cases it is desirable to remove the H
2
S from the recycle
gas. The removal of H
2
S is performed in a scrubber where the recycle gas in contacted
with an amine (generally MEA or DEA) solution. In this manner, the H
2
S content of
the recycle gas can be reduced to the parts per million range.
Another method to increase hydrogen purity is membrane separation. This system
removes the hydrogen from the vent gas and recycles it back with the makeup hydro-
gen.
Catalyst contaminants
Temporary
Process variables influence catalyst life by affecting the rate of carbon deposition on
the catalyst. There is a moderate accumulation of carbon on the catalyst during the
initial days of operation. The rate of increase will be reduced to very low levels under
normal processing conditions. A carbon level of 5 wt% may be tolerated without a
significant decrease in desulfurization. However, denitrogenation activity would be
reduced.
The sulfur and nitrogen found in the feed could be considered contaminants to the
extent that they produce hydrogen sulfide and ammonia which can react to form
ammonium bisulfide. The water injected into the reactor effluent dissolves the am-
monium bisulfide and prevents exchanger fouling. Organic nitrogen in the feed, if
present in amounts higher than expected, will require higher reactor temperatures for
processing, and will lead to a reduction in catalyst life.
If the water injection should be stopped for any period of time, the H
2
S and NH
3
may accumulate in the recycle gas and result in a sudden loss in catalyst activity.
The activity will return to normal once wash water is reestablished. Catalyst bed
temperatures should not be increased to compensate for the temporary activity loss.
Small amounts of molecular nitrogen, CO and CO
2
that enter the system with the
makeup gas are not harmful to the catalyst, but must be vented to prevent accumulation
in the recycle gas. Excessive amounts of CO and CO
2
may have an adverse effect
on catalyst performance, as they may be methanated by the catalyst taking up active
sites and liberating heat. This will raise the outlet temperature and reduce the apparent
catalystactivity. The unit should never be pressured up with high CO +CO
2
containing
makeup gas as a temperature runaway may result.
Permanent
Permanent loss of catalyst activity is usually caused by the gradual accumulation
of inorganic species picked up from the feed, makeup hydrogen or effluent wash
water. Examples include arsenic, lead, calcium, sodium, silicon, and phosphorus. Low
HYDROTREATING 353
concentrations of these elements (and other alkaline metals) can cause deactivation
over time as they are deposited on the catalyst.
Organic metal compounds are decomposed and typically deposit in the upper section
of the catalyst bed as a metal sulfide. The graded catalyst bed, if used, may contain
demetallization catalysts that have a high metals retention capacity. Some of these
catalysts may retain as much as 100 wt% of the fresh catalyst weight as metals from the
feed. These demetallization catalysts typically have a lower activity for desulfurization
and denitrogenation.
Hydrotreating catalysts exhibit a moderate tolerance for metals such as arsenic and
lead. Total metal content of 2–3 wt% of the hydrotreating catalyst have been observed.
However, product analysis frequency should be increased to monitor breakthrough
when calculations show the metals level on the hydrotreating catalyst exceeds 0.5
wt%. Metals cannot be removed by catalyst regeneration. Catalyst replacement should
be considered when metal loading of 1–2 wt% is exceeded on the hydrotreating
catalyst.
Apparent catalyst deactivation may be caused by the accumulation of deposits on
top of the catalyst bed. Solid material, such as corrosion products and coke, will
lead to rapid fouling of the catalyst bed if allowed to enter the reactor. This problem
is remedied by skimming a portion of the catalyst, screening, and reloading. Feed
filtering is quite effective in removing solid material, and as such results in longer
operating cycles due to the lower rate of reactor pressure drop build-up. The use of
feed filtering will depend on the type of feed processed and its source.
Hydrotreaters licensors and catalyst manufacturers
Licensors
Until the 1970s all the types of hydrotreating were offered as licensed processes by
several major licensors. Since that time, more and more of the types of hydrotreating
have been offered without a license and the catalyst used in the process was sold
as a commodity. Today, Chevron, IFP and UOP offer only residue hydrotreating as
a licensed process. A few specialized types of hydrotreating such as some aromatic
saturation units are also offered as licensed units, however, the vast majority of new hy-
drotreaters are sold without a license. Engineering contractors do most of the designs.
Catalyst suppliers
Through the years there have been many manufacturers of hydrotreating catalysts.
Other manufacturers have absorbed some, some have changed names, and some
354 CHAPTER 8
have discontinued hydrotreating catalyst manufacturing. Some suppliers simply sell
catalyst they have re-branded with their name. Following is a list of the current major
suppliers of hydrotreating catalysts: Advanced Refining Technologies LP, Akzo Nobel
Catalysts BV, Axens/Procatalyse SA, Catalysts and Chemicals Industries Co. Ltd.,
Chevron Research and Technology Co., Criterion Catalysts Co. Ltd., Exxon Research
and Engineering Co., Haldor Topsoe AS, Orient Catalyst Co. Ltd., Sud Chemie Inc.,
and UOP.
Chapter 9
Gasoline components
9.1 Motor fuel alkylation
James F. Himes and Robert L. Mehlberg*
9.1.1 Introduction
Motor fuel alkylation in the petroleum refining industry refers to the acid catalyzed
conversion of C
3
-C
5
olefins with isobutane into highly branched C
5
-C
12
isoparaffins
collectively called alkylate, a valuable gasoline blending component. A major con-
stituent of alkylate is 2,2,4-trimethyl pentane which is defined as 100 on the octane
scale.
Alkylation reactions are catalyzed by liquid and solid acids, including H
2
SO
4
, AlCl
3
-
HCl, HF, HF-BF
3
,H
2
SO
4
-HSO
3
F (Fluorosulfuric acid), Trifluoromethane sulfonic
acid chlorided Pt alumina, BF
3
on alumina, zeolites, and ion exchange resins. How-
ever, the catalysts and associated processes commercialized during WWII for aviation
gasoline, HF alkylation, and sulfuric acid alkylation, are the focus of this section as
these remain the primary commercial motor fuel alkylation processes. A solid catalyst
alkylation process (UOP Alkylene™) has been developed and is being offered to the
industry.
History
In 1932–6, alkylation was independently discovered by UOP,
1
Shell, the Anglo Iranian
Oil Company (AIOC), and Texaco whose first publications issue in that order. Herman
Pines told the story of UOP’s discovery of the alkylation of ethylene by pentanes in
1932.
2
At that time leading universities taught that isoparaffins were inert except at
high temperatures and pressures. After finding anomalies in an olefin assay based
upon H
2
SO
4
extraction Pines and his mentor V. I. Ipatieff hypothesized that paraffins
may not be inert to acids. Despite ridicule, they tested that hypothesis by bubbling
ethylene into chilled pentanes over AlCl
3
. All the ethylene was converted into saturated
*UOP LLC.
355
356 CHAPTER 9
hydrocarbons. Over the next few years they tested AlCl
3
-HCl, H
2
SO
4
, HF, and HF-
BF
3
as alkylation catalysts.
Alkylate was found to have excellent aviation gasoline properties. It was the highest
octane fuel component then known, with high motor octane and excellent lead re-
sponse. All of the properties derive from the highly-branched paraffins that form its
composition.
Humble Oil built the first commercial H
2
SO
4
alkylation unit in 1938 at Baytown,
Texas.
3
Alkylation for aviation gasoline grew rapidly with the Allies war effort. In
1939, six petroleum companies formed a consortium to pool their alkylation tech-
nology and develop both sulfuric acid and HF acid processes for 100 octane aviation
fuel. The first commercial HF alkylation unit started up in 1942. During the war 60
alkylation units were built for the Allies’ war effort. Half were built with sulfuric acid
as the catalyst and half with HF.
Following World War II, most alkylation operations were discontinued although a few
refiners continued to use the process for aviation and premium automobile gasolines.
In the mid-1950s, use of higher performance automotive engines required the refining
industry to both increase gasoline production and quality. The developmentof catalytic
reforming, such as UOP Platforming™, provided refiners with an important refining
tool for production of high octane gasolines. However, the motor fuel produced in
such operations, called reformate, is highly aromatic with a higher sensitivity (the
spread between research and motor octane) and a lower lead response than alkylate.
Many refiners expanded their alkylation operations and began to broaden the range
of olefin feeds to both existing and new alkylation units to include propylene and
occasionally even some pentenes along with the butenes.
With the phase-out of leaded gasolines and the advent of environmental gasolines the
lead response of alkylate is no longer valued, but the importance of alkylate and its
production have both grown because of its other properties. Its high unleaded motor
octane, low volatility, low-sulfur, and nearly zero olefins and aromatics make alkylate
critical to the production of quality environmental gasolines. Alkylate can reach 60%
of low-sulfur reformulated premium.
Licensors of motor fuel HF Alkylation processes are UOP LLC and Phillips. Licensors
of H
2
SO
4
alkylation processes are Exxon Mobil and Stratco Engineering.
Process chemistry
The reactions taking place in the alkylation reactor are many and relatively complex.
First the olefin reacts with the acid to form an ester; then the ester is alkylated by a
GASOLINE COMPONENTS 357
t-butyl carbenium ion chain mechanism.
4
The principal overall reactions are described
below.
Primary alkylation reactions
Most of the alkylate product is made by primary alkylation reactions. In these reactions
one mole of olefin reacts with one mole of isobutane to form an isoparaffin exactly
4 carbon numbers heavier. Example primary alkylation reactions, showing only the
carbon framework and one or two of the principal product isomers, are shown for
each of the principal feed olefins in equations (1.1)–(1.5).
ALKYLATION OF BUTENE-2
C—C = C—C + C—C
|
C
—C → C—C
|
C
|
C
—C—C
|
C
—C
cis-Butene-2 + Isobutane → 2,2,4-Trimethylpentane (1.1)
(100 RON)
ALKYLATION OF BUTENE-1
C = C—C—C + C—C
|
C
—C → C—C
|
C
—C—C
|
C
—C—C
Butene-1 + Isobutane → 2,3-Dimethylhexane (1.2)
(71.3 RON)
C = C—C—C+C—C
|
C
—C → C—C
|
C
—C
|
C
—C
|
C
—C
Butene-1 + Isobutane → 2,3,4-Trimethylpentane (1.3)
(102.7 RON)
ALKYLATION OF ISOBUTYLENE (I-BUTENE)
C=C
|
C
—C+C—C
|
C
—C → C—C
|
C
—C—C
|
C
—C
Isobutylene + Isobutane → 2,2,4-Trimethylpentane (1.4)
(100 RON)
358 CHAPTER 9
ALKYLATION OF PROPYLENE
C—C=C+C—C
|
C
—C → C—C
|
C
—C
|
C
—C—C
Propylene + Isobutane → 2,3-Dimethylpentane (1.5)
(91.1 RON)
ALKYLATION OF 2-METHYL-BUTENE-2
C—C = C—C + C
|
C
—C—C → C
|
C
—C—C—C
|
C
—C—C
2-methylbutene-2 +Isobutane → 2,2,4-Trimethylhexane (1.6)
(92 RON)
Complex alkylation reactions
The balance of the alkylate and small amounts of undesirable byproduct conjunct
polymer are formed by more complex reactions of the ionic intermediates. Some of
these reactions are given by equations (2.1)–(2.5). For brevity only a few key steps
and no structures are shown.
HYDRIDE TRANSFER (PROPYLENE EXAMPLE)
C
3
H
6
+ H
+
→ C
3
H
+
7
(Formation of propenium ion)
C
3
H
+
7
+ iC
4
H
10
→ iC
4
H
9
+
+ C
3
H
8
(Hydride transfer from isobutane to
propenium ion)
iC
4
H
+
9
→ iC
4
H
8
+ H
+
iC
4
H
8
+ iC
4
H
10
→ iC
4
H
10
Overall
C
3
H
6
+ 2iC
4
H
10
→ C
3
H
8
+ C
8
H
18
Propylene + 2 Isobutane → Propane + 2,2,4-Trimethylpentane (2.1)
POLYMERIZATION (ISOBUTYLENE EXAMPLE)
iC
4
H
8
+ iC
4
H
+
9
→ iC
8
H
+
17
iC
8
H
+
17
+ iC
4
H
8
→ iC
12
H
+
25
iC
12
H
+
25
+ iC
4
H
10
→ iC
12
H
26
+ iC
4
H
+
9
Overall
2iC
4
H
8
+ iC
4
H
10
→ iC
12
H
26
2 isobutylene + Isobutane → Pentamethylheptane (2.2)
GASOLINE COMPONENTS 359
CRACKING (CRACKING OF HEAVY ENDS FROM ISOBUTYLENE)
2iC
4
H
8
+ iC
4
H
+
9
→ iC
12
H
+
25
iC
12
H
+
25
→ iC
5
H
+
11
+ iC
7
H
14
iC
5
H
+
11
+ iC
4
H
10
→ iC
5
H
12
(isopentane) + iC
4
H
+
9
(t-butyl ion)
iC
4
H
+
9
+ iC
7
H
14
→ iC
11
H
+
23
iC
11
H
+
23
+ iC
4
H
10
→ iC
11
H
24
+ iC
4
H
+
9
(t-butyl ion)
Overall
2iC
4
H
8
+ 2iC
4
H
10
→ iC
5
H
12
+ iC
11
H
24
2 isobutylene + 2 isobutane → isopentane + isoundecane (2.3)
COMPETITION (ISOPENTANE ALKYLATION)
iC
5
H
12
+ C
4
H
8
→ iC
9
H
20
Isopentane + butene-2 → 2,2,5-Trimethlyhexane (2.4)
CONJUNCT POLYMERIZATION
17iC
4
H
8
+ H
+
→→→ C
20
H
+
31
+ 4C
12
H
26
isobutylene → conjunct polymer ion (in acid phase) + Heavy alkylate (2.5)
Isomerization
In addition to the listed reactions the acids catalyze isomerization of olefins or their
esters before alkylation as well as isomerization of the alkylate products. Most of the
product isomerizations are methyl shifts of ionic intermediates.
r
Reaction selectivites—The selectivities of these reactions depend upon the feed-
stock, catalyst, and reaction conditions.
r
Isobutylene and isopentenes are very reactive and are prone to polymerization to
heavy alkylate (reaction (2.2)) and to conjunct polymer (reaction (2.5)), especially
when catalyzed by H
2
SO
4
.
r
Propylene is less reactive and tends to make more conjunct polymer, especially for
the H
2
SO
4
catalyst.
r
Butene-1 makes higher yields of low-octane dimethyl hexanes (reaction (1.2)) than
butene-2 in HF, but there is little difference in these olefins in H
2
SO
4
. To maximize
octane many refiners have added selective hydrogenation units (such as UOP-Huls
SHP™) upstream of the alkylation unit to saturate diolefins reducing the amount
of conjunct polymers as well as isomerizing most of the Butene-1 to Butene-2.
r
HF enhances hydride transfer reactions (2.1) much more than H
2
SO
4
. These re-
actions increase alkylate yield, RVP, and octane. As a result, HF catalyzes higher
yields of isopentane from pentenes than H
2
SO
4
; it also produces low amounts of
n-paraffins from n-olefins while H
2
SO
4
produces none. The additional yield of
hydride transfer reactions naturally comes with increased isobutane consumption.
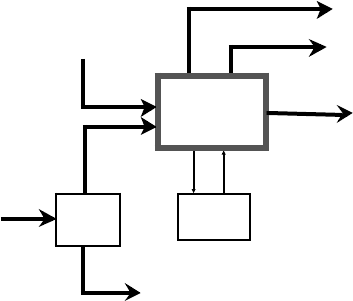
360 CHAPTER 9
FCC
Alkylate
Product
FCC Feed
Alkylation
Unit
n-Butane
Mixed
Butanes
Butamer
Propane
C3/C4 Olefin
Rich Stream
FCC Products
(Gasoline, etc.)
Figure 9.1.1. Alkylation unit location in refinery.
r
H
2
SO
4
produces more heavy ends (reaction 2.2) and more conjunct polymer (reac-
tion 2.5) and less isopentane than HF, especially from iso-olefins and propylene. For
all olefins it produces lower yields, higher endpoints, and consumes less isobutane.
r
Increasing reactor temperature increases cracking and conjunct polymer formation
at the expense of primary alkylation lowering product octane and increasing RVP
and acid consumption.
r
Increasing isobutane concentration increases primary alkylation and hydride trans-
fer reactions while reducing polymer formation, cracking, and competition which
increase yield and octane and reduce acid consumption.
HF alkylation process flow description
Figure 9.1.1 illustrates how an alkylation unit fits into the refinery in a typical FCC-
Alkylation arrangement. The location of the butane isomerization unit (Butamer™)
is also indicated as many refiners have this arrangement.
Figure 9.1.2 is a simplified process flow diagram for the UOP Propylene-Butene HF
Alkylation process.
Feed pretreatment
In the UOP HF alkylation process olefin-rich feeds from the FCC gas plant are
typically deethanized, Merox treated to remove H
2
S and mercaptans and dried. Some
refiners have also added MTBE or Selective Hydrogenation (SHP) plants upstream
the alky.
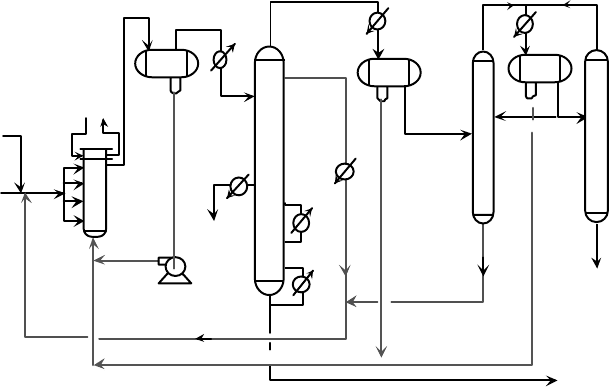
GASOLINE COMPONENTS 361
Olefin
Feed
Recycle
i-C
4
Cooling
Water
Alkylate
Product
Propane
Product
Saturate
Feed
n-C
4
Product
Isostripper
DeC3
HF Stripper
Reactor
Settler
Figure 9.1.2. UOP HF alkylation.
Reaction
After pretreatment, the olefin feeds are combined with a large excess of recycle isobu-
tane to provide an 6–14 isobutane:olefin molar ratio and injected into the circulating
HF acid catalyst at the shell side inlet to the water-cooled reactor. Cooling water
flows through the reactor tubes to remove the highly exothermic heat of reaction
and to maintain reaction conditions at 80–100
◦
F. The alkylation reaction is very fast
with 100% olefin conversion. The excess isobutane, alkylate product, non-reactive
hydrocarbons (propane, n-butane) in the feeds and the acid catalyst pass on to the set-
tler vessel. The dense acid phase separates from the hydrocarbons rapidly by gravity
and is then pumped back to the reactor. The hydrocarbons containing dissolved HF
flow off the top of the settler to the isostripper.
Fractionation
It consists of an isostripper, a depropanizer, and an HF Stripper. The isostripper is
a large tower with two sidedraws with the primary function recycling isobutane to
support the high isobutane:olefin molar ratio of the reactor. The tower typically has
two reboilers; the upper reboiler typically maximizes the use of relatively low cost low
or medium pressure steam and the lower reboiler uses a heating medium that can give
400–450
◦
F process temperatures. Alkylate is drawn off the bottom of the tower, cooled
in exchangers, and sent to product storage. The next product draw up the tower is the
n-butane sidedraw and above that is the isobutane recycle draw. On most UOP units the
isobutane draw is located below the feed tray to minimize HF in the isobutane recycle.
